Abstract
Objectives
To evaluate mineral metabolism markers as potential risk factors for calcific aortic valve disease.
Background
Mineral metabolism disturbances are common among older people and may contribute to cardiac valvular calcification. Associations of serum mineral metabolism markers with cardiac valvular calcification have not been evaluated in a well-characterized general population of older adults.
Methods
We measured serum levels of phosphate, calcium, parathyroid hormone, and 25-hydroxyvitamin D in 1,938 Cardiovascular Health Study participants who were free of clinical cardiovascular disease and who underwent echocardiography measurements of aortic valve sclerosis (AVS), mitral annular calcification (MAC), and aortic annular calcification (AAC). We used logistic regression models to estimate associations of mineral metabolism markers with AVS, MAC, and AAC after adjustment for relevant confounding variables, including kidney function.
Results
The respective prevalences of AVS, MAC, and AAC were 54%, 39%, and 44%. Each 0.5 mg/dl higher serum phosphate concentration was associated with a greater adjusted odds of AVS (odds ratio 1.17, 95% confidence interval 1.04 to 1.31, p = 0.01), MAC (odds ratio 1.12, 95% confidence interval 1.00 to 1.26, p =0.05), and AAC (odds ratio 1.12, 95% confidence interval 0.99 to 1.25, p = 0.05). In contrast, serum calcium, parathyroid hormone, and 25-hydroxyvitamin D concentrations were not associated with aortic or mitral calcification.
Conclusions
Higher serum phosphate levels within the normal range are associated with valvular and annular calcification in a community-based cohort of older adults. Phosphate may be a novel risk factor for calcific aortic valve disease and warrants further study.
Keywords: Phosphate, Aortic Valve, Mitral Valve, Calcification, Epidemiology
Calcific aortic valve disease (CAVD) is a progressive condition involving calcification and fibrosis of the aortic valve leaflets. The disease sequence begins with aortic valve sclerosis (AVS), in which the leaflets thicken and develop microcalcification but do not obstruct left ventricular outflow. Progressive calcification and fibrosis result in obstruction to left ventricular outflow, which characterizes clinical aortic stenosis. Both aortic sclerosis and aortic stenosis are common among older people(1) and share histologic and epidemiologic features with atherosclerosis.(2-4) However, unlike atherosclerosis, there is no known effective medical therapy for CAVD, and a recent large randomized clinical trial of lipid lowering therapy failed to reduce the progression of aortic stenosis.(5) Identification of novel, modifiable risk factors remains an essential next step in developing medical therapies for valvular heart disease.
Disturbances in mineral metabolism are common among older adults,(6,7) and preliminary evidence suggests a potential role in the pathogenesis of cardiac valve calcification. In the setting of kidney disease, in which phosphate metabolism is grossly disturbed, higher serum phosphate concentrations are associated with aortic and mitral valve calcification.(8,9) Moreover, primary hyperparathyroidism is linked with aortic valve calcification,(10) and vitamin D receptor polymorphisms are associated with aortic stenosis.(11) These connections are not yet evaluated in a general population of older people without known kidney impairment or hyperparathyroidism.
We tested the hypotheses that serum concentrations of phosphate, parathyroid hormone, and 25-hydroxyvitamin D would be associated with aortic valve sclerosis (AVS), mitral annular calcification (MAC) and aortic annular calcification (AAC) in a population-based cohort of ambulatory older adults.
Methods
Study Population
We evaluated participants from the Cardiovascular Health Study (CHS), a prospective population-based cohort study of cardiovascular disease among older adults. Details of the study design have been published previously.(12) Briefly, between 1989 and 1990, CHS enrolled 5,201 ambulatory adults aged 65 years or older from 4 US communities (Forsyth County, North Carolina; Washington County, Maryland; Sacramento County, California; and Pittsburgh, Pennsylvania). An additional 687 African Americans were enrolled between 1992 and 1993. Exclusions from CHS included the use of a wheelchair, institutionalized, inability to give informed consent, plans to move away from the area within 3 years, or active treatment for malignancy. Each participating center received institutional review board approval, and all individuals gave informed consent.
We studied CHS participants from the 1992-1993 examination who had available measurements of mineral metabolism markers, which were performed as a part of a different ancillary study of incident cardiovascular events. Participants were excluded from the cardiovascular events study if they had a preexisting clinical history of any one of the following cardiovascular diseases: coronary artery disease, heart failure, aortic stenosis, stroke, claudication, arrhythmias, or presence of a pacemaker or implantable-cardiac defibrillator. Cardiovascular conditions were determined by review of medical records, electrocardiographic findings, and patient questionnaires.(13) There were 4,692 individuals who attended the 1992-1993 CHS examination. A total of 1,428 participants were excluded due to prevalent cardiovascular disease, 948 due to inadequate serum volume to run the mineral metabolism measurements, and 378 due to not completing an echocardiogram, leaving 1,938 participants for analysis.
Measurements
Mineral metabolism measurements were performed using serum samples collected during the 1992-1993 CHS examination. Participants were asked to fast prior to collection. Samples were stored at the Laboratory for Clinical Biochemistry Research (LCBR) at the University of Vermont using established methods to ensure long-term stability,(14) and were assayed at the University of Washington Clinical Nutrition Research Unit laboratory. Total 25-OHD was measured on a Waters Quattro Micro mass spectrometer with an interassay coefficient of variation (CV) <3.4%. Intact PTH was quantified with a two-site immunoassay (Beckman Unicell DxI clinical analyzer) with a reference range of 17-66 pg/mL. Phosphate levels were determined using a timed-rate colorimetric reaction method with ammonium molybdate at acidic pH on a Beckman DxC Synchron analyzer. Serum non-ionized total calcium was measured using indirect potentiometry on a Beckman DxC Synchron clinical analyzer
Trained CHS study personnel conducted standardized interviews to determine participant demographics, past medical history, lifestyle factors, and medication data. Blood pressure was measured in triplicate 5 minutes apart. Prescription medication use was ascertained from a review of prescription bottle labels by interviewers. Lipid measurements were made at the LCBR and low-density lipoprotein was calculated using the Friedwald equation. Estimated glomerular filtration rate (eGFR) was derived from cystatin C measurements using the equation: eGFR = 76.7*(cystatin C)-1.19.(15) Hypertension was defined as a systolic blood pressure greater than or equal to 140 mmHg, diastolic blood pressure greater than or equal to 90mmHg or use of anti-hypertensive medications. Diabetes was defined by fasting glucose levels >7.8 mmol/L (>140 mg/dL) or the use of a diabetic medication.
Echocardiography
Two-dimensional echocardiograms were recorded on videotape using a Toshiba SSH-160A ultrasound machine during the 1994-1995 CHS examination, as detailed previously.(16) The echocardiograms were evaluated at a centralized core laboratory (Georgetown University, Washington, DC) by observers blinded to the participants’ clinical history.
AVS, MAC, and AAC were defined based on previous CHS studies.(17,18) Aortic annulus (AAC) and leaflet calcification (AVS) are considered separate outcomes due to their different cellular components and potential calcification mechanisms. AVS was identified as aortic cusp thickening with normal aortic cusp excursion and a peak trans-aortic valve flow velocity <2.0 m/s. MAC was defined by an intense echocardiograph-producing structure located at the junction of the atrioventricular groove and posterior mitral leaflet on the parasternal long-axis, short axis, or apical four-chamber view. The presence of AAC was similarly defined as increased echodensity of the aortic root at the insertions of the aortic cusps. For 167 participants, individual components of the aortic valve evaluation (peak velocity, cusp excursion, or leaflet thickness) were not evaluated or were considered to be abnormal, but did not meet the definition of AVS; these individuals were excluded from AVS analyses. There were 16 participants without an assessment of MAC and 48 participants without evaluation of AAC, who were excluded from analyses of these outcomes, respectively.
Statistical Analysis
We evaluated mineral metabolism exposure variables continuously, and using previously published categories.(8) We analyzed AVS, MAC, and AAC as binary outcome variables. We used linear regression to explore associations of covariates with the serum phosphate concentration (supplementary appendix). We used logistic regression with robust standard errors to estimate the association of each mineral metabolism variable with the log odds ratio of each binary outcome. We created nested multivariable models to evaluate an a priori set of potential confounding variables: age, sex, and race, estimated GFR, hypertension, diabetes, smoking, body mass index (BMI), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), statin use, serum calcium levels, and clinic site. Individual serum samples at each clinical site were collected during the same season, thus providing seasonally-adjusted estimates. We investigated potential non-linear associations using cubic spline models for continuous mineral metabolism covariates. There were 11 individuals that were self-identified as an ethnicity that was not Caucasian or African-American, these measurements were classified into the Caucasian strata when models were adjusted for race given small numbers. Because of the high prevalence of AVS in this population, odds ratios do not approximate relative risk well, thus we additionally calculated adjusted proportions for easier clinical interpretation.
We tested for whether sex, race, estimated GFR, and/or serum calcium level modified associations of serum phosphorus with study outcomes using the Wald test. Given no statistically significant interactions we present these associations are presented as a pooled analyses. We conducted a sensitivity analysis by excluding patients with overt hyperphosphatemia (>4.5 mg/dL); this exclusion did not change inferences. All analyses were conducted using Stata 10.1 (Stata Corp, College Station, TX)
Results
There were 1,938 individuals with serum phosphate measurements and available echocardiogram data in this analysis. Serum phosphate concentrations were normally distributed, with a mean±SD of 3.6 mg/dL±0.5 mg/dL. Overt hyperphosphatemia was rare in this cohort; >97% of individuals had a serum phosphate level below the upper limit of normal (4.5 mg/dL), and there were only 3 individuals who had a serum phosphate level >5.0 mg/dL. Mean±SD values for serum concentrations of 25-OHD and PTH were 25.8±11.7 ng/mL and 56.0±28.8 pg/mL, respectively. Measurements consistent with primary hyperparathyroidism (calcium >10mg/dL and PTH >65 pg/mL) were found in 25 (1%) participants and 304(16%) individuals had 25-OHD deficiency (<15 ng/mL).
The average age of the study population was 73.5 years. The majority of participants were Caucasian and the mean eGFR was 76.6 mL/min/1.73m2 (Table 1). Excluded patients without available measurments were more likely to be older, male, and have more comorbidities (Supplementary appendix). In multivariable analysis, female sex had the strongest association with serum phosphate levels, which were on average 0.4 mg/dL greater among women. Additionally, LDL cholesterol correlated directly with the serum phosphate concentration, while BMI, systolic blood pressure, eGFR, and PTH were correlated inversely.
Table 1.
Baseline Characteristics of Participants by Serum Phosphate Concentration.
| Serum Phosphate Concentration | ||||
|---|---|---|---|---|
| Characteristic | ≤3.0 mg/dl (n=271) | 3.1 – 3.5 mg/dl (n=657) | 3.6 – 4.0 mg/dl (n=675) | > 4.0 mg/dl (n=335) |
| Age(years) | 73.3± 4.6 | 73.5±4.6 | 73.6±4.4 | 73.6±4.7 |
| Males | 174 (64.2) | 246 (37.4) | 119 (17.6) | 33 (10.9) |
| Race | ||||
| Caucasian | 227 (84) | 564 (86) | 592 (88) | 291 (87) |
| African-American | 44 (16) | 93 (14) | 83 (12) | 44 (13) |
| Hypertension | 170 (62.7) | 360 (54.8) | 342 (50.7) | 206 (61.5) |
| Diabetes | 39 (14.4) | 79 (12.0) | 62 (9.2) | 34 (10.1) |
| Smoking (current or past) | 153 (56.5) | 329 (50.1) | 323 (47.9) | 146 (43.6) |
| Medication use | ||||
| Anti-hypertensive | 101(37.3) | 265 (40.3) | 251 (37.2) | 136 (40.6) |
| Statin | 7 (2.6) | 20(3.0) | 39 (5.8) | 23 (6.9) |
| BMI (kg/m2) | 27.5±4.4 | 27.1±4.6 | 26.6±4.8 | 26.2±4.4 |
| LDL (mg/dL) | 122.5±31.7 | 125.7±30.6 | 130.3±32.9 | 135.3±33.5 |
| HDL (mg/dL) | 50.6±13.6 | 54.7±14.6 | 56.9±14.0 | 58.2±15.2 |
| eGFR (mL/min/1.73m2) | 77.7±18.9 | 76.5±17.2 | 77.0±17.0 | 75.4±18.4 |
| Calcium (mg/dL) | 9.44±0.38 | 9.47±0.36 | 9.49±0.32 | 9.5±0.42 |
| 25-OHD (ng/mL) | 26.3±11.3 | 25.6±10.1 | 26.0±12.6 | 25.4±13.1 |
| PTH (pg/mL) | 67.8±33.9 | 57.9±28.7 | 52.3±24.1 | 50.5±30.4 |
Continuous data presented as means ±standard deviation, Categorical as number (%)
Abbreviations: BMI, Body Mass Index; LDL, low-density lipoprotein; HDL, High-Density Lipoprotein; eGFR, estimated glomerular filtration rate; PTH, Parathyroid Hormone; 25-OHD, 25-hydroxyvitamin D.
The respective prevalences of AVS, MAC, and AAC were 54%, 39%, and 44%. In unadjusted and demographic adjusted models, higher serum phosphate concentrations were associated with greater odds of each valvular calcification outcome (Table 2). Associations of serum phosphate with AAC no longer reached statistical significance in fully adjusted models. The adjusted prevalences of AVS, MAC, and AAC were 12%, 11%, and 7% greater, comparing participants in the highest (>4.0 mg/dl) versus lowest (≤3.0 mg/dl) serum phosphate category. Associations of serum phosphate with valve outcomes appeared to be generally linear (Figure 1)
Table 2.
Association of Serum Phosphate Concentration with Aortic Valve Sclerosis, Mitral Annular Calcification and Aortic Annular Calcification
| Unadjusted OR (95% CI) | P | Model 1* OR (95% CI) | P | Model 2† OR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Aortic Valve Sclerosis | ||||||
| ≤3.0 mg/dL | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |||
| 3.1-3.5 mg/dL | 1.11 (0.82-1.50) | 1.23 (0.91-1.68) | 1.23 (0.90-1.70) | |||
| 3.6-4.0 mg/dL | 1.23 (0.91-1.65) | 1.47 (1.07-2.02) | 1.46 (1.04-2.05) | |||
| >4.0 mg/dL | 1.44 (1.02-2.01) | 1.78 (1.23-2.57) | 1.64 (1.10-2.43) | |||
| per 0.5 mg/dL | 1.12 (1.01-1.23) | 0.03 | 1.20 (1.07-1.33) | 0.001 | 1.17 (1.04-1.31) | 0.01 |
| Mitral Annular Calcification | ||||||
| ≤3.0 mg/dL | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |||
| 3.1-3.5 mg/dL | 1.35 (1.00-1.82) | 1.30 (0.95-1.77) | 1.39 (1.01-1.93) | |||
| 3.6-4.0 mg/dL | 1.44 (1.07-1.94) | 1.34 (0.97-1.84) | 1.31 (0.94-1.85) | |||
| >4.0 mg/dL | 1.70 (1.22-2.38) | 1.58 (1.11-2.27) | 1.62 (1.10-2.38) | |||
| per 0.5 mg/dL | 1.16 (1.06-1.30) | 0.001 | 1.13 (1.02-1.26) | 0.02 | 1.12 (1.00-1.26) | 0.05 |
| Aortic Annular Calcification | ||||||
| ≤3.0 mg/dL | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |||
| 3.1-3.5 mg/dL | 1.03 (0.77-1.38) | 1.07 (0.79-1.44) | 1.06 (0.77-1.45) | |||
| 3.6-4.0 mg/dL | 1.27 (0.95-1.70) | 1.35 (0.99-1.84) | 1.28 (0.92-1.78) | |||
| >4.0 mg/dL | 1.40 (1.01-1.94) | 1.50 (1.05-2.14) | 1.32 (0.90-1.92) | |||
| per 0.5 mg/dL | 1.14 (1.04-1.25) | 0.006 | 1.17 (1.05-1.29) | 0.003 | 1.12 (0.99-1.25) | 0.05 |
OR, Odds Ratio; CI, Confidence Interval
Adjusted for age, sex, and race
Adjusted for age, sex, race, estimated glomerular filtration rate, hypertension, diabetes, smoking, body mass index, low density lipoprotein, high density lipoprotein, statin use, vitamin D, parathyroid hormone, calcium, and site
Figure 1.
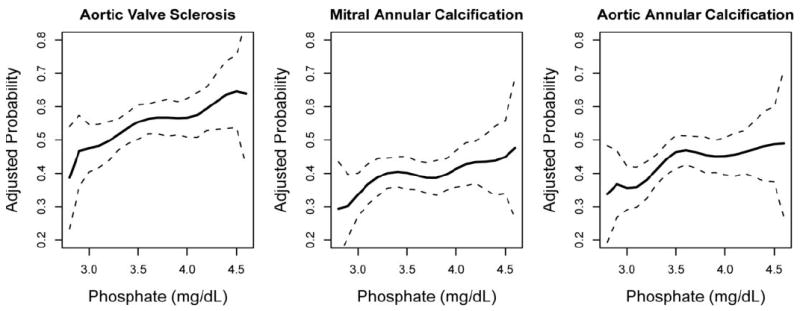
Association of Phosphate and Valve Calcification - Fully adjusted cubic splines showing higher prevalence of aortic valve sclerosis, mitral annular calcification, and aortic annular calcification with higher serum phosphate concentration. Dotted lines indicate 95% Confidence Interval.
Serum concentrations of 25-OHD and PTH were not associated with any of the valvular outcomes (Table 3 and Figure 2). There was no association with calcium and AVS (Odds Ratio: 0.93, 95% CI 0.70 – 1.25), MAC (Odds Ratio: 1.24, 95% CI: 0.91-1.70), or AAC (Odds Ratio:1.02, 95% CI:0.77-1.36). Exclusion of participants with primary hyperparathyroidism did not alter these results.
Table 3.
Odds Ratios (95% CI) of Aortic Valve Sclerosis, Mitral Annular Calcification and Aortic Annular Calcification by Parathyroid Hormone and 25-hydroxyvitamin D Levels
| Aortic Valve Sclerosis | Mitral Annular Calcification | Aortic Annular Calcification | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| Parathyroid hormone(pg/mL) | ||||||
| ≤65 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| >65 | 1.08 (0.87 – 1.35) | 1.21 (0.94 – 1.56) | 0.99 (0.80 – 1.22) | 0.87 (0.68 – 1.12) | 1.01 (0.82 – 1.25) | 0.89 (0.69 – 1.13) |
| 25-hydroxyvitamin D(ng/mL) | ||||||
| ≥30 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| 15 – 30 | 0.93 (0.75 – 1.16) | 0.87 (0.69 – 1.10) | 1.08 (0.88 – 1.33) | 1.00 (0.79 – 1.26) | 1.38 (1.11 – 1.70) | 1.33 (1.06 – 1.67) |
| <15 | 1.04 (0.76 – 1.42) | 0.83 (0.60 – 1.17) | 1.33 (1.01 – 1.77) | 1.21 (0.87 – 1.69) | 1.33 (0.97 – 1.80) | 1.12 (0.81 – 1.56) |
Adjusted for age, sex, race, estimated glomerular filtration rate, hypertension, diabetes, smoking, body mass index, low density lipoprotein, highdensity lipoprotein, statin use, calcium, and site
Figure 2.
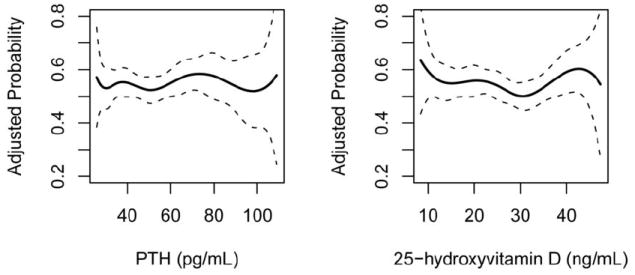
Fully Adjusted Prevalence of Aortic Valve Sclerosis – No association of parathyroid hormone and 25-hydroxyvitamin D with aortic valve sclerosis. Dotted lines indicate 95% Confidence Interval.
Discussion
In a community-based population of older adults without known cardiovascular disease, higher serum phosphate levels that were elevated, but still within the normal range, were associated with aortic valve sclerosis and mitral annular calcification. Associations of phosphate with aortic annular calcification also were observed but did not reach statistical significance after full adjustment. The observed associations were independent of kidney function, parathyroid hormone, calcium, and 25-hydroxyvitamin D, all of which play biological roles in phosphate metabolism and calcification. Importantly, associations were not observed between parathyroid hormone, calcium, or 25-hydroxyvitamin D and calcification outcomes.
To our knowledge, this study is the first to demonstrate an association between serum phosphate levels and cardiac valve calcification in a population-based cohort without CKD. A previous retrospective cross-sectional study of patients with aortic stenosis found an inverse relationship of phosphorus with aortic valve area on echocardiogram.(19) Similarly, a recent case-control study of referred patients with aortic stenosis had a higher unadjusted level of phosphate than age and gender matched controls.(20) Phosphate levels have been associated with calcification of coronary arteries(21,22) and with incidence of future cardiovascular events independent of kidney function.(23-25) However, none of these previous studies were able to concomitantly measure and adjust for parathyroid hormone or vitamin D levels.
The odds of aortic sclerosis per standard deviation of phosphate are similar in magnitude to other well-established modifiable risk factors for aortic valve calcification. Stewart et al, previously reported small associations with LDL cholesterol [OR 1.12, 95% CI:1.03-1.23 (75th vs 25th percentile], hypertension (1.23, 95% CI: 1.1-1.4) and smoking (OR 1.3, 95% CI: 1.1-1.7).(3) Similar magnitudes for LDL have been shown in older adults in the Multiethnic Study of Atherosclerosis. Per 1 standard deviation of LDL-cholesterol for those aged 65-74 and 75-84, the OR of aortic valve calcification was 1.09 and 1.16, respectively.(26)
A potential mechanism explaining the relationship between phosphate and cardiovascular disease is dystrophic calcification. Animal models with deletion of the gene for fibroblast growth factor-23, which controls phosphate metabolism, produce phenotypes characterized by hyperphosphatemia, arteriosclerosis, and ectopic cardiac calcification that can be reversed with restrictions in dietary phosphorus.(27,28) In vitro studies have shown that phosphate levels within the normal range can trigger osteogenic transformation and mineralization of cultured smooth muscle cells.(29) Similar cells that can undergo transdifferentiation into osteoblast-like cells, myofibroblasts, have been identified in the aortic valve interstitial layer.(30) In smooth muscle cells, phosphate uptake via the sodium-dependent phosphate co-transporter, Pit-1, has been shown to induce production of osteogenic markers, Cbfa1 and osteopontin, both of which have increased gene expression in calcified aortic valves.(29,31-33)
This study found a stronger association of phosphate with AVS as compared to MAC. Conversely, CKD has more often been associated with MAC than with AVS or AAC.(8,18,34,35) Mineral metabolism disturbances have been proposed as potential etiologies for valvular and vascular calcification observed in people with CKD. A possible explanation is that, as compared to previously studied populations, our population has less kidney disease and no evidence of coronary heart disease. In CKD, mineral disturbances other than phosphate retention and secondary hyperparathyroidism can develop, such as reduction in serum levels of the calcification inhibitor, Fetuin-A. Fetuin-A has been shown to correlate inversely with valve calcification in dialysis patients.(36) Among patients with coronary heart disease lower Fetuin-A levels appear to be associated more strongly with MAC than with CAVD.(37) We were unable to measure Fetuin-A in this study, and the relationship of Fetuin-A with phosphate levels and cardiac calcification remains a topic for future investigation.
We had hypothesized that there may be an association between valve calcification and lower 25-OHD and higher PTH levels. In kidney failure, disturbances in vitamin D metabolism cause secondary hyperparathyroidism that may accelerate CAVD progression.(38) Furthermore, a hospital-based study in unstable angina patients with preserved kidney function found a significant, though modest, association of more severe CAVD, with higher parathyroid hormone and lower vitamin D levels.(39) High PTH and low vitamin D may promote ectopic calcification and inflammation that could lead to cardiovascular disease.(40,41) Population-based studies of people without chronic kidney disease have shown that higher PTH and lower 25-OHD levels are markers for adverse cardiovascular events and associated with cardiovascular risk factors.(42-44) Additionally, 25-OHD levels have demonstrated an inverse association with risk of incident coronary artery calcification.(45) It is possible that elevated PTH and inadequate vitamin D promotes atherosclerosis but not valve calcification. Alternatively, the lack of association in our study may be related to the exclusion of people with known cardiovascular disease. Strengths of our study include a large sample size from a well-defined community-based cohort with standardized echocardiography measures and risk factor assessment. In addition, we were able to adjust our models for serum cystatin C, which more precisely characterizes kidney function in older adults than does serum creatinine, the traditional serologic marker of GFR.
The study has several limitations. First, the study includes only single measurements of phosphate, PTH, and 25-OHD, all of which exhibit biological variation over time, and all of which were performed two years prior to the echocardiograms. However, these limitations likely would lead to non-differential misclassification that would attenuate associations towards the null hypothesis. A similar limitation includes not being able to account for dietary phosphate intake in this analysis. However, participants were instructed to fast, and serum phosphate levels are tightly regulated, even in the postprandial state.(46,47). Further, because longitudinal echocardiograms were not available, his is a cross-sectional study that reports prevalence, not incidence, of AVS, AAC and MAC. Additionally, because echocardiograms do not allow precise quantification of calcification severity, relationships of phosphate dose-response to calcification severity could not be assessed. Another limitation of this observational study is the possibility that unmeasured confounders might explain observed associations of phosphate with AVS and AAC. Further, phosphate levels were measured only in CHS participants at year 5 without known cardiovascular disease, resulting in survival bias in the study population. Finally, because this is an observational study, and not designed to assess the effect of phosphate levels on clinical outcomes, the results should not be used to guide clinical decision-making in CAVD.
The findings of this study, along with the role of phosphate in ectopic calcification, suggest that phosphate may be a biologically-plausible, novel risk factor for CAVD. If these results are confirmed, they may lead to studies to determine whether novel therapies targeting phosphate metabolism might alter the development or progression of calcific aortic valve disease. Accordingly the role of phosphate in CAVD warrants further study.
Supplementary Material
Acknowledgments
Funding support: The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additionally, the research reported here was supported by Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, TPM #61-034. Jason Linefsky is presently the postdoc fellow at HSR&D Northwest Center for Excellence. Funding from National Institutes of Health R01 HL084443 and R01 AG 027002 was also provided.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Dr. O’Brien reports receiving honoraria from AstraZeneca and Merck in the past 2 years. No other investigator had any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Otto C, Lind B, Kitzman D, Gersh B, Siscovick D. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–7. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 2.Otto C, Kuusisto J, Reichenbach D, Gown A, O’Brien K. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–53. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 3.Stewart B, Siscovick D, Lind B, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 4.Agmon Y, Khandheria B, Meissner I, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001;38:827–34. doi: 10.1016/s0735-1097(01)01422-x. [DOI] [PubMed] [Google Scholar]
- 5.Rossebø A, Pedersen T, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–56. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 6.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15:S5–97. 101. [PubMed] [Google Scholar]
- 7.Sherman S, Hollis B, Tobin J. Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J Clin Endocrinol Metab. 1990;71:405–13. doi: 10.1210/jcem-71-2-405. [DOI] [PubMed] [Google Scholar]
- 8.Adeney K, Siscovick D, Ix J, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–7. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro S, Ramos A, Brandão A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transplant. 1998;13:2037–40. doi: 10.1093/ndt/13.8.2037. [DOI] [PubMed] [Google Scholar]
- 10.Stefenelli T, Mayr H, Bergler-Klein J, Globits S, Woloszczuk W, Niederle B. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med. 1993;95:197–202. doi: 10.1016/0002-9343(93)90260-v. [DOI] [PubMed] [Google Scholar]
- 11.Ortlepp J, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P. The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart. 2001;85:635–8. doi: 10.1136/heart.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried L, Borhani N, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Psaty B, Kuller L, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, Cornell E, Howard P, Bovill E, Tracy R. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–70. [PubMed] [Google Scholar]
- 15.Stevens L, Coresh J, Schmid C, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardin J, Wong N, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 17.Barasch E, Gottdiener J, Larsen E, Chaves P, Newman A, Manolio T. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS) Am Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Asselbergs F, Mozaffarian D, Katz R, et al. Association of renal function with cardiac calcifications in older adults: the cardiovascular health study. Nephrol Dial Transplant. 2009;24:834–40. doi: 10.1093/ndt/gfn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills W, Einstadter D, Finkelhor R. Relation of calcium-phosphorus product to the severity of aortic stenosis in patients with normal renal function. Am J Cardiol. 2004;94:1196–8. doi: 10.1016/j.amjcard.2004.07.095. [DOI] [PubMed] [Google Scholar]
- 20.Akat K, Kaden J, Schmitz F, et al. Calcium metabolism in adults with severe aortic valve stenosis and preserved renal function. Am J Cardiol. 2010;105:862–4. doi: 10.1016/j.amjcard.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 21.Foley R, Collins A, Herzog C, Ishani A, Kalra P. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuttle K, Short R. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4:1968–73. doi: 10.2215/CJN.01250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhingra R, Sullivan L, Fox C, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 24.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 25.Foley R, Collins A, Ishani A, Kalra P. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–63. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Owens D, Katz R, Johnson E, et al. Interaction of age with lipoproteins as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:1200–7. doi: 10.1001/archinte.168.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubbs J, Liu S, Tang W, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–24. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 28.Sitara D, Razzaque M, Hesse M, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–32. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Yang H, Giachelli C. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–12. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 30.Mohler Er, Chawla M, Chang A et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–60. [PubMed] [Google Scholar]
- 31.O’Brien K, Kuusisto J, Reichenbach D, et al. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–8. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 32.Mohler Er, Gannon F, Reynolds C, Zimmerman R, Keane M, Kaplan F. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 33.Rajamannan N, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox C, Larson M, Vasan R, et al. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol. 2006;17:521–7. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 35.Ix J, Shlipak M, Katz R, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2007;50:412–20. doi: 10.1053/j.ajkd.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Wang A, Woo J, Lam C, et al. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20:1676–85. doi: 10.1093/ndt/gfh891. [DOI] [PubMed] [Google Scholar]
- 37.Ix J, Chertow G, Shlipak M, Brandenburg V, Ketteler M, Whooley M. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–9. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kume T, Kawamoto T, Akasaka T, et al. Rate of progression of valvular aortic stenosis in patients undergoing dialysis. J Am Soc Echocardiogr. 2006;19:914–8. doi: 10.1016/j.echo.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Linhartová K, Veselka J, Sterbáková G, Racek J, Topolcan O, Cerbák R. Parathyroid hormone and vitamin D levels are independently associated with calcific aortic stenosis. Circ J. 2008;72:245–50. doi: 10.1253/circj.72.245. [DOI] [PubMed] [Google Scholar]
- 40.Perkovic V, Hewitson TD, Kelynack KJ, Martic M, Tait MG, Becker GJ. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res. 2003;26:27–33. doi: 10.1159/000069761. [DOI] [PubMed] [Google Scholar]
- 41.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 42.Hagström E, Hellman P, Larsson T, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–71. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 43.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 44.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 45.de Boer I, Kestenbaum B, Shoben A, Michos E, Sarnak M, Siscovick D. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isakova T, Gutierrez O, Shah A, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–23. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Boer I, Rue T, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


