Abstract
The contribution of the Wnt pathway has been extensively characterized in embryogenesis, differentiation, and stem cell biology but not in mammalian metabolism. Here, using in vivo gain- and loss-of-function models, we demonstrate an important role for Wnt signaling in hepatic metabolism. In particular, β-Catenin, the downstream mediator of canonical Wnt signaling, altered serum glucose concentrations and regulated hepatic glucose production. β-catenin also modulated hepatic insulin signaling. Furthermore, β-catenin interacted with the transcription factor FoxO1 in livers from mice under starved conditions. The interaction of FoxO1 with β-catenin regulated the transcriptional activation of the genes encoding glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK), the two rate-limiting enzymes in hepatic gluconeogenesis. Moreover, starvation induced the hepatic expression of mRNAs encoding different Wnt isoforms. In addition, nutrient deprivation appeared to favor the association of β-catenin with FoxO family members, rather than with members of the T cell factor of transcriptional activators. Notably, in a model of diet-induced obesity, hepatic deletion of β-catenin improved overall metabolic homeostasis. These observations implicate Wnt signaling in the modulation of hepatic metabolism and raise the possibility that Wnt signaling may play a similar role in the metabolic regulation of other tissues.
INTRODUCTION
The Wnt signaling pathway has classically been studied in the context of normal development, morphogenesis, and stem cell regulation (1–4). Alterations in Wnt signaling are associated with tumor formation (3, 5). In the canonical pathway, extracellular Wnt ligands bind to specific membrane-bound receptors and co-receptors. This binding in turn activates an intracellular signal transduction pathway that ultimately results in the stabilization and subsequent nuclear translocation of β-catenin. In the nucleus, β-catenin binds to members of the T cell factor (TCF) or lymphoid enhancer factor (LEF) family of transcription factors to regulate the transcription of various Wnt target genes.
Although most investigations into Wnt biology have focused on its role in cellular proliferation, cell fate decisions, and stem cell self-renewal, genetic and biochemical evidence suggests that Wnt signaling may also play a role in metabolic disorders. One of the first indications of such a link came from an analysis of a Japanese cohort, which noted an association between a single-nucleotide polymorphism in the WNT5B gene and the risk of developing diabetes (6). A subsequent, larger study implicated a relatively common allele of a TCF family member (TCF7L2, also known as TCF4) in diabetes susceptibility (7). Indeed, the genetic association between TCF4 and diabetes has now been extended to multiple ethnic populations and is currently believed to represent the single strongest genetic association with disease susceptibility (8–11). Although several hypotheses have been advanced, the basis for how these genetic alterations in WNT5B or TCF4 alter the risk for developing diabetes remains poorly understood (9, 10, 12). Individuals with variants of TCF4 associated with increased risk for diabetes also tend to exhibit an unexplained increase in fasting hepatic glucose production (13).
In addition to these genetic association studies, other evidence suggests a potential role for Wnt signaling in regulating various metabolic pathways involved in glucose, cholesterol, and glycogen homeostasis. For instance, mice lacking the Wnt co-receptor LRP5 exhibit increased plasma cholesterol concentrations and impaired pancreatic insulin secretion (14). In at least one human family, mutations in the gene encoding the Wnt co-receptor LRP6 are also associated with impaired glucose metabolism and hyperlipidemia (15). In addition, expression of various members of the Wnt signaling pathway is altered in an experimental mouse model characterized by increased glycogen storage (16). These studies, as well as earlier studies in Drosophila (17), have implicated Wnt signaling in tissue glycogen metabolism. Wnt signaling might also modulate normal insulin signal transduction pathways. The secreted frizzled-related protein 5 (Sfrp5), a soluble inhibitor of Wnt signaling, regulates insulin signaling through activation of noncanonical Wnt signaling (18). In addition, Wnt signaling may also alter the activity of the kinases Akt and AMPK, thereby modulating insulin signal transduction (19). Finally, a small interfering RNA screen looking for modulators of mitochondrial biogenesis in skeletal muscle indicated that Wnt transcriptional activation of insulin receptor substrate-1 (IRS-1) promoted increased mitochondrial mass (20). This is consistent with another study that demonstrated β-catenin transcriptional activation of IRS-1 (21). Together, these observations suggest a potential role for Wnt signaling in modulating multiple metabolic pathways.
A metabolic response that is commonly altered in the diabetic state is the starvation-induced production of glucose by the liver, a process called gluconeogenesis. This process requires a complex network of transcription factors and coactivators, including transducer of regulated cAMP (cyclic aden-osine 3′,5′-monophosphate) response element–binding protein (TORC2; also known as CRTC2), hepatocyte nuclear factor 4α (HNF4α), peroxisome proliferator–activated receptor γ coactivator 1 (PGC-1α), and the Forkhead family member FoxO1 (22–28). The activities of these and other factors are regulated by both transcriptional and posttranslational methods to produce a highly ordered and temporally regulated response to starvation. In addition, enzymes such as the sirtuin family of NAD [nicotin-amide adenine dinucleotide (oxidized form)]–dependent deacetylases also play a critical role in the overall hepatic response to starvation by regulating the acetylation and hence activity of TORC2, PGC-1α, and various FoxO family members (25, 26, 29–31). Under oxidative stress conditions, β-catenin can bind to certain members of the FoxO family (32, 33). Furthermore, β-catenin directly interacts with SIRT1 (34).
Here, we demonstrate that Wnt signaling regulates hepatic metabolism. In particular, we demonstrate a nutrient-sensitive interaction between FoxO1 and β-catenin that appears to regulate the hepatic gluconeogenic response.
RESULTS
β-Catenin regulates glucose concentration
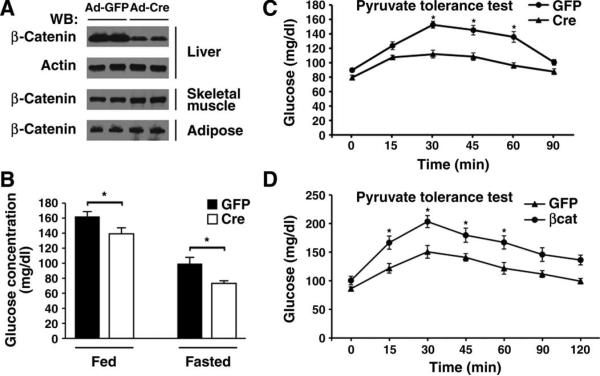
To determine whether Wnt signaling was involved in hepatic metabolism, we took advantage of a previously described mouse model, using loxP sites located in introns 1 and 6 of the CTNNB1 gene (which encodes β-catenin) (35). To avoid potentially confounding effects secondary to the known role of β-catenin in hepatic development (36), we chose to deliver Cre recombinase using replication-deficient adenoviruses administered by tail vein injection into adult mice. This delivery system efficiently and specifically transduces hepatic cells and leads to liver-specific deletion of the floxed allele (37). The abundance of hepatic β-catenin was reduced in mice injected with adenoviral Cre recombinase (Ad-Cre) compared to mice injected with a control adenovirus encoding green fluorescent protein (Ad-GFP) (Fig. 1A). In contrast, the abundance of β-catenin did not appear to be affected in other metabolically active tissues (Fig. 1A). The reduction of hepatic β-catenin in Ad-Cre–treated mice was sufficient to lower resting glucose concentrations under both randomly fed and fasting conditions (Fig. 1B). These differences in fasting and fed glucose concentrations were not due to any significant alterations in circulating insulin concentrations (fig. S1).
Fig. 1.
Regulation of hepatic glucose metabolism by β-catenin. (A) Western blot analysis for β-catenin abundance from various tissues 7 days after tail vein infusion of adenoviruses encoding either Cre recombinase or a GFP control. Shown are protein lysates obtained from two representative mice for each condition from one of three similar experiments. (B) Serum glucose concentrations in β-catenin floxed mice previously infected with an adenovirus encoding GFP or Cre recombinase. Fasting was for 5 hours. n = 8 mice per condition. *P < 0.05, Student's t test. (C) Hepatic gluconeogenesis as assessed by pyruvate tolerance tests (PTTs) in control or β-catenin– deleted (Cre) mice. n = 8 mice per group. *P < 0.05, ANOVA with Bonferroni correction. (D) PTT in control GFP-infected mice or in mice overexpres-sing β-catenin. n = 4 mice per group. *P < 0.05, ANOVA with Bonferroni correction.
The liver contributes to resting glucose concentrations in part through hepatic gluconeogenesis, a process that is deranged in the diabetic state (38). Pyruvate tolerance tests (PTTs) revealed that hepatic deletion of β-catenin resulted in reduced glucose production (Fig. 1C). To complement these loss-of-function studies, we also analyzed the metabolic effects after transient hepatic overexpression of β-catenin. Consistent with our observation after deletion of β-catenin, overexpression of β-catenin led to an increase in fasting glucose concentrations (Ad-GFP: 86.1 ± 6.4 mg/dl, Ad-βcat: 107.6 ± 14.1 mg/dl; n = 8 mice, P < 0.01, Student's t test). When compared to control mice, adenoviral-mediated delivery of β-catenin also resulted in increased hepatic glucose production when assessed with a PTT (Fig. 1D). Thus, in the adult mammalian liver, β-catenin appears to be an important determinant of the overall gluconeogenic response.
β-Catenin regulates hepatic FoxO1 subcellular localization
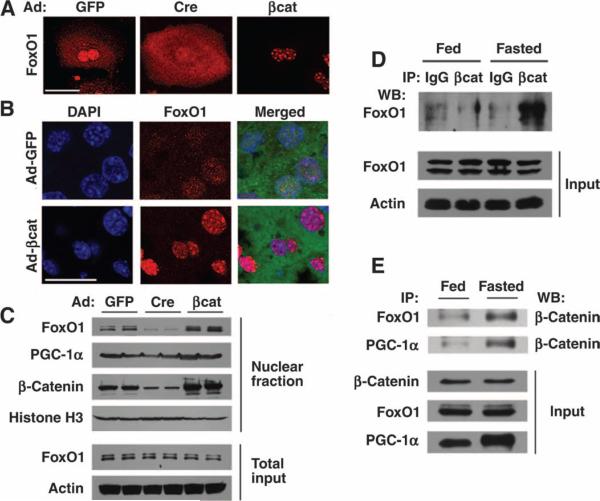
On the basis of the above observations and the previous known association between β-catenin and other FoxO family members (32), we determined whether β-catenin might regulate the function of hepatic FoxO1. Because Forkhead transcription factors are regulated largely through changes in subcellular localization, we determined the distribution of FoxO1 in freshly isolated primary hepatocytes isolated from mice with floxed β-catenin alleles that were subsequently infected with either Ad-GFP or Ad-Cre. In control infected hepatocytes, we routinely observed both nuclear and cytoplasmic FoxO1 staining (Fig. 2A). In contrast, hepatocytes expressing Cre recombinase demonstrated more diffuse and predominantly cytosolic localization of FoxO1 (fig. S2). When β-catenin was overexpressed in isolated hepatocytes, FoxO1 became predominantly nuclear and appeared to be confined within discrete subnuclear foci. At present, we are unsure of the precise importance of the FoxO1 nuclear foci observed in Ad-βcat–infected hepatocytes. Overexpression of β-catenin by tail vein injection of Ad-βcat also stimulated the in vivo nuclear accumulation of FoxO1 (Fig. 2B and fig. S2). We further confirmed the role of β-catenin in regulating FoxO1 nuclear localization by isolating nuclei from the livers of starved mice engineered to have either a gain or a loss of β-catenin expression. Consistent with our immunohistochemical observations, the abundance of nuclear FoxO1 and nuclear β-catenin appeared to be increased in Ad-βcat–infected mice (Fig. 2C). Similarly, deletion of β-catenin by delivering Cre recombinase resulted in an apparent decrease in FoxO1 nuclear accumulation. In contrast, the abundance of nuclear PGC-1α did not appear to be altered by manipulation of β-catenin. This is consistent with previous reports suggesting that nutrient control of PGC-1α activity occurs predominantly through transcriptional regulation and posttranslational modification rather than through alteration in subcellular distribution (25, 27, 30).
Fig. 2.
Interaction of FoxO1 with β-catenin. (A) Primary hepatocytes isolated from β-catenin floxed mice were infected with the indicated adenoviruses, and FoxO1 subcellular localization was subsequently assessed by indirect immunofluorescence. (B) In vivo localization of FoxO1 was determined in mice previously infected with the indicated adenoviruses and starved overnight. The Ad-βcat vector is a bicistronic construct that also encodes GFP. (C) Western blot analysis for the abundance of nuclear FoxO1, PGC-1α, and β-catenin. Nuclear fractions were prepared from mice previously infected with the indicated adenoviruses. Histone H3 was used as a loading control for nuclear proteins and actin as a control for the total hepatic lysate. (D) Lysates were prepared from whole livers of either fed mice or animals starved for 18 hours. Equal amounts of protein were immunoprecipitated (IP) with an antibody directed against β-catenin or with a control IgG serum, and the amount of coprecipitating FoxO1 was assessed by Western blot (WB) analysis. (E) Nutrient-sensitive protein interactions in fed and fasted livers observed between β-catenin and both FoxO1 and PGC-1a. Scale bar in immunohistochemical panels, 20 μm. All Western blots are representative of experiments that were performed at least three times.
We next analyzed fed or starved mice to assess whether the observed β-catenin–dependent alterations in FoxO1 nuclear accumulation result from direct interaction between the two proteins. Protein homogenates were prepared from livers from fed mice or from mice subjected to an 18-hour fast. In livers from starved mice, β-catenin coprecipitated FoxO1 (Fig. 2D). Furthermore, FoxO1 also coprecipitated with β-catenin, an interaction that appeared most evident in livers from starved mice (Fig. 2E). We also noted a nutrient-sensitive interaction between PGC-1α and β-catenin, consistent with previous studies demonstrating that nuclear FoxO1 and PGC-1α can directly interact (22).
β-Catenin modulates hepatic insulin signaling
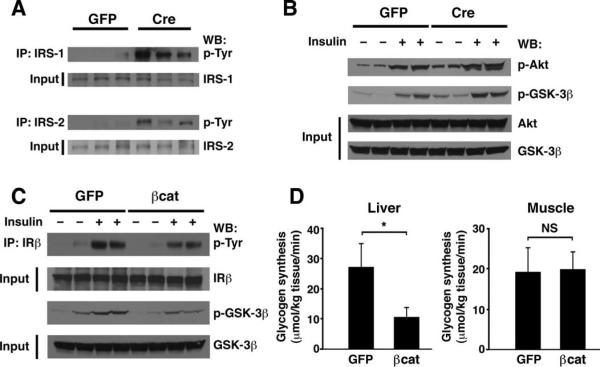
The subcellular localization of FoxO1 is regulated by insulin signaling. Previous studies have demonstrated that stimulation of the insulin receptor leads to Akt activation that in turn phosphorylates FoxO proteins, leading to their nuclear exclusion (39). Several studies have suggested interactions between the Wnt and the insulin signaling pathways (18–21). Combined with our results regarding the ability of β-catenin to regulate the localization of FoxO1, we sought to understand whether manipulation of β-catenin abundance altered insulin signaling. In a skeletal muscle cell line, Wnt stimulation increases transcription of IRS1, which is a direct transcriptional target of β-catenin (20). We therefore analyzed the abundance of IRS-1 and IRS-2 in liver samples from control or β-catenin–deleted mice. Although the abundance of these proteins was not obviously different, we did observe that after Cre-mediated deletion of β-catenin, basal activation of both IRS-1 and IRS-2, as assessed by tyrosine phosphorylation, appeared to increase (Fig. 3A). We also analyzed other downstream targets of insulin signaling in mice. In the absence of β-catenin, both basal and insulin-stimulated phosphorylation of Akt and glycogen synthase kinase–3β (GSK-3β) was modestly but reproducibly enhanced (Fig. 3B). Consistent with these biochemical observations, we noted that β-catenin–deleted mice also exhibited evidence for improved insulin tolerance (fig. S3). In contrast, transient overexpression of β-catenin triggered a corresponding reduction in insulin signaling, which was most evident with insulin-stimulated insulin receptor phosphorylation and downstream GSK-3β phosphoryl ation (Fig. 3C). Furthermore, insulin stimulation appeared to result in the cytosolic localization of FoxO1 in control hepatocytes but not in hepatocytes overexpressing β-catenin (fig. S4). Given that GSK-3β is an intermediate of both insulin and Wnt signaling pathways that has a role in glycogen metabolism, we next directly measured rates of glycogen synthesis in control mice or in mice that overexpressed β-catenin. Overexpression of β-catenin led to decreased de novo insulin-stimulated hepatic glycogen synthesis (Fig. 3D). These observations are consistent with the observed decrease in insulin receptor and GSK-3β phosphorylation after β-catenin overexpression. As expected, we saw no effects of Ad-βcat infusion on the rate of glycogen synthesis measured in skeletal muscle, consistent with the pattern of adenoviral-mediated gene expression after tail vein infusion (Fig. 1A).
Fig. 3.
β-Catenin modulates in vivo hepatic insulin signaling. (A) Assessment of IRS-1 and IRS-2 abundance and tyrosine phosphorylation in GFP control animals or Ad-Cre–infected mice (n = 3). Deletion of β-catenin results in higher basal tyrosine phosphorylation of IRS-1 and IRS-2. (B) Analysis of phosphorylation of Akt Ser473 and GSK-3β Ser9 under basal conditions or 5 min after insulin injection. Signaling was assessed in control GFP mice (n = 4 mice; 2 insulin-stimulated) or a similar number of Ad-Cre–infected animals. (C) Influence of β-catenin overexpression on in vivo insulin signaling. Insulin-stimulated tyrosine phosphorylation of the insulin receptor β chain (IRβ) and serine phosphorylation of GSK-3β were reduced in mice that overexpressed β-catenin. (D) In vivo assessment of de novo glycogen incorporation in control (GFP) or β-catenin–overexpressing mice. Both hepatic and skeletal muscle glycogen syntheses were assessed. n = 8 mice per group. All Western blots are representative of experiments that were performed at least three times. *P < 0.05, Student's t test. NS, not significant.
β-Catenin regulates the hepatic gluconeogenic response
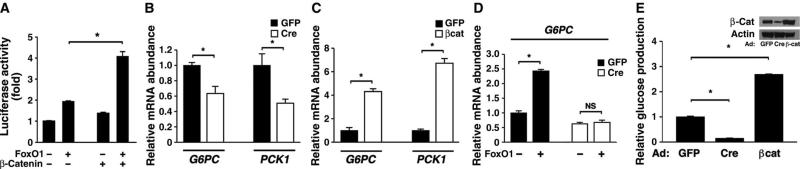
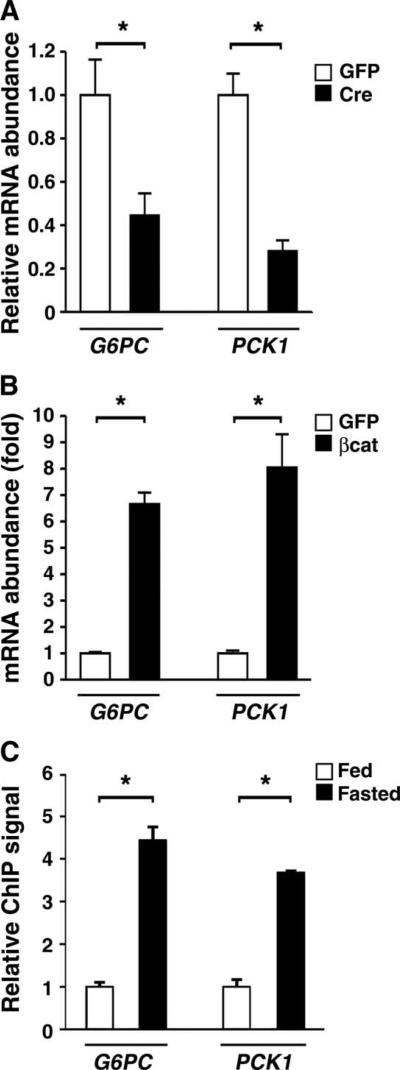
The ability of β-catenin to regulate the localization of FoxO1 through direct interaction suggested that this interaction might increase FoxO1 function. In Hepa1-6 hepatoma cells transiently transfected with a FoxO-dependent luciferase reporter, β-catenin expression did not have a direct stimulatory effect but appeared to synergize with cotransfected FoxO1 (Fig. 4A). Glucose-6-phosphatase (G6Pase, which is encoded by G6PC) and phosphoenolpyruvate carboxykinase (PEPCK, which is encoded by PCK1) are thought to be the rate-limiting enzymes involved in hepatic gluconeogenesis, and FoxO1 and PGC-1α working in concert are required for robust induction of the genes encoding these enzymes under fasting conditions (22–26). Cre recombinase–mediated deletion of β-catenin reduced the mRNA abundance for both gluconeogenic enzymes in isolated primary hepatocytes (Fig. 4B). Similarly, overexpression of β-catenin stimulated hepatocyte expression of both G6PC and PCK1 (Fig. 4C). We next asked whether β-catenin was required for FoxO-dependent expression of these two gluconeogenic enzymes. Although FoxO1 expression was sufficient to stimulate both G6PC and PCK1 expression in control hepatocytes, we did not observe any FoxO-dependent stimulation of expression of these genes in hepatocytes lacking β-catenin (Fig. 4D and fig. S5). Similarly, short hairpin RNA (shRNA)–mediated knockdown of FoxO1 decreased β-catenin–stimulated expression of G6PC and PCK1 (fig. S6). Finally, we directly measured hepatocyte glucose production in the setting of deletion or overexpression of β-catenin. Deletion of β-catenin significantly reduced in vitro hepatic glucose output, whereas overexpression resulted in increased production (Fig. 4E).
Fig. 4.
β-Catenin regulates in vitro transcription of two hepatic gluconeogenic enzymes and glucose production. (A) The hepatoma cell line Hepa1-6 was transiently transfected with expression plasmids encoding FoxO1, β-catenin, or empty vector (−). The activity of a FoxO-dependent luciferase promoter construct was normalized to an internal Renilla control reporter. Shown is one experiment performed in triplicate that is representative of at least three similar experiments. (B) Hepatocytes isolated from β-catenin flox/flox mice were infected with Ad-GFP or Ad-Cre, and the expression of G6PC and PCK1 was determined. n = 6 mice per group. (C) Primary mouse hepatocytes were infected with Ad-GFP or Ad-βcat, and G6PC and PCK1 expression was assessed. n = 6 mice per group. (D) Primary hepatocytes were isolated from β-catenin flox/flox mice and subsequently infected with Ad-Cre or the control Ad-GFP. Hepatocytes were then reinfected with an adenovirus encoding FoxO1, and G6PC gene expression was assessed. The deletion of β-catenin reduces FoxO1-stimulated gene expression. n = 6 mice per group. (E) Hepatic glucose production from primary hepatocytes obtained from β-catenin flox/flox mice that were infected with adenoviruses encoding GFP, Cre recombinase, or β-catenin. n = 6 mice per group. Inset shows corresponding β-catenin protein abundance.
We next assessed the role of β-catenin in regulating the in vivo expression of G6PC and PCK1. As observed in isolated hepatocytes, Cre-mediated deletion of β-catenin also resulted in reduced in vivo expression of G6PC and PCK1 (Fig. 5A). Moreover, β-catenin overexpression resulted in a potent in vivo stimulation of the expression of these enzymes (Fig. 5B). Finally, chromatin immunoprecipitation (ChIP) analysis revealed a nutrient-sensitive increase in the binding of β-catenin to the FoxO1 regulatory region of the promoters for G6PC and PCK1 (Fig. 5C).
Fig. 5.
In vivo regulation of the gluconeogenic program by bcatenin. (A) Relative expression of G6PC and PCK1 after in vivo deletion of hepatic β-catenin. n = 3; triplicate determinations from three animals in each group. (B) Expression of G6PC and PCK1 3 days after delivery of either a control or a β-catenin–expressing adenovirus. n = 4; triplicate determinations from four mice per group. (C) ChIP assay demonstrating increased in vivo β-catenin binding to the G6PC and PCK1 promoter after mice were fasted for 18 hours. n = 6 mice per condition. *P < 0.05, Student's t test.
Wnt signaling is involved in the hepatic response to starvation
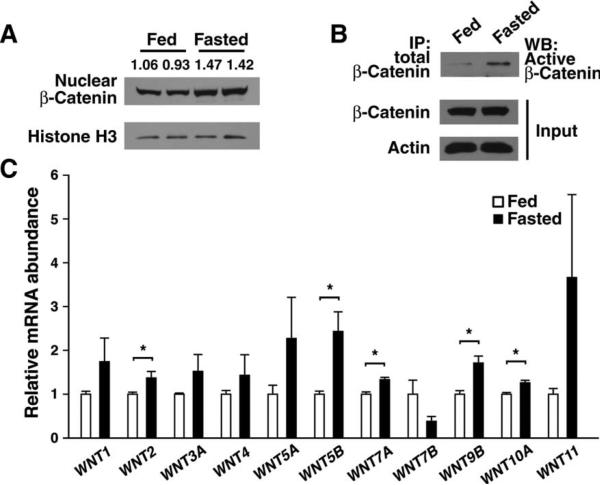
The above data suggest that β-catenin is an important hepatic cofactor for the FoxO1-dependent gluconeogenic response. However, it was unclear whether nutrient availability could directly affect Wnt signaling pathways. We isolated the hepatic nuclear fraction from wild-type mice under fed or fasting conditions. Although β-catenin was evident in the nucleus under fed conditions, the overall amount of nuclear β-catenin appeared to increase after an overnight fast (Fig. 6A). In contrast, the total amount of β-catenin appeared to be unaffected by nutrient status (Figs. 2E and 6B). Similarly, fasting led to an apparent increase in the amount of the active, dephosphorylated form of β-catenin (Fig. 6B). Previous data have demonstrated that multiple Wnt isoforms are present in the adult liver (40). We analyzed the expression pattern of various Wnt isoforms present in adult liver under both fed and starved conditions. Starvation increased the expression of most, but not all, of these Wnt isoforms (Fig. 6C). Furthermore, increased Wnt3 or Wnt7a expression was sufficient to stimulate β-catenin binding to the G6Pase promoter (fig. S7), and exogenous Wnt addition increased the basal metabolism of isolated hepatocytes (fig. S8).
Fig. 6.
Wnt activity is regulated by nutrient status. (A) β-Catenin abundance observed in hepatic nuclei isolated from pairs of fed or starved mice. Numerical values represent the arbitrary ratio of nuclear β-catenin to histone H3 abundance.(B) Abundance of active β-catenin in fed and fasting livers. Total β-catenin was immunoprecipitated from equal amounts (2 mg) of hepatic protein lysate and then assessed by Western blot (WB) analysis using an antibody that recognizes the active (hypophosphorylated) form of β-catenin. All Western blots are representative of experiments that were performed at least three times. (C) In vivo expression of various Wnt ligands under fed and starved conditions. n = 3 to 4 mice per condition, each assessed in triplicate. *P < 0.05, Student's t test.
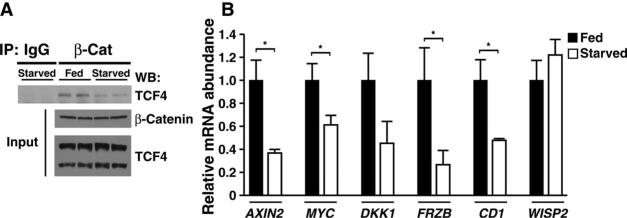
The oxidant-stimulated interaction between FoxO3a and β-catenin results in increased expression of FoxO-regulated stress resistance genes and decreased TCF-dependent transcription (33). This was attributed to competition between FoxO3a and TCF factors for a limited pool of β-catenin. To assess whether a similar competition might exist in the starved liver, we immunoprecipitated β-catenin from fed or starved liver and assessed the amount of coprecipitating TCF4. Although the abundance of β-catenin and TCF4 did not apparently differ under fed and starved conditions, the amount of TCF4 that associated with β-catenin appeared to decrease under starved conditions (Fig. 7A). A survey of six β-catenin and TCF target genes revealed that most of these targets also exhibited a corresponding starvation-induced decrease in expression (Fig. 7B). In contrast, under these conditions, starvation increased the association of β-catenin with FoxO1 and increased FoxO-dependent gene expression (Fig. 2D).
Fig. 7.
Starvation alters β-catenin binding to TCF4. (A) Hepatic lysates were prepared from fed or starved mice to assess the interaction of β-catenin with TCF4. Lysates were immunoprecipitated with a β-catenin antibody or an IgG control serum. Interaction of β-catenin with TCF4 was determined by immunoprecipitation of β-catenin followed by Western blot analysis of TCF4. Although the abundance of β-catenin and TCF4 was not altered by starvation, the interaction of β-catenin with TCF4 was reduced under starved conditions. TCF4 appeared as a long and a short isoform in hepatic lysates, of which only the long form appeared to interact strongly with β-catenin. (B) Analysis of known Wnt target genes under fed or starved conditions. Consistent with the decline in TCF4 binding, most previously identified β-catenin/TCF4 targets decreased in expression under starved conditions. n = 4 to 6 determinations per gene. *P < 0.05, Student's t test.
Deletion of β-catenin improves metabolic homeostasis in a diet-induced obesity model
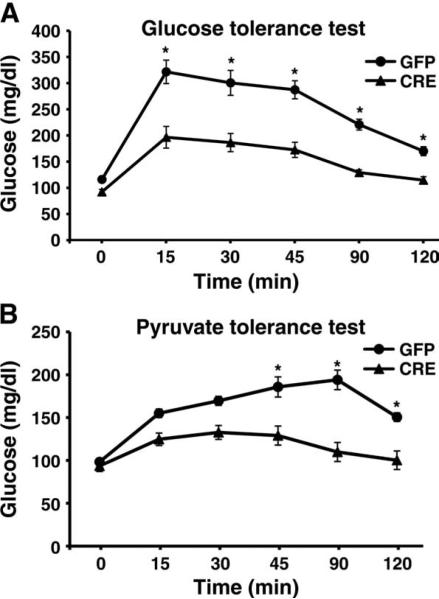
Given that hepatic gluconeogenesis is deranged in diabetes, we next determined whether selective interruption of hepatic Wnt signaling might provide a therapeutic advantage in a model of glucose intolerance. We placed mice bearing floxed β-catenin alleles on a high-fat diet. Ten weeks after being placed on such a diet, animals were randomized to receive tail vein injection of replication-deficient adenoviruses encoding either Cre recombinase or GFP. One week after injection, those animals that received Cre recombinase showed improved overall glucose tolerance (Fig. 8A), which coincided with reduced hepatic gluconeogenesis when assessed by a PTT (Fig. 8B).
Fig. 8.
Deletion of β-catenin improves glucose homeostasis in a model of diet-induced obesity. (A) Glucose tolerance test of β-catenin floxed mice on a high-fat diet randomized to tail vein injection of adenoviruses encoding Cre recombinase or GFP. (B) PTT of control mice or mice with Cre recombinase–mediated deletion of β-catenin. n = 6 mice per group. *P < 0.05 by ANOVA with Bonferroni correction.
DISCUSSION
Our data suggest that hepatic Wnt signaling is an important physiological regulator of mammalian hepatic metabolism. Our models were transient overexpression of β-catenin or the partial deletion of the floxed gene in adult animals, a strategy that avoids the developmental effects of a liver-specific knockout (36) and the massive hepatomegaly and death caused by continuous β-catenin activation (41). We demonstrate that β-catenin regulated the localization and activity of FoxO1. Furthermore, β-catenin can modulate the gluconeogenic response through regulation of the gluconeogenic enzymes G6Pase and PEPCK. We also noted that nutrient availability modified hepatic expression of various Wnt ligands. Further study is required to understand the basis and importance of the complex pattern of induction and suppression of various Wnt ligands. Furthermore, although most of our experiments have centered on the starved state, additional experiments delineating the role of Wnt ligands under fed conditions or after other metabolic perturbations appear warranted. To date, we cannot fully replicate the pattern of starvation-induced Wnt ligand expression by simple glucagon stimulation of isolated hepatocytes (fig. S9). Nonetheless, it is conceivable that circulating peptides such as glucagon or GLP-1 may exert part of their biological effects through the regulation of Wnt expression or through the regulation of hepatic β-catenin activity through non-classical pathways (42). Future experiments in which specific Wnt isoforms expressed in the liver have undergone either a gain or a loss of function may be useful in resolving some of these issues.
The observation that Wnt stimulation increases mitochondrial respiration in skeletal muscle (20), coupled with our observation that exogenous Wnt can alter the metabolism of primary hepatocytes (fig. S8), raises the possibility that Wnt signaling may regulate the basal metabolism of other tissues. The range of metabolic activities regulated by Wnt signaling does not appear to be confined to glucose and glycogen metabolism, because we have observed that hepatic β-catenin activity affects lipid metabolism and hepatic triglyceride concentrations (fig. S10). Whether these lipid alterations occur through FoxO1-dependent or -independent pathways is unknown at this point. The overall scope of these metabolic effects suggests that the capacity of β-catenin to alter intracellular metabolism may be essential for the observed phenotypic alterations induced by Wnt ligands in the context of development and tumorigenesis. In particular, it is intriguing to speculate that the metabolic effects described in this report may somehow relate to the link that we and others have made regarding Wnt signaling in aging and senescence (43–45).
Our data suggest that in the starved liver, β-catenin may increase its binding to FoxO1 while decreasing its binding to the TCF family of transcriptional activators. This situation is reminiscent of the previously described oxidant-stressed induced interaction of FoxO3a with β-catenin, in which expression of FoxO-regulated stress resistance genes increased, whereas TCF-dependent transcription decreased (33). Thus, it may be that the binding partner and transcriptional activity of β-catenin are altered during either redox or metabolic stress. The molecular basis for how this shift occurs requires further study. Nonetheless, our data suggest that nutrient status regulates whether β-catenin interacts with TCF or with FoxO1. Although considerable caution regarding causality must be applied to any genetic association study, these observations are at least provocative as to how variations in TCF7L2 might contribute to diabetes (7). In addition, our observation demonstrating that there is considerable cross-modulation between the Wnt and the insulin signaling pathways provides additional insights regarding the potential role of the Wnt pathway in metabolic ho meostasis. Further study is needed to understand how this cross-regulation is achieved, although our in vivo glycogen synthesis results suggest that the activity of GSK-3β, a molecule common to both pathways, may be a particularly important nodal point.
Finally, although Wnt signaling has been extensively studied in many diverse contexts, the role of this pathway in metabolism has been largely ignored. Our observations that Wnt–β-catenin signaling affects hepatic glucose, glycogen, and lipid metabolism, as well as that partial deletion of β-catenin improves metabolic homeostasis in a model of diet-induced obesity, suggest that modulation of hepatic Wnt signaling may have therapeutic value. Furthermore, given these observations, it will be of interest to test whether other pathways that regulate stem and progenitor biology (such as Notch or transforming growth factor–β) may also be implicated in the regulation of intracellular metabolism.
MATERIALS AND METHODS
Cell culture and adenoviral infection
Primary mouse hepatocytes were isolated and cultured as previously described (46). Hepa1-6 hepatoma cells (American Type Culture Collection) were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). The adenoviruses encoding GFP and Cre recombinase were obtained from the University of Iowa Gene Transfer Center. The adenovirus encoding for β-catenin (Ad-βcat) was made by standard methods with the AdEasy adenoviral vector system (Stratagene). Oxygen consumption was assessed with a Seahorse XF24 analyzer as previously described (47).
In vivo mouse experiments
For the in vivo deletion or overexpression of β-catenin in liver, 1 × 109 plaque-forming units (PFU) of adenoviruses encoding GFP, Cre recombinase, or β-catenin were injected into the tail vein of 8- to 12-week-old floxed β-catenin mice (Jackson Labs). PTTs were routinely performed 3 days after viral infusion for overexpression studies and 7 days after virus delivery for loss-of-function studies. The test consisted of an intraperitoneal injection of 2 g of sodium pyruvate per kilogram of body weight. All tests were conducted after overnight fasting. Serum glucose measurements were made with a Contour glucometer, and insulin concentration was determined with an Insulin Elisa kit (Crystal Chem Inc.). For the insulin tolerance test (ITT), insulin was injected at 0.5 U per kilogram of body weight. Where indicated, mice were placed on a high-fat diet (42% of calories from fat; Harlan Teklad) for 10 weeks before infusion of the indicated adenovirus. To determine the rate of glycogen synthesis, we analyzed mice under hyperinsulemic-euglycemic clamp conditions. In brief, after animals were starved for 5 hours, insulin was infused initially as a bolus of 16 mU/kg over a period of 3 min, followed by continuous insulin infusion at a rate of 2.5 mU/kg per minute (Humulin R; Eli Lilly) to maintain a target plasma insulin concentration of ~2 ng/ml. Simultaneously, a 20% glucose solution was infused at a variable rate to maintain plasma glucose at ~140 mg/dl in control mice. Glycogen synthesis was estimated with a continuous infusion of high-pressure liquid chromatography–purified [3-3H]glucose (0.1 μCi/min; NEN Life Science Products) throughout the clamp and subsequent determination of 3H incorporation into glycogen in both liver and skeletal muscle tissue as previously described (48).
Protein-protein interactions
Liver samples were harvested in lysis buffer (150 mM NaCl, 20 mM tris-HCl, 0.5% Triton X-100, and protease inhibitor cocktail). Protein lysates (2 mg) were then incubated as indicated with antibodies for FoxO1 (Santa Cruz Biotechnology), PGC-1a (Santa Cruz Biotechnology), or β-catenin (BD Pharmingen) along with protein A/G Sepharose beads at 4°C overnight. The following day, immunoprecipitates were washed with lysis buffer five times, resolved by SDS-gel electrophoresis, and subsequently analyzed by Western blotting using the above antibodies or, where indicated, an antibody recognizing the activated, hypophosphorylated form of β-catenin (Upstate Cell Signaling). Nuclear extracts were in general assessed for equal protein loading with histone H3 (Abcam) as a loading control, whereas whole-cell lysate loading was assessed with an antibody directed against actin (BD Pharmingen). To determine the nutrient-sensitive change in the β-catenin–TCF4 interaction, we isolated liver samples of wild-type mice under fed or starved conditions. Samples were lysed and 1 mg of protein liver lysate was immunoprecipitated with a β-catenin–specific antibody (BD Pharmingen) or an immunoglobulin G (IgG) nonspecific control antibody. Immunoprecipitates were resolved on SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and coimmunoprecipitating TCF4 was analyzed by Western blotting with an antibody recognizing TCF4 (Cell Signaling).
Immunofluorescence and nuclear fractionation
Isolated primary hepatocytes or cryosections of harvested liver were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and then rinsed three times (5 min each wash) with PBS. Samples were then blocked with 0.5% bovine serum albumin (BSA) and subsequently incubated with a FoxO1 or β-catenin antibody followed by a fluorescently conjugated secondary antibody (Molecular Probes); nuclei were visualized by DAPI (4′,6-diamidino-2-phenylindole) staining. The hepatic nuclear fractions were prepared with the Nuclear and Cytoplasmic Extraction Re-agent Kit (Pierce). Briefly, ~80 mg of liver tissue was homogenized and lysed in the Cytoplasmic Extraction Reagent for 10 min and the extract was subsequently centrifuged to separate a nuclear pellet from the cytoplasmic supernatant. After washing, the nuclear pellet was resuspended into Nuclear Extraction Reagent and lysed on ice for 40 min combined with 15 s of vortexing every 10 min. The nuclear lysate was then centrifuged to obtain the nuclear extract (supernatant). Routinely, 50 μg of nuclear extract was used for SDS-PAGE and subsequent Western blotting.
Modulation of insulin signaling
For assessment of in vivo insulin signaling, mice with deletion or over-expression of β-catenin were assessed 5 min after intraperitoneal injection of either insulin (1 U/kg) or a control injection of PBS. Harvested liver samples were stored in liquid nitrogen and subsequently solubilized in lysis buffer. For detection of insulin signaling intermediates, 1 mg of liver protein lysate was used for immunoprecipitation with antibodies directed against the insulin receptor (IR) β chain (Santa Cruz Biotechnology), IRS-1 (Santa Cruz Biotechnology), or IRS-2 (Santa Cruz Biotechnology), respectively. After being washed five times with lysis buffer, the protein A and/or G beads were boiled with 1× sample buffer at 95°C for 5 min and the immunoprecipitated proteins were subsequently loaded on SDS-PAGE gels. Phosphorylation of the IR, IRS-1, and IRS-2 was assessed with an anti-phosphotyrosine antibody (Santa Cruz Biotechnology). Assessment of Akt and GSK-3β phosphorylation was measured directly by Western blotting and the use of phosphospecific antibodies for Akt phosphorylated at Ser473 and GSK-3β phosphorylated at Ser9 (Cell Signaling).
Isolated hepatocyte assays
To determine expression of gluconeogenic enzymes in vitro, we isolated primary hepatocytes from β-catenin flox/flox mice. These cells were subsequently infected with Ad-GFP, Ad-Cre, or Ad-βcat virus particles (25 PFU) as indicated. Thirty-six hours after infection, cells were collected and gene expression was measured by quantitative reverse transcription–polymerase chain reaction (RT-PCR) and the primer sets listed below. To determine the requirement of β-catenin for FoxO-mediated gluconeogenic gene expression, we isolated primary hepatocytes from β-catenin flox/flox mice. Isolated cells were infected with 25 PFU of Ad-Cre or, as a control, 25 PFU of Ad-GFP. After 12 hours, cells were reinfected with 25 PFU of Ad-FoxO1 adenovirus (a gift of D. Accili) or, as a control, a similar amount of Ad-GFP virus. After an additional 24 hours, the hepatocytes were trypsinized, and RNA was isolated for quantitative PCR analysis of gluconeogenic enzymes. For the reciprocal experiment demonstrating a role for FoxO1 in β-catenin–stimulated gene expression, we used Hepa1-6 cells. These cells were first infected with a shFoxO1 lentivirus (Open Biosystems) or control shRNA virus and subsequently selected with puromycin (2 μg/ml). Knockdown of FoxO1 was confirmed by quantitative PCR analysis. Control or FoxO1 knockdown cells were subsequently infected with Ad-βcat or Ad-GFP virus (25 PFU). After 24 hours, cells were collected for RNA isolation and the expression of G6Pase and PEPCK was quantified with quantitative RT-PCR.
For the in vitro assessment of glucose production, hepatocytes were isolated from β-catenin flox/flox mice. These hepatocytes were subsequently infected with 25 PFU of Ad-GFP, Ad-Cre, or Ad-βcat. After 20 hours, cells were switched to minimal medium (glucose-free DMEM, 0.1% BSA, and 1 mM sodium pyruvate). After an additional 12-hour incubation in minimal medium, cells were washed with PBS three times and then placed in glucose production buffer (phenol red and glucose-free DMEM, 20 mM sodium lactate, and 2 mM sodium pyruvate). After 4 hours, the supernatant was collected and the glucose concentration was measured with the Glucose (GO) assay kit (Sigma).
FoxO reporter and ChIP assay
Hepa1-6 cells were seeded in 12-well plates 24 hours before transfection. In each well, 100 ng of a FoxO firefly luciferase reporter and 10 ng of a Renilla luciferase reporter were transfected into cells along with constructs (2 μg) expressing β-catenin (Origene) and/or FoxO1 (Addgene). One day after transfection, cells were collected for a luciferase assay with the Dual-Luciferase Assay System (Promega). FoxO reporter activity was normalized in each case to the internal Renilla control. For ChIP analysis, cells or liver samples were fixed with formaldehyde, lysed, and sonicated with the Active Motif ChIP assay kit. Soluble chromatin was immunoprecipitated with antibodies directed against β-catenin or RNA polymerase II. After de-cross-linking, DNA samples were used for quantitative PCR with the following primers: G6PC: 5′-CCTTGCCCCTGTTTTATATGCC-3′ and 5′-CGTAAATCACCCTGAACATGTTTG-3′; PCK1: 5′-AGGTAACACACCCCAGCTAAC-3′ and 5′-GGCTCTTGCCTTAATTGTCAG-3′. Values were normalized to the signal obtained from RNA polymerase II immunoprecipitation of the ubiquitously expressed EF-1a gene according to the manufacturer's instructions (Active Motif). Where indicated, primary hepatocytes were transfected with a Wnt3 expression plasmid as previously described (43) or similarly with a plasmid encoding Wnt7a.
Gene expression analysis
For in vivo gene expression analysis, cellular RNAs from liver samples were extracted with the NucleoSpin RNA II RNA isolation kit (Macherey-Nagel) and then reverse-transcribed into complementary DNA (cDNA) with the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). PCRs were set up with the SYBR Green PCR Master Mix (Applied Biosystems). Primers used for Wnt isoforms in liver were as follows: WNT1: 5′-ATCCATCTCTCCCACCTCCT-3′ and 5′-AGCAACCTCCTTTCCCACTT-3′; WNT2: 5′-AGAGTGCCAACACCAGTTCC-3′ and 5′-TACAGGAGCCACTCACACCA-3′; WNT3A: 5′-GCGGGCATCCAGGAGTGCCAG-3′ and 5′-CTCTGCACAGGAGCGTGTCAC-3′; WNT4: 5′-GAGAAGTGTGGCTGTGACCGG-3′ and 5′-ATGTTGTCCGAGCATCCTGACC-3′; WNT5A: 5′-TGACAACTGGCAGAAAAACAA-3′ and 5′-TCCCTGAATGGAACAACAAA-3′; WNT5B: 5′-GCAGTTGACCTGACCTGCTA-3′ and 5′-TTCTGTCACCTGCTACAGCC-3′; WNT7A: 5′-CCAAATGGGCCTGGACGAGTG-3′ and 5′-CCGGTGGTACTGGCCTTGCTT-3′; WNT7B: 5′-TCTCTGCTTTGGCGTCCTCTAC-3′ and 5′-GCCAGGCCAGGAATCTTGTTG-3′; WNT9B: 5′-GGGTGTGTGTGGTGACAATC-3′ and 5′-TCCAACAGGTACGAACAGCA-3′; WNT10A: 5′-GGCGCTCCTGTTCTTCCTACTGCT-3′ and 5′-GATAGCAGAGGCGGCCACGTCAGG-3′; WNT11: 5′-CTGAATCAGACGCAACACTGTAAAC-3′ and 5′-CTCTCTCCAGGTCAAGCAGGTAG-3′.
Assessment of glucagon-mediated Wnt gene expression was performed in primary hepatocytes that were serum-starved overnight. Cells were stimulated with glucagon (100 nM) for 2 hours before RNA isolation and subsequent Wnt isoform analysis.
For the analysis of both G6Pase and PEPCK, the following primers were used: G6PC: 5′-ATGAACATTCTCCATGACTTTGGG-3′ and 5′-GACAGGGAACTGCTTTATTATAGG-3′; PCK1: 5′-CATAACGGTCTGGACTTCTCTGC-3′ and 5′-GAATGGGATGACATACATGGTGCG-3′.
For Wnt target gene analysis, the following primers were used: AXIN2: 5′-AACCTATGCCCGTTTCCTCT-3′ and 5′-CTGGTCACCCAACAAGGAGT-3′; CD1: 5′-TCTCCTGCTACCGCACAAC-3′ and 5′-TTCCTCCACTTCCCCCTC-3′; MYC: 5′-CTAGTCCGACCAGCGTCAC-3′ and 5′-GTACCCCAATCCTGAACCAC-3′; DKK1: 5′-GAGGGGAAATTGAGGAAAGC-3′ and 5′-GCAGGTGTGGAGCCTAGAAG-3′; FRZB: 5′-TCCAACAAGTGATCCGAGCG-3′ and 5′-CTCCATACAATTGTAAGCCG-3′; WISP2: 5′-ATACAGGTGCCAGGAAGGTG-3′ and 5′-CAAGGGCAGAAAGTTGGTGT-3′.
Analysis of gene expression in lipid metabolism was achieved with the following primers: CPT1A: 5′-TGCACTACGGAGTCCTGCAA-3′ and 5′-GGACAACCTCCATGGCTCAG-3′; ACOX1: 5′-GCCTGCTGTGTGGGTATGTCATT-3′ and 5′-GTCATGGGCGGGTGCAT-3′; ACCA1: 5′-ATGCTTCCATGCTGAGATTGT-3′ and 5′-TCCATCCTTGAAGGCAGGCTT-3′.
All gene expression results were normalized to an internal control with the following primer set: ACTA1: 5′-TGGCATTGTTACCAACTGGGACG-3′ and 5′-GCTTCTCTTTGATGTCACGCACG-3′.
Statistical analysis
For experiments with multiple comparisons, data were first analyzed by analysis of variance (ANOVA) with Bonferroni correction. For single comparisons, a Student's t test was performed on data before normalization.
Supplementary Material
Fig. S1. Deletion of hepatic β-catenin does not alter serum insulin concentration.
Fig. S2. Quantification of FoxO1 subcellular localization.
Fig. S3. Insulin tolerance tests (ITTs) in flox/flox β-catenin mice after injection with adenoviruses encoding GFP or Cre recombinase.
Fig. S4. Subcellular localization of FoxO1 in primary hepatocytes after insulin stimulation.
Fig. S5. Deletion of β-catenin decreases the mRNA abundance of FoxO1 transcriptional targets.
Fig. S6. Knockdown of FoxO1 decreases the mRNA abundance of β-catenin transcriptional targets.
Fig. S7. Wnt stimulation increases binding of β-catenin to the G6PC promoter.
Fig. S8. Wnt3a stimulates hepatocyte oxygen consumption.
Fig. S9. Effects of glucagon stimulation on the abundance of mRNAs encoding Wnt ligands.
Fig. S10. The effect of β-catenin on the abundance of mRNAs encoding lipid enzymes and on triglyceride concentrations.
Acknowledgments
We are grateful to P. Puigserver for helpful comments and D. Accili and M. Quon for providing the FoxO1 adenovirus. Funding: This work was supported by NIH Intramural funds, an NIH Path to Independence award (H.L.), and a grant from the Ellison Medical Foundation (T.F.).
Footnotes
Citation: H. Liu, M. M. Fergusson, J. J. Wu, I. I. Rovira, J. Liu, O. Gavrilova, T. Lu, J. Bao, D. Han, M. N. Sack, T. Finkel, Wnt signaling regulates hepatic metabolism. Sci. Signal. 4, ra6 (2011).
Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 2.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 4.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am. J. Hum. Genet. 2004;75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 8.Hattersley AT. Prime suspect: The TCF7L2 gene and type 2 diabetes risk. J. Clin. Invest. 2007;117:2077–2079. doi: 10.1172/JCI33077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welters HJ, Kulkarni RN. Wnt signaling: Relevance to β-cell biology and diabetes. Trends Endocrinol. Metab. 2008;19:349–355. doi: 10.1016/j.tem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Schinner S. Wnt-signalling and the metabolic syndrome. Horm. Metab. Res. 2009;41:159–163. doi: 10.1055/s-0028-1119408. [DOI] [PubMed] [Google Scholar]
- 11.Cauchi S, El Achhab Y, Choquet H, Dina C, Krempler F, Weitgasser R, Nejjari C, Patsch W, Chikri M, Meyre D, Froguel P. TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: A global meta-analysis. J. Mol. Med. 2007;85:777–782. doi: 10.1007/s00109-007-0203-4. [DOI] [PubMed] [Google Scholar]
- 12.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by β-catenin and glycogen synthase kinase-3β. J. Biol. Chem. 2005;280:1457–1464. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 13.Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjogren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Invest. 2007;117:2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. U.S.A. 2003;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker GE, Pederson BA, Obayashi M, Schroeder JM, Harris RA, Roach PJ. Gene expression profiling of mice with genetically modified muscle glycogen content. Biochem. J. 2006;395:137–145. doi: 10.1042/BJ20051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki H, Yanagawa S. Axin and the Axin/Arrow-binding protein DCAP mediate glucose–glycogen metabolism. Biochem. Biophys. Res. Commun. 2003;304:229–235. doi: 10.1016/s0006-291x(03)00582-5. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abiola M, Favier M, Christodoulou-Vafeiadou E, Pichard AL, Martelly I, Guillet-Deniau I. Activation of Wnt/β-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS One. 2009;4:e8509. doi: 10.1371/journal.pone.0008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bommer GT, Feng Y, Iura A, Giordano TJ, Kuick R, Kadikoy H, Sikorski D, Wu R, Cho KR, Fearon ER. IRS1 regulation by Wnt/β-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype. J. Biol. Chem. 2010;285:1928–1938. doi: 10.1074/jbc.M109.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, Meseck M, Dong HH. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–5652. doi: 10.1210/en.2006-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, III, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 28.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): Requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae J, Cao Y, Daitoku H, Fukamizu A, Ogawa W, Yano Y, Hayashi Y. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J. Clin. Invest. 2006;116:2473–2483. doi: 10.1172/JCI25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman GI. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 33.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J. Biol. Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 34.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 36.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of β-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Kozarsky K. Gene delivery to the liver. Curr. Protoc. Hum. Genet. 2001 doi: 10.1002/0471142905.hg1310s22. Chapter 13,Unit 13.10. [DOI] [PubMed] [Google Scholar]
- 38.Wahren J, Ekberg K. Splanchnic regulation of glucose production. Annu. Rev. Nutr. 2007;27:329–345. doi: 10.1146/annurev.nutr.27.061406.093806. [DOI] [PubMed] [Google Scholar]
- 39.Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr. Diab. Rep. 2009;9:208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 40.Zeng G, Awan F, Otruba W, Muller P, Apte U, Tan X, Gandhi C, Demetris AJ, Monga SP. Wnt'er in liver: Expression of Wnt and frizzled genes in mouse. Hepatology. 2007;45:195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
- 41.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev. Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic β cell proliferation. J. Biol. Chem. 2008;283:8723–8735. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 44.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 45.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase–1. J. Clin. Invest. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira II, Xu XL, van Lohuizen M, Motoyama N, Deng CX, Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Youn JH, Kim JK, Buchanan TA. Time courses of changes in hepatic and skeletal muscle insulin action and GLUT4 protein in skeletal muscle after STZ injection. Diabetes. 1994;43:564–571. doi: 10.2337/diab.43.4.564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Deletion of hepatic β-catenin does not alter serum insulin concentration.
Fig. S2. Quantification of FoxO1 subcellular localization.
Fig. S3. Insulin tolerance tests (ITTs) in flox/flox β-catenin mice after injection with adenoviruses encoding GFP or Cre recombinase.
Fig. S4. Subcellular localization of FoxO1 in primary hepatocytes after insulin stimulation.
Fig. S5. Deletion of β-catenin decreases the mRNA abundance of FoxO1 transcriptional targets.
Fig. S6. Knockdown of FoxO1 decreases the mRNA abundance of β-catenin transcriptional targets.
Fig. S7. Wnt stimulation increases binding of β-catenin to the G6PC promoter.
Fig. S8. Wnt3a stimulates hepatocyte oxygen consumption.
Fig. S9. Effects of glucagon stimulation on the abundance of mRNAs encoding Wnt ligands.
Fig. S10. The effect of β-catenin on the abundance of mRNAs encoding lipid enzymes and on triglyceride concentrations.