Abstract
A large outbreak of dengue virus (DV) infections occurred on Caribbean islands during 2010, with cases peaking during the second half of the year. In conjunction with the outbreak, we observed an unprecedented spike in the number of sera submitted for DV antibody testing between June and December 2010, with a concomitant increase in the number of IgM-positive specimens, indicative of acute DV infection. Analysis of the place of residence of the IgM-positive patients identified from June to December of 2010 revealed that 58.1% were residents of Caribbean islands (Puerto Rico and the U.S. Virgin Islands), whereas 40.6% were residents of the U.S. mainland or Hawaii. The U.S. residents represented 42 states plus the District of Columbia, but most (53%) were from just 3 states (California, Florida, and New York). In comparison to the Caribbean IgM-positive patient group, the U.S. IgM-positive patient group contained proportionately more adults 21 to 60 years old and fewer individuals <21 years old. These findings indicate that the 2010 Caribbean DV outbreak affected many U.S. residents (mostly adults, presumably travelers) from diverse geographic areas and emphasize the potential for a viremic DV-infected returning traveler to spark a local DV outbreak by introducing DV into a community with competent mosquito vectors.
INTRODUCTION
Dengue virus (DV) infections are transmitted among humans by Aedes mosquitoes (mainly Aedes aegypti) in tropical and subtropical areas worldwide; in these areas, dengue fever and dengue hemorrhagic fever are major public health problems (29). The World Health Organization estimates that as many as 50 million DV infections occur annually throughout the world (35); others estimate this number to be 100 million (10, 21). A large DV outbreak occurred in the tropics and subtropics of the Americas during the summer and fall of 2010, with more than 1 million reported cases (8). One of the areas with highest activity was the Caribbean basin, including the U.S. territories of Puerto Rico and the U.S. Virgin Islands (7), with more than 21,000 reported cases. DV infections are also an important medical concern outside the tropics and subtropics, where cases in travelers returning from areas of endemicity have been well documented (1, 6, 19, 20, 26, 31, 36). Measurement of DV immunoglobulin M (IgM) is an important laboratory tool for identifying individuals with recent DV infection; DV IgM develops within 5 days of symptom onset and then wanes to undetectable levels within a few months (10, 11, 16, 17, 34).
Although reports (4, 13, 15, 23, 24, 32, 33) have described the role of DV IgM detection by hospital, medical center, and public health laboratories in conjunction with regional DV outbreaks, little information is available on DV IgM detection in a reference laboratory setting during an outbreak. As part of a large commercial reference laboratory system, our laboratory performs DV antibody testing on specimens submitted from across the United States, as well as from outside the United States. We thus evaluated DV IgM results generated during the 2010 DV outbreak; our goals were to determine the geographic distribution of patients with DV IgM-positive samples and gauge the extent of DV exposure among residents of the U.S. mainland, presumably related to international travel.
MATERIALS AND METHODS
Specimens.
Sera included in the analysis were submitted by hospital, medical center, and regional reference laboratories for DV IgM testing between January 2008 and February 2011, inclusive. No information regarding clinical symptoms, date of disease onset, or travel history accompanied any of the specimens.
DV IgM measurement.
Sera were tested for DV IgM using a laboratory-developed IgM capture background subtraction enzyme-linked immunosorbent assay (ELISA) as previously described (3, 9, 25, 27). Diluted control sera (internally developed), calibrator serum (internally developed), and patient sera were added to duplicate microtiter wells coated with rabbit anti-human IgM (Focus Diagnostics, Cypress, CA). After 1 h at room temperature (RT), the wells were washed three times; one well per specimen then received inactivated DV antigen (containing all 4 DV serotypes; internally developed) and the other well received specimen diluent. After incubation for 2 h at RT and washing, all wells received horseradish peroxidase-conjugated 6B6C antiflavivirus monoclonal antibody (Focus Diagnostics). After 30 min at RT and washing, all wells received tetramethylbenzidine (enhanced K-blue; Neogen Corp., Lexington, KY); after 10 min, the color reaction was stopped by adding sulfuric acid (Ricca Chemicals, Arlington, TX). The absorbance at 450 nM was measured using an ELISA reader (BioTek, Winooski, VT). The absorbance value of the well receiving specimen diluent was subtracted from the absorbance value of the well receiving dengue antigen, and this corrected absorbance value was then used to calculate the index, defined as the specimen absorbance value divided by the calibrator absorbance value; indices of >1.10 were considered positive.
Determination of patient residence.
Clients submitting sera that tested positive for DV IgM were informed of the IgM-positive result and asked to provide the place of residence for the patient supplying the serum specimen.
Statistics.
Differences among proportions were assessed by Chi-square analysis using MedCalc software (Mariakerke, Belgium); significance was defined as a P value of <0.01.
RESULTS
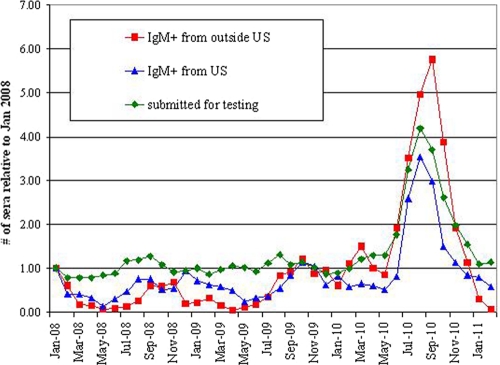
As shown in Fig. 1, we observed a profound spike in the number of specimens submitted for DV antibody testing between June and December 2010, the same time period as the DV outbreak in the Americas (7, 8). Concomitant with this spike in submitted specimens, there was an increase in the number of IgM-positive samples. This 2010 seasonal increase in the number of submitted samples and IgM-positive samples was markedly larger than the subtle increases observed during the summer/fall of the prior 2 years.
Fig. 1.
Fold increases in numbers of monthly samples relative to the number submitted during January 2008 for DV antibody testing, and relative fold increases in numbers of IgM-positive samples segregated by geographic origin.
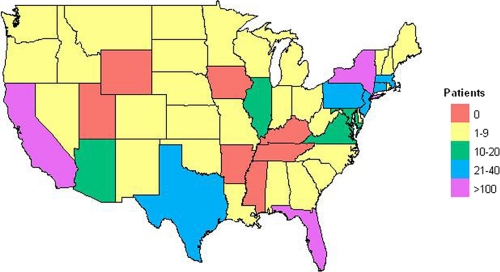
Analysis of the place of residence of IgM-positive patients identified during the period from June to December of 2010 revealed that 58.1% lived on the Caribbean islands of Puerto Rico and the U.S. Virgin Islands and 40.6% were residents of the U.S. mainland or Hawaii; the remaining 1.3% resided in other countries, including some countries in the Americas (Table 1). The geographic distribution of IgM-positive patients from the United States is shown in Fig. 2; patients were from 42 states and the District of Columbia, with the majority (379/709 = 53%) from just 3 states (California, Florida, and New York).
Table 1.
Place of residence of DV IgM-positive patients identified from June to December of 2010
| Geographic area | No. of patients (n = 1,747) | % of all patients |
|---|---|---|
| U.S. mainland plus Hawaii | 709 | 40.6 |
| Puerto Rico | 944 | 54.0 |
| U.S. Virgin islands | 72 | 4.1 |
| Other countries of the Americasa | 14 | 0.8 |
| Countries outside the Americasb | 8 | 0.5 |
Bermuda, Mexico, Peru, and Venezuela.
Guam, France, Japan, Saudi Arabia, and the United Kingdom.
Fig. 2.
Geographic distribution of DV IgM-positive patients residing on the U.S. mainland. The key indicates the number of patients from a given state. Although not shown on the map, there were 6 IgM-positive patients from Hawaii and none from Alaska.
Marked differences were noted in the age distributions of IgM-positive U.S. residents and IgM-positive Caribbean island residents identified during the period from June to December of 2010 (Table 2). The proportions of patients in age groups <21 years old were significantly lower in the U.S. patient group than in the Caribbean island patient group, whereas the proportions of patients in age groups spanning 21 to 60 years were significantly higher in the U.S. patient group than in the Caribbean island patient group.
Table 2.
Age distribution of DV IgM-positive patients in relation to place of residence
| Age group (yr) | Value for patients residing: |
|||
|---|---|---|---|---|
| On Caribbean islands (n = 1,016) |
In the United States (n = 709) |
|||
| No. of samples | % of total | No. of samples | % of totala | |
| <10 | 103 | 10.1 | 34 | 4.8* |
| 11–20 | 275 | 27.1 | 97 | 13.7* |
| 21–40 | 236 | 23.3 | 211 | 29.8* |
| 41–60 | 226 | 22.2 | 257 | 36.2* |
| >60 | 169 | 16.6 | 104 | 14.7 |
| Unknown | 7 | 0.7 | 6 | 0.8 |
*, significantly different (P < 0.01) from the comparable value observed for the Caribbean islands group.
DISCUSSION
In conjunction with the 2010 DV outbreak in the Americas that profoundly affected Caribbean islands (7, 8), we observed a marked increase in the number of specimens submitted to our reference laboratory for DV antibody analysis, as well as the number of specimens testing positive for DV IgM, a marker of recent infection. Although subtle upward shifts in submitted and IgM-positive sample numbers were observed in the summer and fall of 2008 and 2009, these shifts were paltry compared to the profound upward shift observed in 2010.
The majority of IgM-positive specimens submitted during the outbreak period from June to December of 2010 were supplied by patients residing in the U.S. territories of Puerto Rico and U.S. Virgin Islands; however, a sizeable proportion (40.6%) of IgM-positive patients resided on the U.S. mainland or Hawaii. The close proximity of Caribbean islands to the U.S. mainland makes it reasonable to assume that a significant proportion of IgM-positive U.S. mainland patients were exposed in conjunction with travel to this area. However, the actual proportion of IgM-positive U.S. patients exposed to DV on Caribbean islands versus other geographic areas where DV is endemic remains unknown. Likewise, we cannot rule out the possibility that DV IgM detection in some U.S. patients reflected cross-reactive antibodies induced by exposure to other flaviviruses (e.g., Japanese encephalitis virus or yellow fever virus) during international travel (10). We also cannot rule out local exposure to cross-reactive flaviviruses endemic to the United States (e.g., West Nile virus or St. Louis encephalitis virus) (10, 12); this possibility seems unlikely, however, assuming DV antibody testing was ordered based on a history of travel outside the United States.
The differences we observed in the age distribution of DV IgM-positive U.S. residents versus IgM-positive Caribbean island residents supports the expected epidemiological differences in DV infections among travelers to areas of endemicity versus residents of areas of endemicity. Consistent with published findings (20, 30), more than 80% of IgM-positive U.S. residents were adults, presumably recent travelers to destinations outside the United States. In contrast, 37% of IgM-positive residents of Caribbean islands were <21 years old, consistent with local DV transmission among residents of an area where DV is endemic (28).
The DV IgM-positive patients residing in the United States exhibited a wide geographic distribution, representing 42 states and the District of Columbia. Many of these states are also included in the habitat range of Aedes sp. mosquitoes capable of transmitting DV (2, 22). Patients with DV infection are viremic for approximately 5 days after the onset of symptoms (10, 14, 29); thus, the possibility that a recently infected returning traveler may serve as the point source for a local DV outbreak within the United States appears very real. Indeed, this scenario may have already occurred: in 2009/2010, recent DV infections were identified in 26 residents of Key West, FL, who had no history of recent international travel (5). However, the subtropical climate and presence of competent mosquito vectors raise the possibility that DV is endemic to this area and unknown factors triggered its recent reemergence (5).
In addition to the study limitations already mentioned, it must be emphasized that our data only reflect the patient populations served by medical facilities that refer specimens to us for DV antibody testing. There were undoubtedly additional DV IgM-positive individuals, including U.S. residents, identified by other medical center, reference, and public health laboratories during the time period of the 2010 DV outbreak. Approximately 500 cases of DV infections in U.S. residents returning from international travel have been reported to the Centers for Disease Control and Prevention (CDC) for 2009 to 2010 (18), but the number reported for the time period from June to December of 2010 and the overlap with patients identified by our testing are unknown. In addition, many DV infections in travelers are likely not diagnosed because the patient either does not present for medical care or the diagnosis is not considered by the treating physician. Greater awareness by clinicians regarding the risk of DV infection in returning travelers with febrile illness, an understanding of appropriate laboratory tests that aid in diagnosing DV infections, and the utilization of reporting systems, such as ArboNET (6), are needed to accurately ascertain the burden of DV infections among residents of the United States and its territories. DV infection was designated a nationally notifiable disease in the United States in 2009 (5, 6), which should facilitate more accurate tracking of DV-infected patients.
Footnotes
Published ahead of print on 25 May 2011.
REFERENCES
- 1. Bakker R. C., Veenstra J., Dingemans-Dumas A. M., Wetsteyn J. C. F. M., Kager P. A. 1996. Imported dengue in The Netherlands. J. Travel Med. 3:204–208 [DOI] [PubMed] [Google Scholar]
- 2. Benedict M. Q., Levine R. S., Hawley W. A., Lounibos L. P. 2007. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 7:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Branch S. L., Levett P. N. 1999. Evaluation of four methods for detection of immunoglobulin M antibodies to dengue virus. Clin. Diagn. Lab. Immunol. 6:555–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buerano C. C., et al. 2000. IgM-capture ELISA of serum samples collected from Filipino dengue patients. Southeast Asian J. Trop. Med. Public Health 31:524–529 [PubMed] [Google Scholar]
- 5. Centers for Disease Control Prevention 2010. Locally acquired Dengue—Key West, Florida, 2009-2010. Morb. Mortal. Wkly. Rep. 59:577–581 [PubMed] [Google Scholar]
- 6. Centers for Disease Control Prevention 2010. Travel-associated dengue surveillance—United States, 2006-2008. Morb. Mortal. Wkly. Rep. 59:715–719 [PubMed] [Google Scholar]
- 7. Centers for Disease Control Prevention 2011. 2010: largest dengue outbreak in Puerto Rico history. Dengue Update 3:1–2 [Google Scholar]
- 8. Centers for Disease Control Prevention 2011. Outbreak notice update: dengue, tropical and subtropical regions. Centers for Disease Control and Prevention, Atlanta, GA. http://wwwnc.cdc.gov/travel/content/outbreak-notice/dengue-tropical-sub-tropical.aspx Updated 18 January 2011, accessed 4 April 2011
- 9. Groen J., Koraka P., Velzing J., Copra C., Osterhaus A. D. 2000. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin. Diagn. Lab. Immunol. 7:867–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gubler D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guzman M. G., Kouri G. 1996. Advances in dengue diagnosis. Clin. Diagn. Lab. Immunol. 3:621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hogrefe W. R., Moore R., Lapé-Nixon M., Wagner M., Prince H. E. 2004. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J. Clin. Microbiol. 42:4641–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabilan L., Balasubramanian S., Keshava S. M., Satyanarayana K. 2005. The 2001 dengue epidemic in Chennai. Indian J. Pediatr. 72:919–923 [DOI] [PubMed] [Google Scholar]
- 14. Kao C.-L., King C.-C., Chao D.-Y., Wu H.-L., Chang G.-J. J. 2005. Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J. Microbiol. Immunol. Infect. 38:5–16 [PubMed] [Google Scholar]
- 15. Khan N. A., et al. 2008. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop. 105:39–44 [DOI] [PubMed] [Google Scholar]
- 16. Kuno G., Gomez I., Gubler D. J. 1991. An ELISA procedure for the diagnosis of dengue infections. J. Virol. Methods 33:101–113 [DOI] [PubMed] [Google Scholar]
- 17. Lam S. K., Devi S., Pang T. 1987. Detection of specific IgM in dengue infection. Southeast Asian J. Trop. Med. Public Health 18:532–538 [PubMed] [Google Scholar]
- 18. Margolis H. 2011. Dengue diagnostic testing for case management and surveillance. Focusevents Webex presentation, April 26, 2011. https://focusevents.webex.com/mw0306lc/mywebex/default.do?siteurl=focusevents
- 19. Masaki H., et al. 2002. A clinical, serological, and immunological study in a Japanese traveler with dengue fever. J. Infect. Chemother. 8:365–367 [DOI] [PubMed] [Google Scholar]
- 20. Mohammed H. P., et al. 2010. Travel-associated dengue infections in the United States, 1996-2005. J. Travel Med. 17:8–14 [DOI] [PubMed] [Google Scholar]
- 21. Monath T. P. 1994. Dengue: the risk to developed and developing countries. Proc. Natl. Acad. Sci. U. S. A. 91:2395–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morens D. M., Fauci A. S. 2008. Dengue and hemorrhagic fever. A potential threat to public health in the United States. JAMA 299:214–216 [DOI] [PubMed] [Google Scholar]
- 23. Nogueira R. M., Miagostovich M. P., Cavalcanti S. M., Marzochi K. B., Schatzmayr H. G. 1992. Levels of IgM antibodies against dengue virus in Rio de Janeiro, Brazil. Res. Virol. 143:423–427 [DOI] [PubMed] [Google Scholar]
- 24. Osman O., Fong M. Y., Devi S. 2007. A preliminary study of dengue infection in Brunei. Jpn. J. Infect. Dis. 60:205–208 [PubMed] [Google Scholar]
- 25. Porter K. R., et al. 1999. Evaluation of a commercially available immunoglobulin M enzyme-linked immunosorbent assay kit for diagnosing acute dengue infections. Clin. Diagn. Lab. Immunol. 6:741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Potasman I., Scugo I., Schwartz E. 1999. Dengue seroconversion among Israeli travelers to tropical countries. Emerg. Infect. Dis. 5:824–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prince H. E., Yeh C., Lapé-Nixon M. 2008. Development of a more efficient algorithm for identifying false-positive reactivity results in a dengue virus immunoglobulin M screening assay. Clin. Vaccine Immunol. 15:1304–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rigau-Perez J. G., Gubler D. J., Vorndam A. V., Clark G. G. 1994. Dengue surveillance—United States, 1986-1992. Morb. Mortal. Wkly. Rep. 43:7–19 [PubMed] [Google Scholar]
- 29. Solomon T., Mallewa M. 2001. Dengue and other emerging flaviviruses. J. Infect. 42:104–115 [DOI] [PubMed] [Google Scholar]
- 30. Sotir M. J., Johnson D. K., Davis J. P. 2009. Travel-associated dengue illnesses among Wisconsin residents, 2002-2008. Wisconsin Med. J. 108:447–452 [PubMed] [Google Scholar]
- 31. Stephenson I., Roper J., Fraser M., Nicholson K., Wiselka M. 2003. Dengue fever in febrile returning travelers to a UK regional infectious diseases unit. Travel Med. Infect. Dis. 1:89–93 [DOI] [PubMed] [Google Scholar]
- 32. Tang J. W., et al. 2008. A wide spectrum of dengue IgM and PCR positivity post-onset of illness found in a large dengue 3 outbreak in Pakistan. J. Med. Virol. 80:2113–2121 [DOI] [PubMed] [Google Scholar]
- 33. Thomas L., et al. 2010. Prospective and descriptive study of adult dengue cases in an emergency department, in Martinique. Med. Mal. Infect. 40:480–489 [DOI] [PubMed] [Google Scholar]
- 34. Vaughn D. W., et al. 1997. Dengue in the early febrile phase: viremia and antibody responses. J. Infect. Dis. 176:322–330 [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization 2009. Dengue and dengue haemorrhagic fever. Fact sheet no. 117, March 2009. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs117/en/ Accessed 6 April 2011
- 36. Xu G., et al. 2007. An outbreak of dengue virus serotype 1 infection in Cixi, Ningbo, People's Republic of China, 2004, associated with a traveler from Thailand and high density of Aedes albopictus. Am. J. Trop. Med. Hyg. 76:1182–1188 [PubMed] [Google Scholar]