Abstract
Microfabrication and micropatterning techniques in tissue engineering offer great potential for creating and controlling microenvironments in which cell behavior can be observed. Here we present a novel approach to generate layered patterning of hepatocytes on micropatterned fibroblast feeder layers using microfabricated polydimethylsiloxane (PDMS) stencils. We fabricated PDMS stencils to pattern circular holes with diameters of 500 µm. Hepatocytes were co-cultured with 3T3-J2 fibroblasts in two types of patterns to evaluate and characterize the cellular interactions in the co-culture systems. Results of this study demonstrated uniform intracellular albumin staining and E-cadherin expression, increased liver-specific functions, and active glycogen synthesis in the hepatocytes when the heterotypic interface between hepatocytes and fibroblasts was increased by the layered patterning technique. This patterning technique can be a useful experimental tool for applications in basic science, drug screening, and tissue engineering, as well as in the design of bioartificial liver devices.
Keywords: hepatocytes, co-culture, layered cell patterning, cellular interactions, fibroblasts
Introduction
Hepatic tissue engineering has emerged as a novel therapeutic approach to treat damaged or diseased liver tissue (1–3). The reconstruction of functional hepatic tissue is dependent on the ability to control factors that influence the cell environment, including cell-matrix interactions, soluble stimuli, and cell-cell interactions (4–8). Cell-cell interactions play a critical role in tissue morphogenesis, embryogenesis, and organ development. In vivo, the liver is a structurally and functionally heterocellular construct, composed of primary hepatocytes, endothelial cells, Kupffer cells, stellate cells, and fibroblasts (4). In an effort to reconstruct liver tissue in vitro for therapeutic applications, several studies have demonstrated that co-culturing primary hepatocytes with non-parenchymal cells such as fibroblasts or endothelial cells maintains hepatocyte viability and function, whereas hepatocytes cultured alone rapidly lose their function (9–12). However, these conventional co-culture methods (i.e., mixing the two cells types at random) are not able to control the cellular interactions and spatial signaling that occur in the in vivo hepatic microenvironment.
Microfabrication and micropatterning technologies have been used to control cell-cell and cell-surface interactions for applications in tissue engineering (13–18). Various techniques have been used to create micropatterned cells including photolithography, elastomeric stencils, and cell printing. Two-dimensional spheroid microarrays using microfabrication techniques have been utilized to mimic in vivo-like tissue structure (19–21). These cell micropatterning techniques allow co-cultures to be created in which the cell density and the total length of contact between the two cell populations (“heterotypic interface”) can be controlled independently of the cell-cell ratio. Using photolithography to create micropatterned islands of hepatocytes with surrounding 3T3-J2 fibroblasts, we and others demonstrated that albumin and urea levels increased as the length of the heterotypic interface increased (13,22). In addition, intracellular albumin staining of the hepatocytes by day 6 in the micropatterned co-cultures revealed that albumin induction was strongest in hepatocytes that were in close proximity to fibroblasts (i.e., at the heterotypic interface) compared with that in hepatocytes that were far (i.e., >5 cell diameters) from the heterotypic interface. These studies indicate that heterotypic cell-cell interactions play an important role in the maintenance of hepatocellular function.
In the present study, we present a novel culture method to generate layered, patterned hepatocytes on micropatterned fibroblast feeder layers that significantly increases the heterotypic interface using microfabricated polydimethylsiloxane (PDMS) stencils. Based on the previous studies, we hypothesize that hepatocellular structure and function can be improved with increasing heterotypic interface between hepatocytes and fibroblasts by the layered cell patterning method. Micropatterned PDMS stencils were fabricated using a soft lithography approach. The morphologic, phenotypic, and functional characteristics of hepatocytes in the micropatterned co-cultures were evaluated. Results of this study indicate that increasing the heterotypic interface using the layered cell patterning technique in micropatterned hepatic co-cultures significantly enhances the liver-specific functions of hepatocytes, including intracellular albumin staining, urea synthesis, albumin secretion, E-cadherin expression, and glycogen storage. This cell culture technique can be useful for generating a stable in vitro hepatic co-culture model with enhanced function, enabling better understanding of cellular interactions.
Materials and methods
Cell isolation and culture
Hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Wilmington, MA, USA) using a two-step collagenase perfusion procedure as described previously (23). Hepatocyte viability was greater than 90% as determined by trypan blue exclusion. Hepatocyte culture medium consisted of DMEM supplemented with 10% fetal bovine serum (Gibco, Gaithersburgh, MD, USA), 7 ng/mL glucagon (Bedford Laboratories, Bedford, OH, USA), 7.5 µg/mL hydrocortisone (Pharmacia Corporation, Kalamazoo, MI, USA), 0.5 U/mL insulin (Eli Lilly, Indianapolis, IN, USA), 20 ng/mL epidermal growth factor (Sigma-Aldrich, St. Louis, MO, USA), 200 U/mL penicillin, and 200 µg/mL streptomycin (Gibco). Culture medium was changed daily and medium samples were collected for functional analysis.
Murine 3T3-J2 fibroblasts (purchased from Howard Green, Harvard Medical School, Boston, MA, USA), were maintained in T175 tissue culture flasks in DMEM (Gibco) plus 10% FBS and 2% penicillin and streptomycin. Culture medium was changed twice a week. In some cases, the fibroblasts were growth-arrested by treatment with 12 µg/mL of mitomycin-C (Sigma-Aldrich) for 2.5 h prior to cell seeding. Hepatocyte and fibroblast co-cultures were maintained in hepatocyte culture medium and culture medium was changed daily. The 3T3-J2 fibroblasts were used in these co-culture experiments because they have been shown to induce the high levels of hepatic functions (6,13,24).
Microfabrication of the silicon master and PDMS stencil
The PDMS stencils with 500 µm diameter holes were fabricated as described in our earlier study (25), and is schematized in Figure 1. Briefly, SU-8 photoresist (Microchem, Newton, MA, USA) was spun onto the silicon wafers. The wafers were then baked at 65°C for 5–20 min, followed by pre-baking at 100°C for 20–90 min. Next the wafers were exposed to UV light through a mask, followed by post-baking at 100°C for 10–20 min. The SU-8 patterns on the substrates were developed in SU-8 developer (Microchem) for 10 min and rinsed with isopropyl alcohol three times. After drying the substrates using nitrogen gas, they were overexposed to UV light without a mask and baked at 150°C for 1 h. The PDMS prepolymer was made from a mixture of Sylgard 184 Silicone Elastomer Base and Curing Agent at 10:1 ratio (Sylgard 184 kit, Dow Corning, Midland, MI, USA). The mixture was poured on the wafers after degassing bubbles created during mixing. On the applied PDMS, a polyacetate film was placed and clamped with an aluminum block, making the plastic film contact the top surface of the SU-8. The PDMS and aluminum block were heated to 90°C for curing for 12 h. The next day, the PDMS stencil was detached, cut into 10 cm2 pieces to fit into a 35 mm tissue culture dish (Cat. no. 353001; BD Biosciences, San Jose, CA, USA), and sterilized with 70% ethanol prior to patterning cells. Each stencil contained ~550 circular holes and the center-to-center distance between the holes was 1500 µm.
Figure 1. Schematic illustration of the procedure for the fabrication of micropatterned PDMS stencils.
The silicon wafer substrate with patterned SU-8 photoresist was covered with PDMS prepolymer. A polyacetate film was placed on the PDMS and clamped with an aluminum block. After curing, the microfabricated PDMS stencils were peeled off from the master, cut into 10 cm2 pieces to fit into a 35 mm tissue culture dish.
Patterning of cells
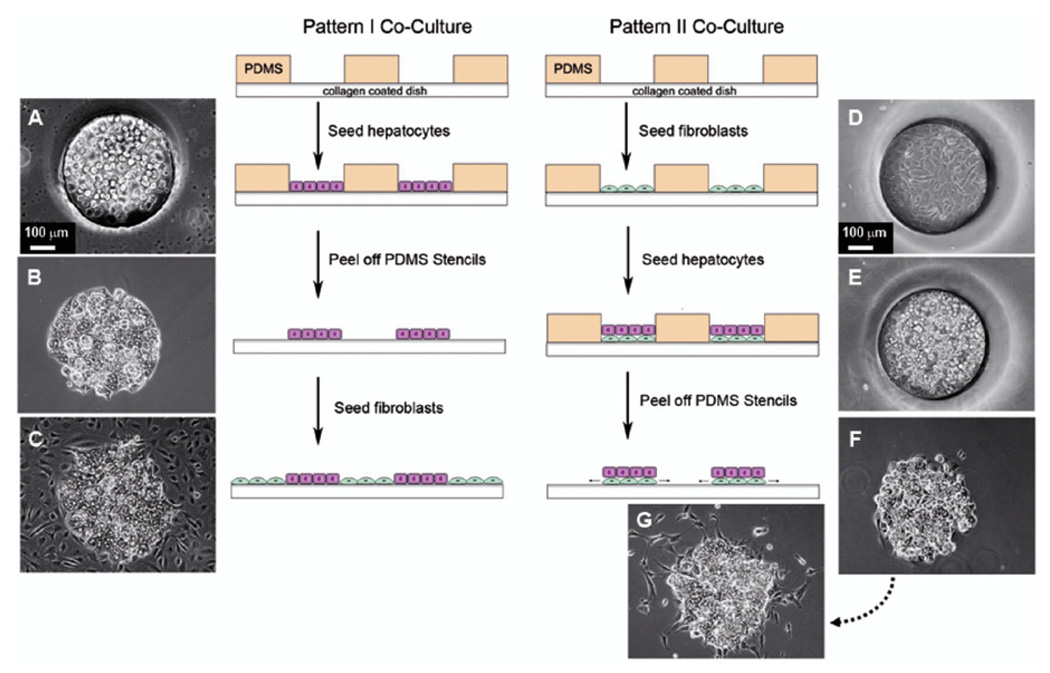
In order to better understand the role of heterotypic cellular interactions in hepatic co-cultures, two different micropatterning methods were used and compared (Figure 2). The first micropatterning method (Pattern I co-culture) was modified from our earlier work (6,25). Using this method, hepatocytes were seeded within the patterned islands, which thereby enabled heterotypic cell-cell contact to occur at the edge of the patterned hepatocyte islands. Briefly, tissue culture dishes (35 mm) were coated with 1 mL diluted type I collagen solution (0.11 mg/mL) for 30 min at 37°C and dried after washing with PBS. Type I collagen was purified from rat tail tendons as described previously (23). The microfabricated stencil with 500 µm–diameter holes was applied to each dish, covered with DMEM, and the air bubbles removed by gentle pipetting. Prior to cell seeding, DMEM was replaced with hepatocyte culture medium. Two milliliters of primary cell suspension (1 × 105 hepatocytes/mL) was added into each stencilcontaining dish, and incubated for 24 h at 37°C to allow for cell attachment. After peeling off the stencil, cultures were washed with DMEM twice to remove unattached cells. The total number of hepatocytes patterned in each dish was ~150,000. Two milliliters of secondary cell suspension (2.25 × 105 fibroblasts/mL) was added into each dish, giving an initial hepatocyte to fibroblast ratio of 1 to 3. Culture medium (1 mL) was changed daily.
Figure 2. Schematic diagram showing sequence of steps in Pattern I and Pattern II co-culture techniques with corresponding micrographs of the cultured cells.
The PDMS stencil, with holes 500 µm in diameter, is applied to each dish. Pattern I co-culture: (A) hepatocytes seeded in the wells of the PDMS stencil, (B) hepatocyte island after removal of PDMS stencil, (C) hepatocyte island with surrounding fibroblasts (culture day 2). Pattern II co-culture: (D) fibroblasts seeded in the wells of the PDMS stencil, (E) hepatocytes seeded in the wells on top of the fibroblasts, (F) island of fibroblasts and hepatocytes after removal of PDMS stencil, (G) fibroblasts proliferating from under the hepatocytes.
The second micropatterning method (Pattern II co-culture) is a new technique that increases the heterotypic interface between hepatocytes and fibroblasts by culturing hepatocytes on micropatterned fibroblast feeder layers. Briefly, tissue culture dishes (35 mm) with PDMS stencils were prepared as described in the Pattern I method. The first cell suspension (2 mL of 1 × 105 fibroblasts/mL) was added into the stencil-containing dish, and incubated for 24 h at 37°C. After changing the medium, the second cell suspension (2 mL of 1 × 105 hepatocytes/mL) was applied into the stencil, and incubated for 24 h at 37°C. After incubation, the stencil was removed and cultures were washed with DMEM twice to remove unattached cells. In Pattern II co-culture, there were ~150,000 hepatocytes patterned in each dish. Culture medium (1 mL) was changed daily.
Immunofluorescence and immunohistochemical staining
For immunofluorescence staining, cultures in 35 mm dishes were washed twice with PBS, fixed in 2% paraformaldehyde in PBS at room temperature for 20 min, washed twice in PBS, followed by addition of 0.2% Triton X-100 in PBS to permeabilize cells for intracellular staining. After 5 min of incubation at room temperature, the cells were washed twice in PBS and incubated in blocking buffer (PBS/3% BSA/5% donkey serum) for 60 min at room temperature to block non-specific antibody binding. After incubation, the cells were stained for 1 h at room temperature with mouse anti-E-cadherin (Invitrogen, Carlsbad, CA, USA) or rabbit anti-N-cadherin (Calbiochem, San Diego, CA, USA). After washing twice in blocking solution, the cells were incubated with fluorescein isothiocyanate (FITC) or Cy3-conjugated mouse IgG or rabbit IgG (ICN Pharmaceuticals, Costa Mesa, CA, USA) for 60 min at room temperature, and washed twice at room temperature. Cells were visualized by fluorescence microscopy on a Zeiss 200 Axiovert microscope (Zeiss, Thornwood, NY, USA).
For immunohistochemical staining, the fixed and permeabilized cultures were incubated with 0.3% hydrogen peroxide in methanol for 30 min followed by incubation in blocking buffer for 30 min. The primary antibody (rabbit anti-rat albumin; ICN Pharmaceuticals, Aurora, OH, USA) was applied for 30 min. For diaminobenzidine (DAB) staining, a biotinylated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA) was used followed by a streptavidin-conjugated horseradish peroxidase (Vector Laboratories). Reaction was stopped with distilled water. All incubations were performed at room temperature. The glycogen storage was detected by periodic acid-Schiff (PAS) reaction (Sigma-Aldrich) according to the manufacturers’ instructions.
Functional assays
The culture medium samples were collected and tested for albumin and urea secretion. The albumin secretion was determined by enzyme-linked immunosorbent assay (ELISA) using purified rat albumin and a peroxidase-conjugated antibody (MP Biomedicals, Aurora, OH, USA). The urea concentration was determined using the Stan Bio urea nitrogen (BUN) kit (Stan Bio Laboratory, Boerne, TX, USA). Standard curves were generated using purified rat albumin or urea dissolved in culture medium. Absorbances were measured with a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Each value represents mean ± sd from triplicate dishes. Statistical significance was determined by a two-tailed Student’s t-test. A p-value of <0.05 was used for statistical significance. Data presented are representative of two or more separate experiments.
Results and discussion
To assess the hepatocelluar characteristics of micropatterned hepatocytes, we evaluated morphologic, phenotypic, and functional characteristics of hepatocytes patterned in single culture and when co-cultured on fibroblast feeder layers. The sequence of micrographs in Figure 2 illustrate step-by-step how the two different micropattern configurations were generated using the PDMS stencil. In Pattern I co-culture, the hepatocytes are initially seeded in the 500 µm wells of the PDMS stencil (Figure 2A). Figure 2B shows an individual island of hepatocytes after removing the PDMS stencil. Once the stencil is removed, the fibroblasts are seeded surrounding the hepatocyte island (Figure 2C). In Pattern I co-culture, the heterotypic interface between the hepatocytes and fibroblasts occurs at the perimeter of the hepatocyte island. In Pattern II co-culture, the fibroblasts are initially seeded in the wells of the PDMS stencil, which formed a feeder layer (Figure 2D). Next, hepatocytes are seeded in the wells on top of the fibroblast feeder layer (Figure 2E). After removing the PDMS stencil (Figure 2F), fibroblasts proliferate from under the hepatocytes (Figure 2G). In Pattern II co-culture, since the hepatocytes are seeded on top of the fibroblasts, the heterotypic interface between the two cell types occurs throughout the area of the cell island as well as at the perimeter of the cell island. This results in an increased heterotypic interface compared with that in Pattern I co-culture.
The hepatocyte morphology for the micropatterned cultures was monitored for 7 days. The micropatterned hepatocytes in single culture exhibited a rapid loss of hepatocelluar morphology (Figure 3A). In contrast, the micropatterned hepatocytes co-cultured with fibroblasts retained their characteristic phenotypic morphology in culture. In Pattern I co-culture, the polygonal morphology with distinct nuclei and well demarcated cell borders was observed in hepatocytes at the heterotypic interface (i.e., island perimeter) with fibroblasts (Figure 3B). In Pattern II co-culture with increased heterotypic interface, the hepatocytes exhibited well developed hepatocellular morphology with uniform distribution of well demarcated cell borders throughout the hepatocyte islands (Figure 3C).
Figure 3. Morphology and albumin staining by immunohistochemistry after 7 days of culture in micropatterned co-cultures.
(A,D) Hepatocytes cultured alone. (B,E) Pattern I co-culture. (C,F) Pattern II co-culture. Insets in panels B and C show higher magnification.
To assess the phenotypic characteristics of the micropatterned hepatocytes, we performed immunohistochemical analysis for intracellular albumin (a hepatic marker). A similar trend was observed for intracellular albumin staining as was observed above for hepatocyte morphology. We observed a very low level of albumin expression for micropatterned hepatocytes cultured alone (Figure 3D), indicating the loss of hepatic characteristics. High levels of albumin staining were observed at the boundaries of the hepatocyte islands, where hepatocytes directly contact the 3T3-J2 fibroblasts in Pattern I co-culture (Figure 3E). Decreased levels of albumin staining were observed at the center of the hepatocyte islands, where there’s no heterotypic contact between hepatocytes and fibroablasts. These results are consistent with those of Bhatia et al. which demonstrated that hepatocytes close to the heterotypic interface in micropatterned co-culture express high levels of intracellular albumin (13). With the increased heterotypic interface using Pattern II co-culture technique, there was a strong and uniform distribution of albumin staining in the hepatocytes throughout the micropatterned islands (Figure 3F), indicating stable maintenance of hepatocyte characteristics.
Next, we measured urea synthesis and albumin secretion to investigate the liver-specific functions for the hepatocytes in each micropatterned condition. As shown in Figure 4, the liver-specific functions for hepatocytes in Pattern II co-culture were increased compared with those in Pattern I co-culture. Urea synthesis rates were approximately 85 µg/24 h for Pattern I co-culture and 108 µg/24 h for Pattern II co-culture on culture day 7 (Figure 4A). The increased urea synthesis rates on days 3 and 7 for the Pattern II co-cultured hepatocytes were both statistically significant compared with the corresponding values for the Pattern I co-cultured hepatocytes. The albumin secretion rates on days 3 and 7 for the Pattern II co-cultured hepatocytes were increased compared with the corresponding values for the Patterned I co-cultured hepatocytes. The monocultures of patterned hepatocytes rapidly lost their functional characteristics. These results indicate that increasing the heterotypic interface by the Pattern II co-culture technique correlates with an increase in liver-specific functions.
Figure 4. Functional analysis of hepatocytes on day 3 and day 7 in Pattern I and Pattern II co-cultures.
(A) Urea synthesis per 35 mm dish. (B) Albumin secretion per 35 mm dish. Hepatocytes cultured alone were used as a control. Data are expressed as the mean ± sd. *P < 0.05.
To investigate the expression pattern of cell-cell junctional proteins in hepatocyte-fibroblast co-cultures, the micropatterned co-cultures were probed with antibodies to E-cadherin and N-cadherin. We observed E-cadherin adhesion protein was primarily expressed on heterotypic contact sites in Pattern I co-culture (Supplementary Figure S1A), whereas strong and uniform E-cadherin expression was observed throughout the micropatterned hepatocyte islands in Pattern II co-culture (Supplementary Figure S1B), indicating that heterotypic cell-cell contact is an important factor for hepatocellular polarity and organization. In contrast, uniform expression of N-cadherin at both heterotypic and homotypic contact sites in the two different micropatterning techniques was observed. One possible explanation is that N-cadherin in hepatic co-cultures may be mediated by growth factors, which are mainly secreted by fibroblasts. It is known that N-cadherin plays an important role in epithelial cell motility and is affected by growth factors (26,27). Previous studies in hepatic co-culture systems have also shown that E-cadherin has a positive correlation with hepatic function (28) but N-cadherin has negative correlation with function (24). Further studies are needed to better understand the mechanism of regulating cadherin expression in hepatic co-culture systems. To assess the ability of the micropatterned hepatocytes to produce and store glycogen, we performed periodic acid-Schiff stain for glycogen. After 7 days of culture, hepatocytes in Pattern II co-culture exhibited more intense glycogen stores than those in Pattern I co-culture (Supplementary Figure S2).
Hepatocytes have high metabolic activity and are known to have ~5–10× higher oxygen consumption rates than most other cell types (29,30). In addition, hepatocytes in co-cultures have higher hepatic function and oxygen consumption rates than those in mono-culture (31). In a 96-well plate format, oxygen transport to the underlying co-cultured cells occurs via diffusion of oxygen through the column of medium directly above the cells, possibly resulting in oxygen depletion for a given cell density, and therefore decreased hepatocyte function. In the layered patterning technique, oxygen transport to the co-cultured cells occurs not only via diffusion of oxygen through the column of medium directly above the cells—as in the 96-well format—but also via diffusion from the medium surrounding each island, which potentially causes improved oxygen transport to the cells. In addition, this technique can easily be used to modulate the hepatocyte density in co-cultures by carefully controlling island size and spacing, as well as provide controlled, enhanced homotypic and heterotypic cell-cell interactions.
In summary, we describe a new cell micropatterning technique to increase the heterotypic cell interactions in co-cultures of hepatocytes and fibroblasts. Hepatocytes were patterned on micropatterned fibroblast feeder layers using microfabricated stencils. Increasing the heterotypic contact area improved the function of the co-cultured hepatocytes. This culturing technique is easy to implement and is reusable, and may be useful for studying the role of cell-cell interactions in tissue engineering of organ systems.
Supplementary Material
Acknowledgments
This study was partially supported by the National Institutes of Health (NIH; grant nos. R01 DK43371, K08 DK066040, and K18 DK076819), the NIH BioMEMS Resource Center (grant no. P41 EB02503), and the Shriners Hospitals for Children. The authors would like to thank Avrum Leeder and Chris Pohun Chen for the isolation of hepatocytes from rat livers. This paper is subject to the NIH Public Access Policy.
Footnotes
Supplementary material for this article is available at www.BioTechniques.com/article/113317.
Competing interests
The authors declare no competing interests.
References
- 1.Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, Amanuma T, Iwata H, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 2.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 3.Chua KN, Lim WS, Zhang P, Lu H, Wen J, Ramakrishna S, Leong KW, Mao HQ. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–2547. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 5.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho CH, Berthiaume F, Tilles AW, Yarmush ML. A new technique for primary hepatocyte expansion in vitro. Biotechnol. Bioeng. 2008;101:345–356. doi: 10.1002/bit.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 8.Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J. Cell Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corlu A, Ilyin G, Cariou S, Lamy I, Loyer P, Guguen-Guillouzo C. The coculture: a system for studying the regulation of liver differentiation/proliferation activity and its control. Cell Biol. Toxicol. 1997;13:235–242. doi: 10.1023/a:1007475122321. [DOI] [PubMed] [Google Scholar]
- 10.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J. Clin. Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesnil M, Fraslin JM, Piccoli C, Yamasaki H, Guguen-Guillouzo C. Cell contact but not junctional communication (dye coupling) with biliary epithelial cells is required for hepatocytes to maintain differentiated functions. Exp. Cell Res. 1987;173:524–533. doi: 10.1016/0014-4827(87)90292-8. [DOI] [PubMed] [Google Scholar]
- 12.Fraslin JM, Kneip B, Vaulont S, Glaise D, Munnich A, Guguen-Guillouzo C. Dependence of hepatocyte-specific gene expression on cell-cell interactions in primary culture. EMBO J. 1985;4:2487–2491. doi: 10.1002/j.1460-2075.1985.tb03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 14.Chen CS, Mrksich M, Huang S, White-sides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 15.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 16.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YC, Ho CC. Micropatterning of proteins and mammalian cells on biomaterials. FASEB J. 2004;18:525–527. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 18.Folch A, Toner M. Microengineering of cellular interactions. Annu. Rev. Biomed. Eng. 2000;2:227–256. doi: 10.1146/annurev.bioeng.2.1.227. [DOI] [PubMed] [Google Scholar]
- 19.Otsuka H, Hirano A, Nagasaki Y, Okano T, Horiike Y, Kataoka K. Two-dimensional multiarray formation of hepatocyte spheroids on a microfabricated PEG-brush surface. ChemBioChem. 2004;5:850–855. doi: 10.1002/cbic.200300822. [DOI] [PubMed] [Google Scholar]
- 20.Tamura T, Sakai Y, Nakazawa K. Two-dimensional microarray of HepG2 spheroids using collagen/polyethylene glycol micropatterned chip. J. Mater. Sci. Mater. Med. 2008;19:2071–2077. doi: 10.1007/s10856-007-3305-1. [DOI] [PubMed] [Google Scholar]
- 21.Torisawa YS, Takagi A, Nashimoto Y, Yasukawa T, Shiku H, Matsue T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials. 2007;28:559–566. doi: 10.1016/j.biomaterials.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Probing heterotypic cell interactions: hepatocyte function in microfabricated co-cultures. J. Biomater. Sci. Polym. Ed. 1998;9:1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- 23.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 24.Khetani SR, Szulgit G, Del Rio JA, Barlow C, Bhatia SN. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology. 2004;40:545–554. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Cho CH, Parashurama N, Li Y, Berthiaume F, Toner M, Tilles AW, Yarmush ML. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip. 2007;7:1018–1028. doi: 10.1039/b704739h. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. 2004;10:4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 27.Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J. Cell Biol. 2000;151:1193–1206. doi: 10.1083/jcb.151.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brieva TA, Moghe PV. Functional engineering of hepatocytes via hetero-cellular presentation of a homoadhesive molecule, E-cadherin. Biotechnol. Bioeng. 2001;76:295–302. doi: 10.1002/bit.10041. [DOI] [PubMed] [Google Scholar]
- 29.Rotem A, Toner M, Tompkins RG, Yarmush ML. Oxygen-uptake rates in cultured rat hepatocytes. Biotechnol Bioeng. 1992;40:1286–1291. doi: 10.1002/bit.260401020. [DOI] [PubMed] [Google Scholar]
- 30.Foy BD, Rotem A, Toner M, Tompkins RG, Yarmush ML. A device to measure the oxygen-uptake rate of attached cells—importance in bioartificial organ design. Cell Transplant. 1994;3:515–527. doi: 10.1177/096368979400300609. [DOI] [PubMed] [Google Scholar]
- 31.Cho CH, Park J, Nagrath D, Tilles AW, Berthiaume F, Toner M, Yarmush ML. Oxygen uptake rates and liver-specific functions of hepatocyte and 3T3 fibroblast co-cultures. Biotechnol. Bioeng. 2007;97:188–199. doi: 10.1002/bit.21225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.