Abstract
A quantitative duplex time-resolved fluorescence assay, dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA), was developed to measure Norwalk virus (NV)-specific IgA and IgG antibodies simultaneously. The duplex assay showed superior performance by detecting seroconversion following experimental NV infection at an earlier time point than a reference total immunoglobulin enzyme-linked immunosorbent assay (ELISA).
TEXT
Noroviruses (NoVs) are the principal etiological agents in acute nonbacterial gastroenteritis worldwide (1, 5). Human NoVs are noncultivable in vitro, and there is currently no small-animal model for NoV-induced gastroenteritis. Our understanding of immunity is limited and comes primarily from human experimental infections, such as those that have been conducted with the prototype NoV strain Norwalk virus (NV) (6, 7, 13), and studies of natural outbreaks (4, 12). The NV genome is encapsidated within an icosahedral (T=3) capsid consisting of the major and minor capsid proteins, VP1 and VP2, respectively. When these proteins are expressed in vitro using the baculovirus expression system, they spontaneously assemble into virus-like particles (VLPs) that are morphologically and antigenically indistinguishable from the native virion (3, 8, 11). These VLPs have been used as antigens in enzyme-linked immunosorbent assays (ELISA) to study humoral immunity to NV infection (6, 7, 16).
Dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) has emerged as a superior alternative to the ELISA platform. DELFIA technology is based on the time-resolved fluorescence of lanthanide chelates (15), and replacement of the colorimetric label employed by ELISA with lanthanide fluorescent labels utilized by DELFIA allows the detection of up to four labels simultaneously in a single well (10, 17, 18). Furthermore, the large linear range of the fluorescent signal requires testing of fewer sample dilutions, making the assay more suitable for a high-throughput format. In this report we describe the development and performance of a DELFIA-based duplex (dual-label) immunoassay to detect NV-specific IgG and IgA in response to experimental infection.
We collected serum samples during a Norwalk virus challenge study, conducted as described previously (2, 14), before (day 0) and at 7, 14, 28, and 180 days after inoculation. NV infection was defined as excretion of virus in stool (by antigen ELISA or reverse transcription-PCR [RT-PCR]) or a ≥4-fold increase in serum antibody titer by total immunoglobulin (IgG, IgA, and IgM) ELISA (preinoculation to 28 days postinoculation), performed as described previously (6, 14), and infection as determined by these assays was used as the gold standard reference for the serological studies reported here.
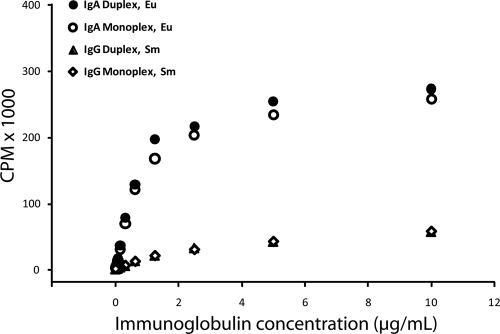
To develop a duplex DELFIA, the monoclonal antibodies used as secondary reagents were custom labeled with either Eu3+ (anti-human IgA) or Sm3+ (anti-human IgG) lanthanide chelates using commercially available labeling kits (Perkin Elmer, Waltham, MA). A monoclonal mouse anti-human IgG (Southern Biotech, Birmingham, AL) was labeled with Sm-N1-ITC chelate and a monoclonal mouse anti-human IgA (Southern Biotech) was labeled with the Eu-N1-ITC chelate for use in the IgG and IgA assays, respectively. We detected the NV-specific IgA with the label possessing the strongest fluorescent intensity (Eu3+) and IgG with the weaker label (Sm3+). Labeling was reproducible, as evidenced by the results from the labeling of two different lots of mouse anti-human IgA antibody with Eu3+ (data not shown). Labeling yields were 7.2 Eu3+ per anti-IgA (IgA-Eu) molecule and 10.4 Sm3+ per anti-IgG (IgG-Sm) antibody. Serial dilutions of purified human immunoglobulins IgG and IgA (Sigma-Aldrich, St. Louis, MO) were titrated against IgG-Sm and IgA-Eu conjugates either mixed together in a duplex assay format or separately as monoplex assays. The Eu3+ signal was approximately 10-fold stronger than the Sm3+ signal (Fig. 1). The monoplex and duplex readouts for IgG-Sm were overlapping, suggesting that the addition of IgA-Eu to the IgG-Sm conjugate did not interfere with the assay. The same observation was seen with the IgA-Eu dose-response curve in the presence or absence of the IgG-Sm within the linear range of the assay (Fig. 1). Furthermore, parameter estimates for the maximum specific binding (Bmax) and equilibrium constants (Kd), as determined with GraphPad Prism software (La Jolla, CA), for saturation curves were similar for the monoplex and duplex assays for both IgG and IgA. The estimates for IgG were as follows: Kd = 2.12 (95% confidence interval [CI] = 0.95, 3.29), and Bmax = 47 (95% CI = 30, 64) versus Kd = 1.95 (95% CI = 0.96, 2.94) and Bmax = 49 (95% CI = 33, 65) for monoplex and duplex assays, respectively. The estimates for IgA were as follows: Kd = 0.86 (95% CI = 0.67, 1.05) and Bmax = 282 (95% CI = 254, 309) versus Kd = 0.83 (95% CI = 0.60, 1.06) and Bmax = 304 (95% CI = 267, 341) for monoplex and duplex assays, respectively. The goodness of fit for all saturation binding equations was greater than 0.99. The linear range values of the duplex conjugate-purified immunoglobulin dose-response curves were used to estimate antibody concentration and were calculated by linear regression analysis. Both the IgG (r = 0.995) and IgA (r = 0.984) standard curves were linear from 0.0049 to 1.25 μg/ml (0.4 ng to 94 ng per well) immunoglobulin. The upper limit of detection of IgA was 127,000 cpm, and that for IgG was 12,700 cpm.
Fig. 1.
Comparison of the IgG and IgA dose-response curves for single- (monoplex) and dual-label DELFIAs. Purified IgG or IgA were serially 2-fold diluted (10.0 to 0.0049 μg/ml) and were detected using either monoclonal mouse anti-human IgG-labeled Sm3+ (IgG-Sm) or anti-human IgA-labeled Eu3+ (IgA-Eu). For the duplex assay, the Sm3+ and Eu3+ monoclonal conjugates were added simultaneously to the same well at the same concentration as for the monoplex assay. The maximum specific binding (Bmax) and equilibrium binding constants were not significantly different when comparing the monoplex and duplex assays for either IgG or IgA.
The duplex DELFIA used to measure NV-specific IgG and IgA in human sera was a modification of the purified immunoglobulin DELFIA described above. Recombinant NV virus-like particles (rNV VLP) purified from Spodoptera frugiperda (Sf9) insect cells were used as the solid-phase antigen (11). Alternative rows of black Fluotrac-200 microtiter plates were coated with 75 μl of NV VLP at a concentration of 1.0 μg/ml in phosphate-buffered saline (PBS) or 75 μl of PBS (blank) overnight at 4°C. After the contents of the plate were dumped, the wells were blocked with 400 μl 5% Blotto in Tris-buffered saline (TBS) for 2 h at 37°C in a humidified chamber. After the plate was washed, 75 μl of test serum samples diluted at 1/100 and 1/1,000 in 10% Blotto in TBS-Tween were added to each NV VLP-coated and PBS blank well in triplicate and incubated for 2 h at 37°C. After being washed, plates were incubated with 75 μl of monoclonal mouse anti-human IgG-labeled Sm3+ (1/1,000) and anti-human IgA-labeled Eu3+ (1/1,000) in DELFIA buffer (Perkin Elmer) for 2 h at 4°C. After plates were washed, 100 μl of DELFIA enhancement buffer (Perkin Elmer) was added to each well, and the plates were shaken gently for 30 min at 22 to 25°C to allow dissociation of the fluorescent lanthanide chelates. The fluorescence (in counts per minute [cpm]) was read using the Eu/Sm dual-label time-resolved program set in the VICTOR2 multilabel plate reader (Perkin Elmer). Results given as cpm were corrected by subtracting the blank-well cpm from the NV VLP-coated-well cpm for each sample. The corrected values were expressed as immunoglobulin concentrations by interpolation from standard curves generated using purified IgG or IgA and adjusted for the dilution factor. Only test sample cpm values that fell within the linear range were used to determine immunoglobulin concentrations. If the test sample cpm was greater than the highest value of the working range, the sample was rerun at higher dilutions (e.g., 1/1,000 and 1/10,000) until the cpm fell within the working range. Each plate included a positive- and negative-control human serum sample. The negative cutoff value for assay was calculated using the arithmetic mean and three standard deviations (SD) of the negative-control human serum sample.
By day 14 postchallenge, all subjects (11 blood type O secretors, 7 blood type A secretors) who met the definition of infection had a ≥4-fold rise in IgG and IgA antibody as measured with the duplex DELFIA (sensitivity, 100%) (Table 1). Assay specificity was assessed using serum samples from persons who were not infected (including nonsecretors, persons with blood type B or AB [n = 8], and persons challenged with placebo [n = 5] or challenged but not infected [n = 3]). None of 16 noninfected persons had a ≥4-fold increase in IgG or IgA antibody levels as measured by duplex DELFIA at any time point (specificity, 100%; data not shown).
Table 1.
Comparison of antibody responses measured with different assays following NV infection of human volunteers (n = 18)
| Assay and parameter | Result on study day |
|||||
|---|---|---|---|---|---|---|
| Pred | 2 | 7 | 14 | 28–30 | 180 | |
| Total ELISA (IgG, IgA, IgM) | ||||||
| No. (%) seropositivea | 18 (100) | 18 (100) | 18 (100) | 18 (100) | 18 (100) | 18 (100) |
| No. (%) with seroconversionb,c | 0 (0) | 9 (50) | 18 (100) | 18 (100) | 16 (89) | |
| Geometric mean fold riseb | 1 | 4 | 94 | 128 | 17 | |
| IgG | ||||||
| No. (%) seropositivea | 16 (89) | 16 (89) | 18 (100) | 18 (100) | 18 (100) | 18 (100) |
| No. (%) with seroconversionb,c | 0 (0) | 7 (39) | 18 (100) | 18 (100) | 14 (78) | |
| Geometric mean fold riseb | 1 | 4 | 70 | 97 | 18 | |
| IgA | ||||||
| No. (%) seropositivea | 9 (50) | 10 (56) | 17 (94) | 18 (100) | 18 (100) | 18 (100) |
| No. (%) with seroconversionb,c | 1 (6) | 15 (83) | 18 (100) | 18 (100) | 13 (72) | |
| Geometric mean fold riseb | 1 | 40 | 636 | 260 | 24 | |
| IgM | ||||||
| No. (%) seropositivea | 1 | 2 (11) | 13 (72) | 15 (83) | 1 (6) | |
| No. (%) with seroconversionb,c | 2 (11) | 12 (67) | 14 (78) | 0 (0) | ||
| Geometric mean fold riseb | 1 | 24 | 33 | 1 | ||
Seropositive if IgG or IgA is >1.4 or >1.6 μg/ml, respectively, or ELISA reciprocal titer is >25 (total or IgM).
Compared to prechallenge serum titer.
Seroconversion is defined as a ≥4-fold rise in antibody levels between pre- and postchallenge samples.
Pre, prechallenge.
Concentrations of NV-specific IgG and IgA were determined by interpolation along the linear range of the relevant purified immunoglobulin DELFIA standard curve and adjusted for the dilution factor. The negative cutoffs of the IgG and IgA assays (cutoff = corrected mean negative-control serum cpm + 3 SD) were estimated to be 1.4 μg/ml and 1.6 μg/ml, respectively. Of 216 samples tested, 10 (4.6%) had to undergo repeated tests at higher serum dilutions (1/1,000 and 1/10,000), including 7 samples that exceeded both the IgG and IgA detection limits, 2 samples that exceeded the IgG assay detection limit, and 1 sample that exceeded the IgA assay detection limit. No test serum sample dilution exceeded 1/10,000.
Interassay variability was determined using a positive-control human serum sample known to contain NV-specific IgG and IgA. The control was run on each of the 18 plates (3 replicates per run) needed to screen all the samples. The IgA assay had a percent coefficient of variation (%CV) of 11.7%, and the IgG assay had a %CV of 13.8% over the period of the study. The intra-assay variability was calculated using the means and standard deviations for 18 replicates of the positive-control serum on the same plate. The %CV of the IgA assay was 2.9%, and that of IgG was 1.9%.
We were also interested in serum IgM responses following infection, and these were measured using an IgM capture ELISA. Clear 96-well microtiter plates (Greiner Bio One) were coated with 100 μl of rabbit anti-human IgM (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) at a dilution of 1/20,000 in 0.05 M sodium carbonate/bicarbonate buffer (pH 9.6) overnight at 4°C. Plates were blocked with 200 μl 10% Blotto for 1 h at 37°C. After plates were washed, 2-fold serial dilutions of human serum samples, from 1/50 to 1/6,400 in 10% Blotto, were added to the wells and incubated for 2 h at 37°C. Following washing of plates, NV VLPs at a concentration of 1.0 μg/ml in 10% Blotto were added to the wells for 1 h at 37°C. Binding of NV VLP to test serum IgM was detected with the monoclonal antibody NV8812, raised against NV VLPs (9), at a concentration of 0.1 μg/ml in 10% Blotto for 1 h at 37°C. After being washed, the wells were incubated with a 1/5,000 dilution of horseradish peroxidase-conjugated goat anti-mouse antibody (Sigma-Aldrich) in 10% Blotto for 1 h at 37°C. The colorimetric reaction was developed with tetramethylbenzidine peroxidase (TMB) liquid substrate (Kirkegaard & Perry Laboratory, Gaithersburg, MD) and stopped after 10 min incubation at 22°C by adding an equal volume of 1 M phosphoric acid. Optical density was measured at 450 nm using the SpectraMax M5 (Molecular Devices, Sunnyvale, CA) plate reader. A positive result corresponded to a reciprocal endpoint titer of ≥50, where the endpoint titer was defined as the greatest serum dilution giving an optical density value twice that of the corresponding blank well.
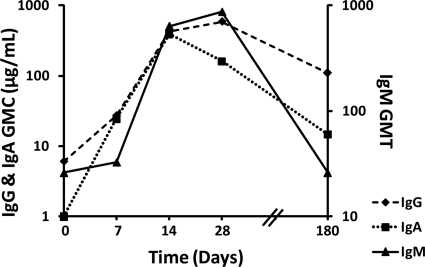
We analyzed the kinetics of the antibody responses in serum from human volunteers following virus challenge by the IgG/IgA duplex DELFIA and total and IgM ELISA. The results are expressed as the geometric mean titer (GMT) (total plus IgM ELISA) or as the geometric mean concentration (GMC) of the IgG and IgA concentrations (μg/ml). Serum samples from the 18 infected subjects collected at various time points from prechallenge to 180 days postchallenge were evaluated for NV-specific isotype responses; 100% of infected volunteers had detectable levels of NV-specific immunoglobulins prechallenge as measured by total ELISA (Table 1). Of 18 infected persons, 16 (89%) possessed NV-specific IgG in the prechallenge samples (IgG GMC, 6.1 μg/ml). Nine (50%) had measurable preexisting virus-specific IgA levels (IgA GMC, 0.6 μg/ml). The two infected persons who were seronegative for IgG were also negative by IgA analysis. One person had measurable preexisting NV-specific serum IgM (reciprocal titer, 50).
The kinetics of the different NV-specific isotype responses to experimental infection is shown in Fig. 2 as GMC/GMT and outlined in Table 1 as geometric mean fold rise (GMFR). The NV-specific IgG peaked at day 28 before falling by day 180. NV-specific IgA was detected earlier than IgG, peaking by day 14 and falling at later time points. An infection was detected in one individual by the IgA assay as early as day 2 postchallenge (219-fold increase in IgA compared to prechallenge result). Of the 18 infected volunteers, 15 (83%) seroconverted by day 7, compared to results with the ELISA, which detected only 9 infections at the same time point (day 7; P = 0.031, McNemar's test), and 7 infections were detected by the IgG assay. Both IgG and IgA DELFIA and total ELISA detected infections in all the infected volunteers by day 14 and also at day 28. Virus-specific IgM was detected in only 2 individuals by day 7 postchallenge and peaked on day 28. No measurable virus-specific IgM was detected on day 180.
Fig. 2.
Comparison of the kinetics of Norwalk virus (NV)-specific serum antibody isotype responses in human volunteers experimentally infected with NV. Samples were collected from persons infected (n = 18) with NV on prechallenge and on days 7, 14, 28, and 180 postchallenge and were analyzed for NV-specific IgG and IgA by duplex DELFIA and IgM by ELISA. Results are expressed as log10 of geometric mean titer (GMT) for IgM and micrograms of immunoglobulin per milliliter for geometric mean concentrations (GMC) of IgG and IgA.
The magnitude of antibody response (log2 fold rise) at day 28 correlated with the total estimated fecal virus burden (2, 14) for both the IgA (r = 0.59, P = 0.01) and IgG (r = 0.53, P = 0.025) assays but not for the total ELISA (r = 0.37, P = 0.13). However, there were no significant correlations between mean fold rise and peak fecal virus titer for any of the serological assays.
This study demonstrates the ability of the duplex DELFIA to robustly detect and quantify the levels of NV-specific IgG and IgA serum antibodies with a high degree of diagnostic sensitivity and specificity. To our knowledge, this is the first time DELFIA has been applied to simultaneously measure virus-specific IgG and IgA responses in human serum. We compared the DELFIA to a gold standard ELISA to detect serum antibody responses generated in human volunteers experimentally infected with NV. The volunteers were followed for 6 months postchallenge, allowing us to describe the kinetics of the NV-specific antibody responses generated. DELFIA detected infections earlier than ELISA postchallenge due to the rapid rise in IgA following infection, suggesting that this antibody subclass may be a useful indicator of disease if paired serum samples are taken within 14 days postinfection.
Acknowledgments
We thank Frederick Neill for laboratory technical support and Timothy Palzkill for helpful discussions.
This work was conducted with support from the National Institutes of Health (P01 AI 57788, N01 AI 25465, P30 DK56336, and M01 RR-000188).
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Atmar R. L., Estes M. K. 2006. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. N. Am. 35:275–290 [DOI] [PubMed] [Google Scholar]
- 2. Atmar R. L., et al. 2008. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14:1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertolotti-Ciarlet A., Crawford S. E., Hutson A. M., Estes M. K. 2003. The 3′ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J. Virol. 77:11603–11615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinker J. P., et al. 1998. Detection of Norwalk virus and other genogroup 1 human caliciviruses by a monoclonal antibody, recombinant-antigen-based immunoglobulin M capture enzyme immunoassay. J. Clin. Microbiol. 36:1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glass R. I., Parashar U. D., Estes M. K. 2009. Norovirus gastroenteritis. N. Engl. J. Med. 361:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graham D. Y., et al. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34–43 [DOI] [PubMed] [Google Scholar]
- 7. Gray J. J., et al. 1994. Detection of immunoglobulin M (IgM), IgA, and IgG Norwalk virus-specific antibodies by indirect enzyme-linked immunosorbent assay with baculovirus-expressed Norwalk virus capsid antigen in adult volunteers challenged with Norwalk virus. J. Clin. Microbiol. 32:3059–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green K. Y., Lew J. F., Jiang X., Kapikian A. Z., Estes M. K. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardy M. E., et al. 1996. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology 217:252–261 [DOI] [PubMed] [Google Scholar]
- 10. Huhtinen P., et al. 2005. Synthesis, characterization, and application of Eu(III), Tb(III), Sm(III), and Dy(III) lanthanide chelate nanoparticle labels. Anal. Chem. 77:2643–2648 [DOI] [PubMed] [Google Scholar]
- 11. Jiang X., Wang M., Graham D. Y., Estes M. K. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noel J. S., et al. 1997. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J. Med. Virol. 53:372–383 [DOI] [PubMed] [Google Scholar]
- 13. Parrino T. A., Schreiber D. S., Trier J. S., Kapikian A. Z., Blacklow N. R. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 297:86–89 [DOI] [PubMed] [Google Scholar]
- 14. Reeck A., et al. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 202:1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siitari H., Hemmila I., Soini E., Lovgren T., Koistinen V. 1983. Detection of hepatitis B surface antigen using time-resolved fluoroimmunoassay. Nature 301:258–260 [DOI] [PubMed] [Google Scholar]
- 16. Treanor J. J., Jiang X., Madore H. P., Estes M. K. 1993. Subclass-specific serum antibody responses to recombinant Norwalk virus capsid antigen (rNV) in adults infected with Norwalk, Snow Mountain, or Hawaii virus. J. Clin. Microbiol. 31:1630–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu Y. Y., et al. 1992. Simultaneous quadruple-label fluorometric immunoassay of thyroid-stimulating hormone, 17 alpha-hydroxyprogesterone, immunoreactive trypsin, and creatine kinase MM isoenzyme in dried blood spots. Clin. Chem. 38:2038–2043 [PubMed] [Google Scholar]
- 18. Zerwes H. G., Peter J. C., Link M., Gubler H., Scheel G. 2002. A multiparameter screening assay to assess the cytokine-induced expression of endothelial cell adhesion molecules. Anal. Biochem. 304:166–173 [DOI] [PubMed] [Google Scholar]