Abstract
The efficacy of vaccines depends on the presence of an adjuvant in conjunction with the antigen. Of these adjuvants, the ones that contain aluminium, which were first discovered empirically in 1926, are currently the most widely used. However, a detailed understanding of their mechanism of action has only started to be revealed. In this Timeline article, we briefly describe the initial discovery of aluminium adjuvants and discuss historically important advances. We also summarize recent progress in the field and discuss their implications and the remaining questions on how these adjuvants work.
Introduction
Vaccines that consist of attenuated pathogens, such as the Sabin ‘live’ polio vaccine, or killed pathogens, such as the Salk inactivated polio vaccine, contain endogenous adjuvants. However, vaccines that contain purified antigens, such as the diphtheria–tetanus–pertussis vaccine or the hepatitis A and hepatitis B vaccines, usually require the addition of an exogenous adjuvant to increase the immune response to the antigens following immunization.
In the early 1900’s, infections by Clostridium tetani and Corynebacterium diphtheriae were serious health issues due to the pathology that is induced by tetanus and diphtheria toxins, respectively (Timeline). Immunization with conjugates of toxin and antibody, which were referred to as toxin–antitoxin, yielded better protection and fewer side effects than low-dose toxin alone1, and it was hypothesized that the antitoxin enhanced the immune response of the recipient by slowly releasing antigen over time. In the following years, further improvement came with the production of toxins that had been inactivated with formalin or heat (referred to as toxoids) that could be used for immunization2,3.
Timeline. History and major scientific advances of aluminium adjuvants.
1910-1919: New York City averages 14,000 cases of diphtheria and 1,290 deaths per year58
1913: Toxin-antitoxin first use for human immunization1.
1921: Immunization with inactivated diphtheria toxin (toxoid) is protective against diphtheria toxin2.
1926: Aluminium precipitation enhances antibody response to diphtheria toxoid in guinea pigs 4
1932: Alum enhances diphtheria toxoid immunization in humans 59
1934: Immunization of guinea pigs with alum plus pollen extracts increases allergic sensitization60.
1939: Alhydrogel becomes commercially available (Erik Lindblad personal communication).
1942-1944: New York City averages 300 cases of diphtheria and 10 deaths per year, >99% reduction in mortality from 191058.
1944: Alexander T. Glenny elected to the Royal Society61.
1953: Alexander T. Glenny awarded the Addingham Gold Medal and Jenner Medal61.
1955: Characterization of cells surrounding the ‘alum granuloma’11.
1985: Aluminium salts enhance antigen presentation by APCs15.
1989: Th2-type CD4 T cells are preferentially primed by aluminium adjuvants5.
1996: Aluminium salt-induced IgE requires IL-435.
2001-2006: Aluminium adjuvant effects do not require IL-1, IL-18, MyD88 or TRIF 19, 20, 30, 44
2003: Alum ‘primes’ B cells to flux calcium when stimulated through MHC II 38
2003: Uric acid acts as an adjuvant for co-administered particulate antigen 49
2008: Uric acid and NLRP3 inflammasome implicated in aluminium adjuvant effects in vivo. 7, 8, 14, 23
Footnote: Biography of Alexander Glenny61.
In 1926, Alexander T. Glenny and colleagues reported that precipitation of antigen onto insoluble particles of aluminium potassium sulphate (Box 1), known as ‘potash alum,’ before immunization produced better antibody responses than soluble antigen alone, providing the first clue to the adjuvant properties of aluminium salts4. Following this discovery, aluminium salts were used in vaccine preparations with tetanus and diphtheria toxoids to protect against C. tetani and C. diphtheriae, respectively, and today insoluble aluminium salts are used worldwide as the principle adjuvants in clinical vaccines.
Box 1. Different insoluble aluminium salts have different characteristics.
Several insoluble aluminium salts are used as adjuvants in human and animal vaccines, but the term ‘alum’ only applies to aluminium potassium sulphate (reviewed in REF.46). Alum was used by Alexander Glenny, and captures antigen by precipitation when the solution is neutralized. Due to problems in manufacturing reproducibility, alum has been almost compeltely replaced by aluminium hydroxide and aluminium phosphate for commercial vaccines. These can be prepared in a more standardized way and capture antigen by direct adsorption. Many types of adsorptive interactions occur between antigen and adjuvant but two adsorption forces seem to predominate46. The first, electrostatic interactions, occurs most strongly between negatively charged proteins and aluminium hydroxide, and between positively charged proteins and aluminium phosphate. The second, anionic ligand exchange, is a covalent interaction that occurs when phosphates from an antigen substitute for hydroxyl groups on the adjuvant.
| Common Name | Used in humans? | Chemical Formula | Chemical name |
|---|---|---|---|
| Alum | Yes | AlK(SO4)2 | Aluminium potassium sulfate |
| Alhydrogel | Yes | Al(OH)3 | Aluminium hydroxide |
| Adju-Phos | Yes | Al(PO4)3 | Aluminium phosphate |
| Imject Alum | No | Al(OH)3 + Mg(OH)2 | Aluminium hydroxide and magnesium hydroxide |
Glenny believed that aluminium salts were effective adjuvants because they allowed antigen to remain in the body for a long time and because the antigen was slowly released from the insoluble salt particles, which allowed prolonged and effective stimulation of the immune system, an effect referred to as the ‘depot effect.’ For approximately 60 years, this explanation was dogmatically accepted within the field and only a few basic research papers on aluminium salts were published, often decades apart.
In the past two decades or so, interest in aluminium salts has reignited, and since 2007 there has been a flurry of activity aimed at understanding the adjuvant action of these compounds. In addition to or in contrast to the depot effect, insoluble aluminium salts activate innate immune cells in a manner that ultimately results in a T helper 2 (Th2)-type immune response5. In 2008 it was suggested that the cytotoxicity of aluminium salt leads to release of uric acid in vivo, which acts as a damage associated molecular pattern (DAMP, reviewed in REF. 6) that is required for the adjuvant activity of aluminium7. A separate paper showed a requirement for caspase-1 activation in vivo, which is mediated by NLR family, pyrin domain containing 3 (NLRP3; also known as NALP3) and apoptosis-associated speck-like protein containing a CARD (ASC; also known as PYCARD), collectively known as the NLRP3 inflammasome8 (Box 2).
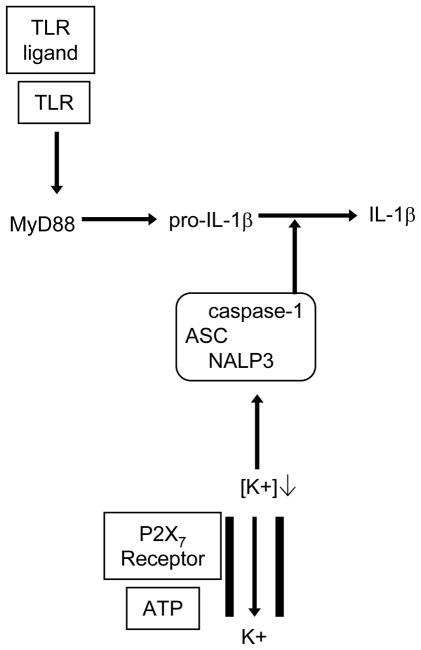
Box 2. The NLRP3 inflammasome and the immunomodulatory activity of uric acid.
NLR family, pyrin domain containing 3 (NLRP3; also known as NALP3) is a member of the nucleotide-binding domain and leucine-rich repeat containing gene (NLR) family and is an intracellular protein that can sense various pathogens, such as Listeria monocytogenes and Staphylococcus aureus, pathogen products, such as lipopolysaccharide (LPS) and muramyl dipeptide (MDP), and crystals, such as monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD)47. When activated, NLRP3 associates with the adapter protein apoptosis-associated speck-like protein containing a CARD (ASC), which interacts with inactive pro-caspase-1. This facilitates the autocatalysis of pro-caspase-1 and results in the generation of active caspase-1. In vitro, expression of pro-interleukin-1β (pro-IL-1β) and activation of caspase-1 depend on Toll-like receptor (TLR) signalling, usually delivered by lipopolysaccharide (LPS). Caspase-1 cleaves pro-IL-1β, pro-IL-18 and pro-IL-33 to generate the active cytokines, which are then secreted through a non-canonical secretion pathway that is poorly understood. For a simplified version of what is known about the action of the NLRP3 inflammasome, see Figure 3.
Uric acid is a catabolic product of nucleotides and is soluble at high concentrations within cells. Cell damage or death causes uric acid release into the extracellular milieu where it is much less soluble and forms MSU crystals, which are the causative agent of gout48. Uric acid has been shown to have adjuvant activity when co-injected with particulate antigen49. However, this activity only occurred above saturating levels, strongly implying that MSU crystals, and not soluble uric acid, function as the adjuvant. Uric acid promotes CD4+ and CD8+ T-cell responses50,51, as well as antibody responses28. In vitro, stimulation of a monocytic cell line or peritoneal macrophages with MSU crystals led to the secretion of interleukin-1β (IL-1β) and IL-18, and this required all three components of the NLRP3 inflammasome: NLRP3, ASC and pro-caspase-152.
However, the field is not without controversy. Other studies have failed to confirm a requirement for uric acid in vitro or for the NLRP3 inflammasome in vivo, and research in these areas is ongoing. In addition, several unresolved issues remain. For example, it is not completely clear how aluminium salts activate the inflammasome, why the Toll-like receptor 4 (TLR4) ligand lipopolysaccharide (LPS) is sometimes required for inflammasome activation in vitro, what the potentially relevant downstream targets of caspase-1 are, and why aluminium salts induce Th2-type rather than Th1-type responses. Here, we describe what is currently known and discuss what remains to be discovered about the effects of aluminium salts on innate and adaptive immune responses.
Challenges to the depot theory
Glenny’s proposal that the adjuvant activity aluminium salts was due to antigen persistence and prolonged release was not without basis. Initial evidence showed that precipitated toxoid persisted for several days9, much longer than soluble toxoid. A subsequent study found that 7 weeks after immunization, alum nodules that were excised from one guinea pig and were ground up could be used to immunize a second guinea pig10.
Experiments by White et al.11 showed that immunization of rabbits with antigen plus aluminium salts induced the appearance of B-cell blasts in the draining lymph nodes within 7 days and at the site of the granuloma by day 14. However, few B-cell blasts remained in the draining lymph nodes after 3 weeks, which implies that antigen is no longer being presented in the lymph nodes and that negligible amounts of antigen escape the site of injection after ~2 weeks11. These results are consistent with data from other studies that showed that removal of the antigen–aluminium salt nodules 14 days after immunization had no effect on the subsequent antibody titres12. Therefore, these data indicate that aluminium salts do not function simply by providing a long-lived antigen depot.
More recent experiments have shown that within hours of administration of aluminium salts to mice, pro-inflammatory mediators such as interleukin-1β (IL-1β) 13, CC-chemokine ligand 2 (CCL2; also known as MCP-1), CCL11 (also known as eotaxin-1)7, histamine and IL-5 (A.S.M., unpublished observations) are detectable. Also, innate inflammatory cells such as neutrophils, eosinophils, inflammatory monocytes, myeloid dendritic cells (DCs) and plasmacytoid DCs are recruited to the site of aluminium salt injection within 1 day7. Although the mediators that contribute to the recruitment of these infiltrating cell types have not yet been completely defined, these data clearly show that aluminium salts have effects, in addition to the depot effect, that account for their adjuvant properties.
Aluminium salts and innate immunity
Effects of aluminium salts on antigen-presenting cells
The ability of aluminium salts to promote antigen uptake and presentation by human macrophages was first shown over 20 years ago15. More recently, it has been shown that exposure of peripheral blood mononuclear cells (PBMCs) to aluminium salts in vitro leads to the upregulation of MHC class II molecules, CD40 and CD8616. Studies using mouse DC in vitro have yielded some conflicting results, with one study finding that aluminium salts did not induce expression of co-stimulatory molecules or enhance antigen presentation17, but another showing that CD86 was upregulated and antigen presentation by DCs was increased18. An in vivo study found that peritoneal DCs and B cells internalized antigen that was adsorbed to aluminium salts more readily than soluble antigen alone, and aluminium salt increased the expression of CD86 by DCs7. In addition, peritoneal injection of aluminium salt induced the recruitment of monocytes to the peritoneum. In the presence of aluminium salts, these monocytes acquired antigen, trafficked to the draining lymph node and differentiated into CD11c+ MHC class II+ DCs in a myeloid differentiation primary-response gene 88 (MyD88)-dependent manner. These recruited monocytic DC precursor cells, but not lymph-node-resident or spleen-resident DCs, were shown to be responsible for priming naive CD4+ T cells in the lymph node.
Is there a specific receptor?
The activity of many immunological adjuvants, for example LPS and unmethylated CpG motifs, requires signalling through TLRs and activation of one or both of their downstream adaptor molecules, MyD88 and TIR-domain-containing adaptor protein inducing IFNβ (TRIF; also known as TICAM2). Therefore, investigators thought that aluminium salts might function by acting as a ligand for one or more TLRs. However, two studies indicate that aluminium salts do not act through TLRs19,20. In the first study, MyD88-deficient mice produced normal amounts of IgG1 and, interestingly, excessive amounts of IgE in response to immunization with antigen and aluminium salts19. The second study used mice that were deficient for both MyD88 and TRIF, which therefore could not signal through TLRs20. In this case, antigen-specific antibody responses of all isotypes induced following vaccination with the antigen trinitrophenyl (TNP)–hemocyanin and aluminium salt were comparable to those observed in control mice. Although the use of hemocyanin slightly confounds the interpretation of these results, as hemocyanin itself has adjuvant properties that might be TLR independent, together these studies strongly suggest that the antigen-specific antibody response to aluminium salts does not depend on TLRs.
It was recently shown that monosodium urate (MSU) crystals, which are formed when the concentration of uric acid is saturating (Box 2), bind to DCs in an unconventional manner21. Using atomic force microscopy to measure interaction force, MSU crystals were found to interact strongly with the plasma membrane, even when surface proteins were removed. This occurred through a direct interaction with cholesterol and potentially other lipids. So, it is possible that DCs or other phagocytic cells bind to aluminium salts through a similar mechanism, but this has yet to be determined experimentally. Latex beads were also shown to interact strongly with the membrane of phagocytes, but basic calcium phosphate (BCP) crystals and allopurinol crystals did not produce detectable interaction forces21. Therefore it is currently an open question whether aluminium salts interact with cells through a specific unidentified receptor or through a less conventional manner, similarly to MSU crystals.
NLRP3 inflammasome activation: direct or indirect?
Several reports showed that in vitro exposure of cells to aluminium salts induced the activation of caspase-1 and the release of its known downstream targets, IL-1β, IL-18 and IL-3318, 22,23. These reports and subsequent studies8,14,24,25 showed that NLRP3 and ASC are required for the activation of caspase-1 and its target cytokines. Macrophages primed with LPS and exposed to aluminium salts in vitro produced IL-1β, as well as IL-18 and IL-33 in studies that examined the production of these cytokines, in a NLRP3- and caspase-1-dependent manner8, 23, 24 (Figure 1). Intact endocytic machinery8,22 and subsequent potassium efflux8, which is a known co-activator of caspase-1, are required for this IL-1β production. However, the ATP receptor P2X7 (a purinergic receptor), which is known to also have a role in the activation of the NLRP3 inflammasome, was not involved in IL-1β production in these studies8,24. Related reports have shown that other particulate materials, such as silica, asbestos and MSU crystals, also act through the NLRP3 inflammasome to produce IL-1β14,26,27,52. Together, these data suggest that aluminium salts and other particulates can initiate caspase-1 activation through the NLRP3 inflammasome. However, given that aluminium salts can induce an antibody response in the absence of TLR signalling adaptor molecules19,20, it remains unclear why a TLR signal, such as that induced by LPS, is required for the production of pro-IL-1β and the activation of caspase-1 in vitro8, 22–24.
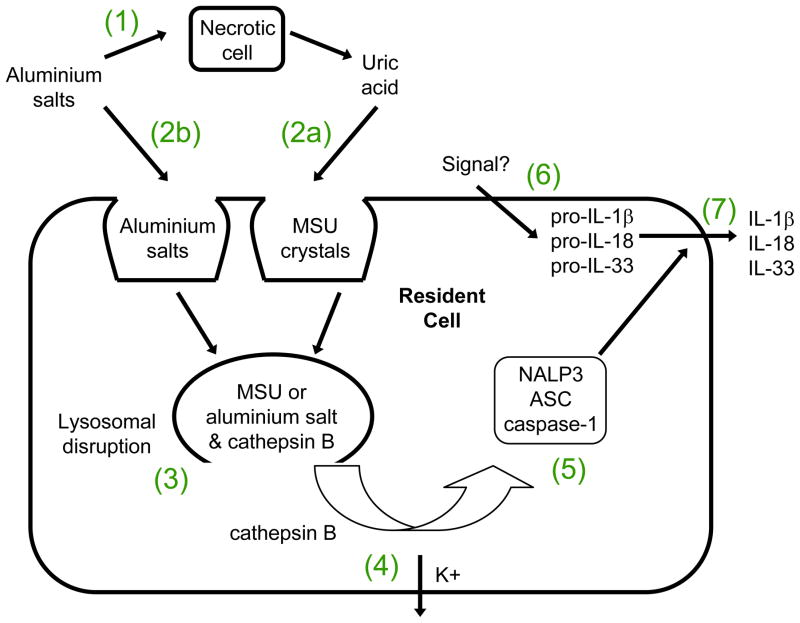
Figure 1. Activation of the NLRP3 inflammasome by aluminium salts.
(1) Aluminum salt cytotoxicity leads to release of DAMPs such as uric acid by the necrotic cell. At high concentrations, uric acid forms monosodium urate (MSU) crystals. (2a) MSU crystals are phagocytosed by resident cells. (2b) In addition or alternatively, aluminum adjuvants are directly phagocytosed by resident cells. (3) MSU or aluminium salts disrupt lysosomes, releasing cathepsin B. (4) Cathepsin B may directly or indirectly induce K+ efflux which (5) co-activates the NLRP3 inflammasome. (6) An unknown signal induces the production of pro-IL-1β, pro-IL-18 and pro-IL-33. (7) caspase-1, following activation by the NLRP3 inflammasome, cleaves pro-IL-1β, pro-IL-18 and pro-IL-33 to their active forms thereby promoting their secretion.
How do aluminium salts activate the NLRP3 inflammasome? At present there are two different models, both of which are compatible with the finding that phagocytosis is required for this process. In the direct activation model, phagocytic cells directly engage and engulf aluminium salt particles14. It is currently unclear if this occurs through the interaction of aluminium salt particles with a specific receptor on the surface of phagocytic cells or through their non-specific adsorption to cell-surface molecules (see above), as has been shown with MSU crystals21. This engulfment leads to lysosomal damage and rupture, followed by the release of antigen and lysosomal enzymes such as cathepsin B into the cytoplasm. In some studies, the release of IL-1β was found to depend partially on cathepsin B activity and to depend strongly on NLRP3 and ASC14,13. Rupture of endolysosomes by silica crystals and osmotic shock produced similar results, suggesting that aluminium-salt-induced lysosomal damage may activate a default pathway that senses intracellular danger.
The indirect activation model proposes that aluminium salt cytotoxicity leads to the release of endogenous DAMPs such as uric acid (Box 2), which results in the activation of the NLRP3 inflammasome. Following intraperitoneal injection of aluminium adjuvant and antigen into mice, the local concentration of uric acid was shown to increase substantially7. Furthermore, pre-treatment of mice with uricase to degrade uric acid inhibited CD4 T-cell priming. Presumably, aluminium salt cytotoxicity causes uric acid release and the formation of MSU crystals, which could then be sensed by macrophages or other leukocytes to initiate innate and adaptive immune responses.
Notably, the study did not report what effect, if any, the uricase pre-treatment had on antibody titers, even though MSU crystals had been previously reported to enhance antibody responses to co-injected antigen28 and antibodies are thought to provide the primary protective mechanism induced by aluminium salts. There are also other questions regarding the role of uric acid. First, in vitro treatment of cells with uricase, which breaks down uric acid, did not prevent aluminium-salt-induced IL-1β release8,14,25. Second, the induction of inflammation and the adjuvant action of MSU crystals is reported to require IL-1–IL-1 receptor (IL-1R) engagement and the MyD88 signalling pathway29. However, the adjuvant activity of aluminium salts does not seem to require either MyD8820 (see above) or IL-1–IL-1R engagement in vivo30, 31 (see below). Therefore, it remains to be elucidated why uric acid is dispensable for aluminium-salt-induced inflammation in vitro but is required in vivo and whether there is any requirement for uric acid in antibody production following immunization with aluminium salts.
Aluminium salts and adaptive immunity
IL-4 and the Th2-type bias
It has been known for many years that aluminium salts induce robust antibody responses. The discoveries that antibody production depends on T-cell help32 and that Th-cell subsets with different functions exist33 led to studies to determine the effects of aluminium salts on different TH-cell subsets. Aluminium salts were found to preferentially induce Th2 cells5, which mediate the differentiation of B cells that secrete Th2-cell-associated antibody isotypes IgG1 and IgE.
Because IL-4 production is a key feature of Th2-cell responses in vivo34, its role in immune responses that are induced by aluminium salts was evaluated35,36. Although immunization of IL-4-, IL-4Rα- or signal transducer and activator of transcription 6 (STAT6)-deficient mice with antigen in the presence of aluminium salts did not trigger the production of IgE, the Th2-cell associated antibody isotype IgG1 was present in the serum, albeit at reduced levels. However, these mice also produced high titres of the Th1-cell-associated isotype IgG2a in response to aluminium salt, which was in contrast to wild-type mice. Mirroring this change in antibody isotype production, antigen-specific T cells from IL-4-, IL-4Rα- or STAT6-deficient mice produced normal levels of the Th2-type cytokine IL-5, but more IFNγ than control mice. Therefore, IL-4 induced by aluminium salts has some role in promoting Th2-type antibody and T-cell responses, but has a more important role in inhibiting Th1-type antibody and T-cell responses. Subsequent work has shown that following immunization with antigen and aluminium salts, IL-6 also inhibits Th1-type antibody and T-cell responses. Unlike IL-4-deficient mice, however, IL-6-deficient mice had increased Th2-type T-cell responses, suggesting that IL-6 suppresses both Th1-type and Th2-type responses to aluminium-salt-containing vaccines37.
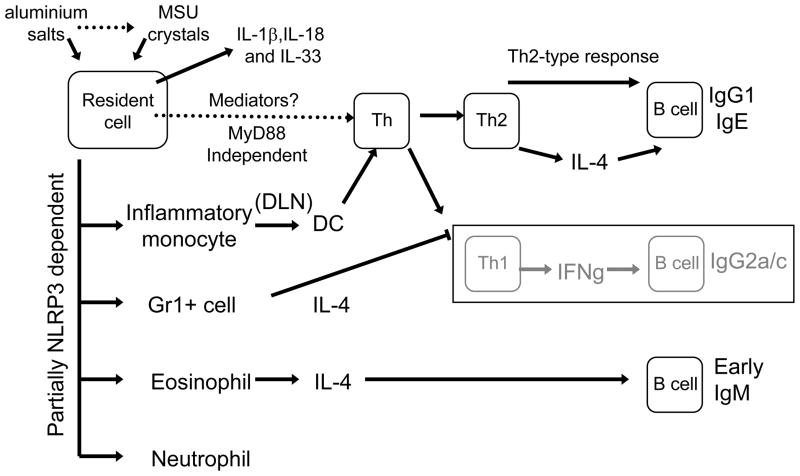
But what cells produce IL-4 early in the response to aluminium salts? One study38 showed that aluminium salts induce the accumulation of a population of Gr1+ IL-4-producing cells in the spleen 6 days following immunization. This population comprised eosinophils and a myeloid-cell type, although more recent evidence argues that this population is composed mainly (~80%) of eosinophils39. These cells appear at the injection site within 24 hours40, and IL-4 produced by these cells was shown to increase B-cell proliferation and promoted an increase in the production of IgM38,39. In addition, depletion of Gr1+ cells in mice followed by administration of an aluminium-salt-containing vaccine resulted in the generation of IgG2c-producing B cells40; neutralization of IL-4 had the same effect.
Together, these studies indicate that aluminium salts promote the recruitment of IL-4-producing eosinophils to the spleen, which in turn mediate the production of early IgM. IL-4 may also be involved in increasing the accessibility of the B-cell IgE locus to factors required for class switching and in suppressing the events required for Th1 cells to develop during priming41 (Box 3, Figure 2).
Box 3. Clues to the adjuvant action of aluminium salts from other adjuvants.
Important clues about aluminium salts can be gained from substances with related properties. Other particles, such as seemingly inert sepharose beads or the structural biopolymer chitin, have been shown to induce rapid eosinophilia and basophilia53,54. Importantly, degradation of chitin in vitro or in vivo abrogated leukocyte infiltration54. Egg extract from the helminth parasite Schistosome mansoni also induces eosinophilia40. Similar to the adjuvant action of aluminium salts (see main text), these extracts induce interleukin-4 (IL-4)-dependent B-cell priming and enhance the early IgM response to co-administered antigen, although they were shown not to act as a CD4+ T-cell adjuvant40.
Furthermore, parasite proteases, such as GP63 proteins of Leishmania species, are important for parasite growth and infective ability in vivo55. In addition, many protein allergens are proteases, and a recent study found that the protease papain directly activates basophils to secrete IL-4 and thymic stromal lymphopoietin (TSLP)56, which are known to promote Th2-cell responses. In line with this observation, Leishmania mexicana lacking cysteine-protease activity induced less antigen-specific IgE production than a wild-type parasite in BALB/c mice57.
Together, it seems that these particles and proteases share some similar properties with those of aluminium salts. This suggests that the adjuvant activity of aluminium salts may be partially due to their particle properties, which induce the release of lysosomal host proteases, and/or some as-yet-unknown mechanisms that work synergistically to induce its Th2-cell biased adjuvant effects.
Box 2.
The NALP3 inflammasome
A controversy on the NLRP3 inflammasome
A link between aluminium-salt-induced inflammasome activation and the induction of antibody responses was first provided by a study by Eisenbarth et al.8 showing that vaccination with antigen and aluminium salt required NLRP3, ASC and caspase-1 to induce antigen-specific IgG1 production8. Soon after, a minor but important role for NLRP3 in aluminium salt-induced IgG1 production was reported by Li et al.23. These studies suggested that unlike IL-4, which primarily inhibits Th1-type responses, the NLRP3 inflammasome promotes Th2-type responses. However, a subsequent study by Franchi et al.24 reported the opposite result: NLRP3 deficiency did not alter production of IgM, IgA or IgG antibodies following immunization with aluminium salt and antigen. Our own experiments in caspase-1-deficient mice have also failed to find a defect in either T-cell or antibody responses in response to aluminium salts (A.S.M., unpublished observations). Adding further confusion, Kool et al.25 recently showed that NLRP3-deficient mice were partially defective at priming antigen-specific TCR Tg T cells, but had normal IgG1 titers. It was also shown that IgG2c antibodies were slightly increased, whereas IgE antibodies were completely absent, suggesting that NLRP3 promotes class-switching to IgE at the expense of IgG2c. Therefore, many outcomes have been reported for mice defective in the same signalling pathway.
The reason for these contrasting results is unclear, but could be the result of differences in the timing of immunizations, the forms of aluminium adjuvant used or the genetic background of the mice. All five studies examined secondary antibody responses after priming and boosting with antigen and adjuvant. The three papers that showed a role for NLRP3 in the antibody response to aluminium salts (Eisenbarth et al.8, Li et al.23, and Kool et al.25) used mice on a C57BL/6 background, whereas Franchi et al.24 used mice of a mixed C57BL/6–129 background. However, in our study we used caspase-1-deficient mice also on a C57BL/6 background (A.S.M. unpublished observations) and did not find a role for caspase-1 in the induction of antibody responses. The Eisenbarth et al., Li et al., and Kool et al. papers also used Imject Alum (Pierce, USA), which is a non-clinical adjuvant containing magnesium hydroxide in addition to aluminium hydroxide (Box 1), whereas Franchi et al. used aluminium hydroxide as the adjuvant, and we used aluminium potassium sulphate. Current work is underway in our laboratory to determine if differences in immunization protocols, the form of the adjuvant or the genetic background of the mice contribute to the discrepant results.
Based on the data discussed above, we conclude that activation of the NLRP3 inflammasome is sometimes, but not always, required for aluminium salts to induce Th2-type T-cell and antibody responses in vivo.
Role for caspase-1 signalling mediators
As discussed earlier, the activation of the inflammasome by aluminium salts results in the production of IL-1β and IL-18, and therefore it was thought that these cytokines may be involved in aluminium-salt-induced adaptive immune responses. Indeed, these cytokines are known to have a role in asthma, a disease that is generally considered to be driven by Th2 cells. In addition, airway hyperresponsiveness (AHR) can be induced by sensitization with antigen and aluminium salts42.
However, earlier studies on the role of IL-1–IL-1R signalling in AHR in the presence or absence of aluminium salt indicated that this signalling pathway was only required for the development of airway inflammation in the absence of aluminium salt30,31. In the presence of aluminium salt, IL-1–IL-1R signalling was not required for bronchoconstriction, pulmonary eosinophilia, T-cell priming or antibody responses.
IL-18, which is a pro-inflammatory member of the IL-1 family, can have either Th1- or Th2-cell-promoting properties depending on the system used in the study43. Following vaccination with antigen and aluminium salt, IL-18 was found to be partially required for IL-4 production, whereas it was not required for antigen-specific IgG1 and IgE production, or for T-cell responses44.
So at present, the requirement for the NLRP3 inflammasome and caspase-1 for the induction of Th2-cell-associated antibodies in response to aluminium salts is still a matter of debate. However, it seems that IL-1β and IL-18, which are the best-studied caspase-1 targets, are not required for the induction of adaptive immune responses in response to aluminium salts. It is possible that IL-1β and IL-18 act redundantly in this capacity or that another member of the family (for example IL-33) or a target of caspase-1 that has yet to be identified45 is the crucial factors.
Outstanding questions
Recent studies have increased our knowledge of the biological events that are induced following the administration of aluminium adjuvants. However, the mechanisms that are required for the subsequent induction of the adaptive immune response are still debated. Some additional questions remain to be answered and some important discrepancies need to be resolved: Is there a specific cellular receptor that recognizes aluminium salts? In vivo, does aluminium salt-induced inflammation occur indirectly by causing cytotoxicity and release of DAMPs such as uric acid, which forms MSU crystals, or directly by triggering phagocytosis and lysosomal disruption? LPS is required to upregulate Il1b mRNA expression and to activate caspase-1 in vitro, but what induces these effects in vivo? Is there a biological role for the induction of IL-1β and neutrophil accumulation? Is activation of the NLRP3 inflammasome and activation of caspase-1 crucial for the adjuvant activity of aluminium salts or not? If they do have a role, which caspase-1 target (or targets) is required for the induction of an adaptive immune response? Finally, after over 80 years of research, there is still no definitive proof for Alexander Glenny’s original hypothesis that the depot effect of aluminium salts contributes to their adjuvant action.
Aluminium adjuvants have been successfully used in hundreds of millions of humans since 1932, greatly decreasing morbidity and mortality with minimal toxicity. Although there are instances where aluminium adjuvants are not protective, such as vaccination against pathogens that require Th1-type immunity, it is hoped that the answers to these questions will aid in improving the effectiveness of aluminium adjuvants and speed development of alternative adjuvants.
Acknowledgments
This work was partially supported by USPHS grants A1-18785 & A1-22995.
Biographies
Philippa Marrack is an Investigator of the Howard Hughes Medical Institute and Professor at National Jewish Health in Denver, Colorado, USA. She performed her doctoral work with Alan Munro at Cambridge University, England, and her postdoctoral work with Richard Dutton at the University of California in San Diego, USA. She is a member of the United States National Academy of Sciences and a Fellow of The Royal Society of London. Her laboratory collaborates closely with that of her husband, John Kappler, studying the adjuvant action of aluminium salts as well as various aspects of T cell biology including development, apoptosis, memory, senescence and autoimmunity.
Amy McKee is a postdoctoral fellow in Philippa Marrack’s lab. She performed her doctoral work with Ed Pearce at Cornell University in Ithaca, New York, USA, studying the role of dendritic cells in host responses to helminth infection. She is currently investigating the cell types and signaling pathways that are involved in the adjuvant effects of aluminium salts.
Michael Munks is a postdoctoral fellow in Philippa Marrack’s lab. He performed his doctoral work with Ann Hill at Oregon Health & Science University in Portland, Oregon, USA, characterizing the CD8 T cell response to mouse cytomegalovirus. He is currently examining the role of fibrinogen in aluminium salt-induced inflammation.
References
- 1.von Behring E. Ueber ein neues diphtherieschutzmittel. Dtsch Med Wschr. 1913;39:873–876. [Google Scholar]
- 2.Glenny AT, Sudmersen HJ. Notes on the production of immunity to diphtheria toxin. J Hyg. 1921;20:176. doi: 10.1017/s0022172400033945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramon G. Sur le pouvoir floculant et sur les proprietes immunisantes d’une toxine diphtherique rendue anatoxique. CR hebd Seances Acad Sci. 1923;177:1338–1340. [Google Scholar]
- 4.Glenny AT, Pope CG, Waddington H, Wallace U. Immunological Notes: XVII–XXIV. J Pathol Bacteriol. 1926;29:31–40. [Google Scholar]
- 5.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 6.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenny AT, Buttle GAH, Stevens MF. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea-pigs: toxoid precipitated with alum. J Pathol Bacteriol. 1931;34:267–287. [Google Scholar]
- 10.Harrison WT. Some Observations on the Use of Alum Precipitated Diphtheria Toxoid. Am J Public Health Nations Health. 1935;25:298–300. doi: 10.2105/ajph.25.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White RG, Coons AH, Connolly JM. Studies on antibody production. III The alum granuloma. J Exp Med. 1955;102:73–82. doi: 10.1084/jem.102.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt LB. Developments in diphtheria prophylaxis. London: Wm. Heinemann, Ltd; 1950. [Google Scholar]
- 13.Sharp FA, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clinical & Experimental Immunology. 1985;61:143–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The Common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69:1151–1159. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21:849–855. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 18.Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25:4575–4585. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Schnare M, et al. Toll-like receptors control activation of adaptive immune responses. Nature Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 20.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng G, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kool M, et al. Cutting Edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 26.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassel SL, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behrens MD, et al. The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity. Blood. 2008;111:1472–1479. doi: 10.1182/blood-2007-10-117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CJ, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz N, Kurrer M, Kopf M. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur J Immunol. 2003;33:991–1000. doi: 10.1002/eji.200323801. [DOI] [PubMed] [Google Scholar]
- 31.Nakae S, et al. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol. 2003;15:483–490. doi: 10.1093/intimm/dxg054. [DOI] [PubMed] [Google Scholar]
- 32.Claman HN, Chaperon EA, Triplett RF. Immunocompetence of transferred thymus-marrow cell combinations. J Immunol. 1966;97:828–832. [PubMed] [Google Scholar]
- 33.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 34.Kopf M, et al. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 35.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 36.Brewer JM, et al. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–6454. [PubMed] [Google Scholar]
- 37.Brewer JM, et al. Neither interleukin-6 nor signalling via tumour necrosis factor receptor-1 contribute to the adjuvant activity of Alum and Freund’s adjuvant. Immunology. 1998;93:41–48. doi: 10.1046/j.1365-2567.1998.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 39.Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKee AS, et al. Gr1+IL-4-producing innate cells are induced in response to Th2 stimuli and suppress Th1-dependent antibody responses. Int Immunol. 2008;20:659–669. doi: 10.1093/intimm/dxn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berton MT, Uhr JW, Vitetta ES. Synthesis of germ-line gamma 1 immunoglobulin heavy-chain transcripts in resting B cells: induction by interleukin 4 and inhibition by interferon gamma. Proc Natl Acad Sci USA. 1989;86:2829–2833. doi: 10.1073/pnas.86.8.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kung TT, et al. Characterization of a murine model of allergic pulmonary inflammation. Int Arch Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 43.Reddy P. Interleukin-18: recent advances. Curr Opin Hematol. 2004;11:405–410. doi: 10.1097/01.moh.0000141926.95319.42. [DOI] [PubMed] [Google Scholar]
- 44.Pollock KG, Conacher M, Wei XQ, Alexander J, Brewer JM. Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology. 2003;108:137–143. doi: 10.1046/j.1365-2567.2003.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 46.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6:685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 47.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Martinon F, Glimcher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116:2073–2075. doi: 10.1172/JCI29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci USA. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Rock KL. Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur J Immunol. 2002;32:155–162. doi: 10.1002/1521-4141(200201)32:1<155::AID-IMMU155>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 52.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira SH, Costa CH, Ferreira SH, Cunha FQ. Sephadex induces eosinophil migration to the rat and mouse peritoneal cavity: involvement of mast cells, LTB4, TNF-alpha, IL-8 and PAF. Inflamm Res. 2002;51:144–153. doi: 10.1007/pl00000286. [DOI] [PubMed] [Google Scholar]
- 54.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol. 2006;1:497–536. doi: 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- 56.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunol. 2007 doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollock KG, et al. The Leishmania mexicana cysteine protease, CPB2.8, induces potent Th2 responses. J Immunol. 2003;170:1746–1753. doi: 10.4049/jimmunol.170.4.1746. [DOI] [PubMed] [Google Scholar]
- 58.Panisset M. Gaston Ramon decouvrait les anatoxines. Can J Comp Med Vet Sci. 1949;13:60–63. [PMC free article] [PubMed] [Google Scholar]
- 59.Park WH, Schroder MC. Diphtheria Toxin-Antitoxin and Toxoid: A Comparison. Am J Public Health Nations Health. 1932;22:7–16. doi: 10.2105/ajph.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison WT. Effect of alum-precipitated ragweed pollen extract on guinea pigs. Public Health Reports. 1934;49:462–464. [Google Scholar]
- 61.Oakley CL. Alexander Thomas Glenny. 1882–1965. The Royal Society; London: 1966. [Google Scholar]