Abstract
Oral immunization is a goal in vaccine development, particularly for pathogens that enter the host through the mucosal system. This study was designed to explore the immunogenic properties of the Taenia crassiceps protective peptide GK-1 administered orally. Mice were orally immunized with the synthetic GK-1 peptide in its linear form with or without the Brucella lumazine synthase (BLS) protein adjuvant or as a chimera recombinantly bound to BLS (BLS-GK-1). Mice were boosted twice with GK-1 only at 15-day intervals. A significant rate of protection of 64.7% was achieved in GK-1-immunized mice, and that rate significantly increased to 91.8 and 96% when mice were primed with GK-1 coadministered with BLS as an adjuvant and BLS as a carrier, respectively. Specific antibodies and T cell activation and proliferation accompanied the protection induced, revealing the potent immunogenicity of GK-1. Through immunohistochemical studies, GK-1 was detected in T and B cell zones of the Peyer's patches (PP) and mesenteric lymph nodes. In the latter, abundant proliferating cells were detected by 5′-bromo-2′-deoxyuridine incorporation. No proliferation was detected in PP. Altogether, these results portray the potent immunogenic properties of GK-1 administered orally and reinforce the usefulness of BLS as an adjuvant and adequate vaccine delivery system for oral vaccines.
INTRODUCTION
Taenia solium cysticercosis continues to be a health problem in most Latin American countries and other undeveloped countries worldwide (4). Its life cycle includes a larval phase (cysticercus) that affects both humans (definitive host) and pigs (intermediate host), and it is acquired by the ingestion of eggs present in food contaminated with human feces. When humans ingest undercooked cysticercotic meat, cysticerci can develop to the adult tapeworm stage, which produces hundreds of thousands of eggs, thus spreading the infection.
Diverse vaccines have been developed to prevent porcine cysticercosis as a measure to reduce the emergence of tapeworm carriers (1, 5, 8, 9, 12, 15, 19, 20, 23, 24, 29). Notwithstanding the success of the anti-porcine cysticercosis vaccines, they have certain drawbacks. For a nationwide interruption of transmission, vaccines have to be sustainable and they must be applied over wide geographic areas to millions of fierce free-ranging pigs, a costly and logistic limitation of parenteral vaccines. To circumvent the drawbacks of injected vaccines, efforts to develop oral vaccines using the S3Pvac anticysticercosis vaccine epitopes are ongoing, with promising results (11, 27). SP3vac is based on three synthetic peptides of 18 amino acids (aa) (GK-1), 12 aa (KETc1), and 8 aa (KETc12) shared by T. solium and Taenia crassiceps (12). Of the S3Pvac components, the potential of GK-1 to be used in oral immunization is presently being studied because of its exceptional immunogenic properties when it is administered systemically. Subcutaneous immunization with the linear synthetic GK-1 plus saponin as adjuvant reduced by 96 to 99% the expected parasite load of mice challenged with T. crassiceps cysticerci; and it elicited a specific antibody response, an increment in CD3+ proliferative cells, and an important increase in gamma interferon (IFN-γ) levels in the supernatants of mononuclear cells from immunized mice stimulated in vitro with GK-1 (32). The immunogenicity of systemically administered GK-1 has been proved not only in its linear synthetic form but also in a form in which it is recombinantly expressed in filamentous phages (18, 20) and embryogenic papaya calli (11). In addition to the specific immunity elicited by GK-1 immunization, it can be used as a parenteral adjuvant. GK-1 also possesses the ability to increase the immunogenicity of the influenza vaccine (30), enhancing CD4+ T cell proliferation in vivo and in vitro, upregulating the expression of costimulatory molecules on dendritic cells (DCs), and inducing the secretion of proinflammatory cytokines and chemokines (31).
On the other hand, Brucella lumazine synthase (BLS) is a highly immunogenic polymeric protein that can be used to fuse foreign peptides and proteins at the 10 amino-terminal ends of the molecule without disrupting its general folding (35). The efficacy of BLS as a delivery system to elicit systemic and oral immunity without the use of additional adjuvants has been demonstrated (14). Moreover, BLS has the ability to stimulate bone marrow-derived mouse dendritic cells in vitro through Toll-like receptor 4 (TLR4), upregulating costimulatory molecules (CD80 and CD86), activation molecules (CD40, major histocompatibility complex class II, mRNA levels of the chemokines macrophage inflammatory protein 1 [MIP-1], MIP-2, monocyte chemoattractant protein 1, RANTES), and the secretion of proinflammatory cytokines (3). All these properties possibly underlie the observed adjuvant capacity of BLS (27).
Considering that oral administration is a major goal in vaccine development for better public acceptance, a considerable reduction in the cost of vaccine administration and logistic problems, and the direct triggering of the mucosal immune system, the present study was aimed at investigation of the capacity of GK-1 to induce protection against murine cysticercosis when it is orally administered, either with or without BLS as an adjuvant and carrier during the priming. Boosts were performed only with GK-1 with the aim of evaluating its effectiveness at induction of an in vivo recall immune response capable of protecting against this infection.
The remarkable immunogenic properties of the linear form of GK-1 administered orally in the absence of adjuvant are described. Moreover, its immunogenic and protective properties were enhanced when, in the priming, GK-1 was coadministered with BLS or was recombinantly bound to BLS. Results encourage the use of GK-1 for oral immunization against cysticercosis.
MATERIALS AND METHODS
Mice.
Six- to 10-week-old female mice of strain BALB/cAnN (H-2d), previously characterized to be a strain susceptible to cysticercosis, were used for vaccine trials. Mice were obtained from the animal facilities at the Biomedical Research Institute, National Autonomous University of Mexico (UNAM), where they were bred and maintained in microisolators by use of a single-line breeding system. The experiments reported here were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (12a). This research study was approved by the Ethical Committee of the Biomedical Research Institute.
Parasites.
The Taenia crassiceps ORF strain (7) has been maintained by serial intraperitoneal (i.p.) passage in BALB/cAnN female mice since 1986 at the Biomedical Research Institute, UNAM. Parasites for experimental infections were harvested from the peritoneal cavity of stock female mice after 1 to 3 months of infection. Parasites for infections were selected according to their high motility.
Peptide.
GK-1 (KETc7 aa 69 to 85 [Gly Tyr Tyr Tyr Pro Ser Asp Pro Asn Thr Phe Thr Ala Pro Pro Tyr Ser Ala]) was prepared by solid-phase synthesis by Sigma Chemical Co., St. Louis, MO. The boldface letter indicates the extra amino acid that was coupled to the commercial resin. The peptide was 95% pure, as judged by high-pressure liquid chromatography on analytical C18 reversed-phase columns (3.9 by 150 nm; Delta Park; Waters). The correct amino acid sequence of GK-1 was confirmed by protein sequencing on a pulsed liquid-phase protein sequencer (Applied Biosystems).
Construction and expression of the recombinant chimera BLS-GK-1.
A pET11a plasmid containing the open reading frame of Brucella spp. lumazine synthase was digested with the restriction enzymes BamHI and XbaI and subcloned in the vector pALTER-Ex1 (Promega, Madison, WI). Using an Altered Sites II site-directed mutagenesis kit (Promega), two new restriction sites were introduced: an NsiI site in the first two codons of the 5′ extreme and an AflII site in the two codons comprising residues 8 and 9 of the wild-type amino acid sequence. The resulting sequence contains a His residue instead of an Ala residue at position 1 and a Leu residue instead of an Asn residue at position 8 of the coding sequence of the wild-type protein. After sequencing the mutations, the construction was subcloned again in the pET11a vector. The mutated pET11a plasmid was digested with the restriction enzymes NsiI and AflII, removing the coding sequence of the first 8 residues. The wild-type sequence was exchanged for the sequences pertaining to the peptide GK-1. To insert this sequence, two oligonucleotides were synthesized (Integrated DNA Technologies, Coralville, IA) in such way that, after annealing, they contained the coding sequence Gly Tyr Tyr Tyr Pro Ser Asp Pro Asn Thr Phe Thr Ala Pro Pro Tyr Ser Ala, followed by two additional residues (GS) for spacing, plus the corresponding overhangs for ligation at the NsiI (5′) and AflII (3′) ends. The double-stranded oligonucleotide thus generated was ligated with the previously digested mutated pET11a by overnight incubation at 16°C with T4 DNA ligase (Promega). The ligation mix was used to transform competent Escherichia coli DH5α cells. The insertion was verified by colony PCR using as primers one of the peptide-specific oligonucleotides and the T7 terminator primer. The construction was finally checked by automated sequencing.
Protein purification and refolding.
BLS protein was successfully expressed as inclusion bodies by transformation of E. coli BL21(DE3) competent cells. The inclusion bodies were solubilized in 50 mM Tris-5 mM EDTA-8 M urea, pH 8.0, at room temperature overnight with agitation. The solubilized material was refolded by dialysis against phosphate-buffered saline (PBS) containing 1 mM dithiothreitol (DTT) for 72 h. This preparation was purified in a MonoQ column in a fast protein liquid chromatography apparatus (Pharmacia, Uppsala, Sweden) using a linear gradient of buffer B (50 mM Tris, 1 M NaCl, pH 8.5). The peak enriched with BLS was further purified on a Superdex-200 column with PBS-1 mM DTT buffer. The purity of the BLS preparation was determined on SDS-15% (wt/vol) polyacrylamide gels. Purified BLS was concentrated (10 mg/ml), frozen in liquid N2, and stored at −20°C. For purification of the BLS-GK-1 chimera, similar procedures were performed, except that the buffer of refolding was prepared without DTT.
Immunization protocol.
For the different experiments, groups of 9 to 10 BALB/cAnN female mice were orally immunized with 50 μg of BLS-GK-1 per mouse. The GK-1 synthetic peptide was also used at a dose equivalent to the amount of peptide included in the chimera (2.5 μg per mouse). To test BLS as an adjuvant, another group of mice was immunized with BLS (25 μg per mouse) with or without the synthetic peptide. The antigen doses were estimated according to previously published results (27). Mice under anesthesia with sevoflurane were orally immunized three times at 15-day intervals and challenged 2 weeks after the last immunization. The primary immunization was performed with GK-1, priming with BLS-GK-1, or coimmunization with GK-1 plus BLS. Boosts were performed only with GK-1. Control mice received saline solution only.
Infection of mice.
Mice were infected with 10 small (2-mm) nonbudding cysticerci of T. crassiceps with high motility in 0.5 ml of PBS 2 weeks after the last immunization. Mice were killed 30 days after infection, and cysts were harvested from the peritoneal cavities and counted to determine the effect of vaccination, as previously reported (32, 33). Organs inside the peritoneal cavity were carefully inspected to detect any remaining T. crassiceps larvae.
Antibody detection by ELISA.
Anesthetized mice from each experimental group were bled from the orbital plexus, before they were killed 15 days after the third immunization. The sera were separated and stored at −70°C until used. Antibody levels were assessed by enzyme-linked immunosorbent assay (ELISA) using 96-well flat-bottom microtitration plates (Costar; Corning Incorporated, Corning, NY) coated overnight with GK-1 expressed on the surface of the M13 bacteriophage (1010 PFU per well) previously prepared as described elsewhere (29). The plates were then incubated with sera diluted 1:100 in PBS containing 1% bovine serum albumin (BSA). Bound mouse immunoglobulins (Igs) were detected by using alkaline phosphatase-conjugated sheep anti-mouse IgG (whole molecule; Sigma) or alkaline phosphatase-conjugated anti-mouse IgA, following the previously described procedure (27). Color was developed at room temperature with p-nitrophenyl phosphate disodium (Sigma Diagnostics, Poole Dorset, United Kingdom), and the absorbance at 405 nm was measured in a Humareader ELISA processor (Human Gessellschaft für Biochemica und Diagnostica, Taunnusstein, Germany). GK-1 recombinantly displayed on the filamentous phage surface was used as antigen in order to identify only the specific antibodies for GK-1 and not those induced by BLS. Recombinant GK-1 was produced by inserting the coding sequence of GK-1 at the amino terminus of the major coat protein pVIII using the procedure described elsewhere (29).
Ex vivo analysis of lymphocyte activation and proliferation by flow cytometry.
For evaluation of cell activation, cells from spleen, Peyer's patches (PP), and mesenteric lymph nodes (MLN) from mice primed with the different treatments were recovered 48 h after the last boost; stained with anti-CD8, CD4, or CD19 and CD25 or CD69 monoclonal antibodies (MAbs); and analyzed by flow cytometry. PP and MLN were removed, cut into small pieces, and incubated at 37°C for 5 min in RPMI 1640 medium with collagenase (0.5 mg/ml; Sigma), DNase (40 μg/ml; Sigma), and 5% fetal bovine serum (FBS). After incubation, the remaining tissue was cut further and passed through a stainless steel mesh with washing solution containing RPMI 1640 medium, 5 mM EDTA, and 5 μg/ml DNase (Sigma). The cell suspension was washed twice with isotonic saline solution, and the cells were counted, suspended at 106 cells/ml in PBS containing 5% FBS and 0.02% sodium azide (binding buffer), and kept at 4°C. On the other hand, the spleen was removed and perfused with isotonic saline solution, the cell suspension was washed twice with the same solution, and red blood cells were removed by incubating the cells with ammonium chloride buffer (150 mM) for 5 min on ice. The reaction was stopped by adding 9 ml of isotonic saline solution, and cells were washed twice with the same solution. Cells were suspended in cold PBS containing 5% FBS and 0.02% NaN3 and left to react for 30 min at 4°C with the corresponding MAbs: fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8a (clone 53-6.7), FITC-conjugated rat anti-mouse CD4 (clone H129.19), phycoerythrin (PE)-conjugated rat anti-mouse CD19 (clone ID3), FITC- and PE-conjugated hamster anti-mouse CD69 (clone H1.2F3), and FITC- and PE-conjugated rat anti-mouse CD25 (clone PC61) (all from Pharmingen, San Diego, CA). Parallel samples of the cells were stained with the corresponding isotype control to account for nonspecific staining. Nonspecific binding of antibodies through Fc receptors was blocked with Fc Block (Pharmingen). Stained lymphocytes were fixed with 2% paraformaldehyde, and 10,000 cells were analyzed with a lymphocyte gate as defined by light scatter in a FACScan flow cytometer (Becton Dickinson, Palo Alto, CA). Results are expressed as the percentage of positive cells.
To study cell proliferation, at 24 h after the last immunization, 0.5 ml of 5′-bromo-2′-deoxyuridine (BrdU; 1 mg/ml; Sigma Chemical Co., St. Louis, MO) was administered orally and i.p. to each mouse. A similar dose of BrdU was given 2 h later. Two hours later, mice were killed and cells from PP, MLN, and spleen were obtained from each mouse following the procedure described above. Cells were suspended at 106 cells/ml in binding buffer. Cells stained with PE-conjugated anti-CD4, anti-CD8, or anti-CD19 were fixed, permeabilized, and stained intracellularly with FITC-conjugated anti-BrdU according to the manufacturer's instructions (BD Biosciences). All samples were subsequently analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Evaluation of cytokines in supernatants of lymphocyte cultures by ELISA.
To study the cytokines induced by an in vitro recall antigen assay, at 15 days after the third immunization, mice were killed and cells from spleen, PP, and MLN of control mice and mice primed with GK-1, BLS plus GK-1, or BLS-GK-1 were recovered as previously described. A total 2.5 × 106 cells/ml of spleen or PP cells of each group were placed in 12-well cluster plates (Costar), and the plates were incubated at 37°C 5% in a CO2 humidified atmosphere. RPMI 1640 medium supplemented with l-glutamine (0.2 mM), nonessential amino acids (0.01 mM), penicillin (100 U/ml), streptomycin (100 mg/ml), FBS (10%), and 50 μM 2-mercaptoethanol was used. Cells were incubated for 72 h at 37°C in 5% CO2 atmosphere in the presence or absence of BLS or GK-1, according to the immunization protocol. Twelve hours before supernatant collection, 20 μl of a 1-mg/ml solution of concanavalin A (ConA) was added to the cultures. Supernatants were collected and stored at −80°C until they were used to determine the cytokine levels.
Interleukin-4 (IL-4) and IFN-γ concentrations were measured by ELISA using Duoset ELISA kits (R&D Systems), according to the manufacturer's instructions. The concentrations of each cytokine were calculated from calibration curves using individual recombinant proteins as standards, according to the manufacturer's instructions. Readings of the optical density (OD) at 405 nm were obtained at 30 and 60 min of incubation.
Immunolocalization of GK-1 and BrdU in lymphoid organs.
At 24 h after the last immunization, 3 mice of each experimental group were orally given BrdU as described above. Mice were killed and the spleen, PP, and MLN were fixed for 72 h in Zamboni solution (1.6% [wt/vol] paraformaldehyde, 19 mM KH2PO4, and 100 mM Na2HPO4·7H2O in 240 ml of saturated picric acid and 1,600 ml of H2O) and embedded in paraffin. The presence of GK-1 and BrdU was localized on 7-μm sections. Endogenous peroxidase was inhibited by incubation with 0.3% (vol/vol) H2O2 in PBS for 10 min. With the aim of detecting the presence of GK-1 in the different lymph organs, sections were incubated with anti-GK-1 rabbit serum 1:100 in 1% BSA-PBS 1 h at 37°C. A serum sample from a nonimmunized rabbit was employed as a negative control. Sections were then incubated with a goat antirabbit antibody (MP Biomedicals) for 30 min at 37°C, followed by incubation with streptavidin-peroxidase (MP Biomedicals) for 1 h at room temperature. Labeled cells were visualized with 3′3-diaminobenzidine (DAB; Zymed, Carlton Court, CA), and slides were counterstained with hematoxylin. Images were taken with a Nikon Eclipse 80i microscope and analyzed using MetaMorph imaging system (version 4.5) software.
Cellular proliferative activity was monitored by BrdU incorporation into cellular DNA. For this, tissue sections were treated as follows: three washes were performed for 10 min each with PBT (0.1 M phosphate buffer, 0.3% [vol/vol] Triton X-100, and 3% H2O2), and sections were incubated for 2 h at 65°C in 50% formamide in 50% 2× SSC buffer (0.3 mol/liter NaCl and 0.03 mol/liter sodium citrate). Sections were treated with 1 N HCl at 37°C for 30 min, followed by washing in 0.1 M boric acid at pH 8.5 for 10 min. Thereafter, sections were washed three times with PBT for 10 min each time. Endogenous peroxidase was inhibited as described above. To prevent nonspecific binding, the slides were treated for 30 min with 1% BSA 3%-0.3% Triton X-100 in PBS. The sections were then incubated overnight with mouse anti-BrdU monoclonal antibody (1:500; Roche) diluted in PBT and 3% BSA at room temperature, followed by three washes in PBT. As secondary antibody, biotinylated goat antimouse antibody (1:500; Chemicon) diluted in PBT and 3% BSA was used. Finally, after three 10-min washes, the sections were incubated with the streptavidin-peroxidase complex for 1 h 30 min at room temperature. Once again, the sections were thoroughly washed, and peroxidase activity was revealed using 3,3-diaminobenzidine (Vector Laboratories, Inc.) for 4 min at room temperature. Sections were counterstained with eosin, and a coverslip was placed and sealed with Cytoseal mounting medium (Richard-Allan Scientific). Images of the sections were captured and analyzed as described above.
Statistical analysis.
The statistical comparisons of individual parasite intensities between groups and the stimulation index data and activation of CD4 and CD8 T cells were performed by the nonparametric Tukey-Kramer multiple-comparisons test. Fisher's exact test was employed to compare the percentages of totally protected mice. Data for the antibodies were compared using an unpaired t test. A P value of <0.05 was considered statistically significant. All analyses were performed using the Instat software program (GraphPad).
RESULTS
Protection induced by oral vaccination.
To evaluate if a heterologous prime-boost trial with GK-1 with or without adjuvant induces protective immunity against Taenia crassiceps cysticerci by oral immunization, mice were assigned to one of three groups. One group was primed with the linear synthetic GK-1 peptide. Mice in the second group were first coimmunized with GK-1 plus BLS, and mice in the third group were primed with GK-1 recombinantly bound to BLS (BLS-GK-1). The three groups were boosted twice with GK-1 alone at 15-day intervals. Two weeks after the last immunization, mice were intraperitoneally infected with 10 small T. crassiceps cysticerci, and 30 days after infection they were killed and the parasite load was determined. Results were compared with those obtained with naïve mice challenged under conditions similar to those for the other three groups. Table 1 shows that oral priming and boosting with GK-1 significantly reduced the number of parasites (64.7%). A significantly higher level of protection was observed when mice were primed with BLS-GK-1 (91.8%) or GK-1 was coadministered with BLS (96.3%). As the results show, none of the mice immunized with GK-1 were fully protected; in contrast, priming with GK-1 with BLS as an adjuvant or as a chimera induced total protection in 43% of the mice, albeit statistical significance was not attained (P = 0.09) (Table 1).
Table 1.
Effective protection induced by oral immunization with GK-1 using different delivery systemsa
| Group | Individual no. of cysticerci | Median no. (95% confidence limit) | % protection | % TpM |
|---|---|---|---|---|
| Nonimmunized | 88, 101, 91, 62, 71, 82, 100a | 88 (72–98) | 0 | |
| Immunized with priming/boost of: | ||||
| GK-1/GK-1 | 20, 23, 30, 35, 55, 19, 28b | 28 (19–41.5) | 64.7 | 0 |
| GK-1 + BLS/GK-1 | 6, 5, 5, 10, 0, 0, 0c | 5 (0.14–7.3) | 96.3 | 43 |
| GK-1-BLS/GK-1 | 16, 11, 15, 7, 0, 0, 0c | 7 (0.4–14) | 91.8 | 43 |
Cysticerci from 7 mice of each group were recovered 40 days after challenge. Different letters indicate the significant differences between parasite intensity using the Tukey-Kramer multiple-comparisons test (P < 0.001). No significant differences were noted between control and groups of totally protected mice according to Fisher's exact test (P > 0.05). TpM, totally protected mice.
Specific IgG and IgA induced by GK-1 after oral immunization.
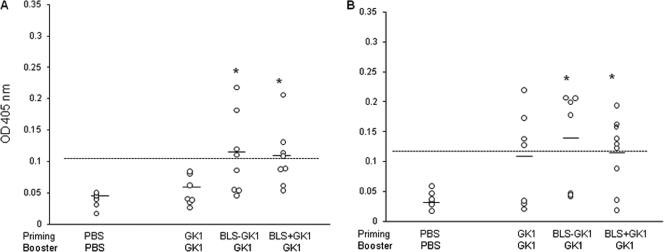
Sera from nonimmunized and immunized mice were collected 15 days after the last immunization, and anti-GK-1 IgG and IgA antibodies were determined by ELISA. A low but significant increase in both antibody classes was detected in sera from mice primed with BLS-GK-1 and BLS plus GK-1 (Fig. 1A and B). Although sera from mice immunized with GK-1 alone did not significantly increase the level of anti-GK-1 antibodies, 50 to 60% of the mice showed IgA levels higher than the cutoff value.
Fig. 1.
Levels of IgG (A) and IgA (B) antibodies against GK-1 in sera developed in mice orally primed with GK, BLS-GK-1, or BLS plus GK-1 and boosted with GK-1. Data are for only 7 or 8 mice because some mice were lost during the last bleeding. Sera were obtained 15 days after the last boost. The mean value of each group is depicted by a line. The dotted line represents the cutoff value, defined as the mean of the OD of the nonimmunized group + 2 standard deviations. *, significant differences (P ≤ 0.05) between naïve and immunized mice were calculated using the nonparametric Mann-Whitney U test. Data are representative of three independent experiments.
Activation and proliferation of CD4 and CD19 cells induced by oral immunization in different lymphoid organs.
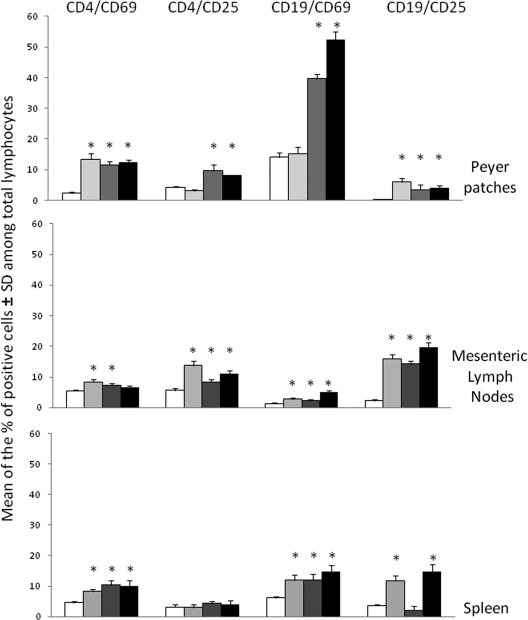
To ascertain if protection was associated with the cellular immune response, the ex vivo activation and proliferative states of CD8+ and CD4+ T cells and CD19+ B cells from PP, spleen, and MLN were evaluated. Activation was measured as the expression of the early CD69 and the late CD25 markers by flow cytometry 48 h after the last GK-1 boost. As shown in Fig. 2, a heterogeneous level of expression of the activation markers in the different lymphoid organs was observed. Significant increases in the percentages of cells expressing CD25 and CD69 (early and late activation markers, respectively) were observed in CD4 T cells and CD19 B cells of MLN and PP of mice primed with BLS-GK-1 or BLS plus GK-1 and boosted with GK-1. A similar response was induced in mice immunized only with GK-1, except that no increase in the percentages of CD4+ CD25+ and CD19+ CD69+ cells was observed in the spleen and PP, respectively. A significantly higher percentage of CD8+ CD25+ lymphocytes was found only in the spleens of mice primed with GK-1 and BLS (data not shown).
Fig. 2.
Oral immunization with GK-1 induced the activation of CD4 and CD19 cells in mucosal and peripheral lymphoid tissues. Groups of nine mice were orally primed with GK-1 ( ), BLS-GK-1 (
), BLS-GK-1 ( ), or BLS plus GK-1 (▪) and boosted with GK-1 (prime/boost). At 15 days after the third immunization, mice were primed in vivo, and 24 h later lymphocytes were recovered from PP, MLN, and spleen. The lymphocytes from groups of three mice were pooled and analyzed by fluorescence-activated cell sorter analysis. *, significant differences compared with mice treated only with saline (□) (P < 0.05, Tukey-Kramer multiple-comparisons test). Data are representative of three independent experiments.
), or BLS plus GK-1 (▪) and boosted with GK-1 (prime/boost). At 15 days after the third immunization, mice were primed in vivo, and 24 h later lymphocytes were recovered from PP, MLN, and spleen. The lymphocytes from groups of three mice were pooled and analyzed by fluorescence-activated cell sorter analysis. *, significant differences compared with mice treated only with saline (□) (P < 0.05, Tukey-Kramer multiple-comparisons test). Data are representative of three independent experiments.
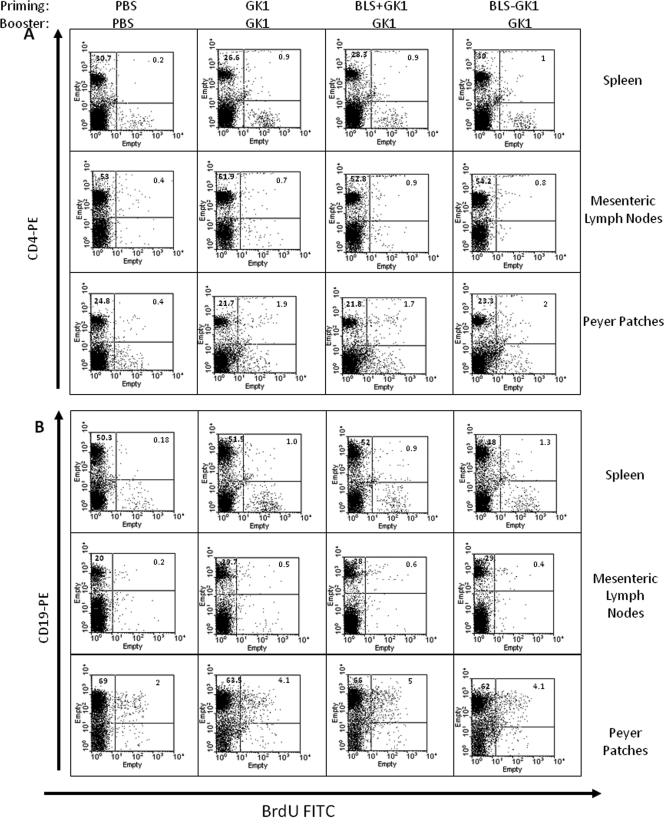
Cell proliferation was assessed by BrdU incorporation into CD4, CD8, and B lymphocytes from mice primed with GK-1, BLS-GK-1, or GK-1 plus BLS and boosted only with GK-1. Proliferation was analyzed in spleen, PP, and MLN lymphocytes. As shown in Table 2 and Fig. 3A and B, proliferation of CD4+ was observed in the three lymphoid organs of the three groups of immunized mice to a greater extent in spleen and MLN than in PP. No CD8+ proliferation was detected in any of the lymphoid organs assayed (data not shown).
Table 2.
Lymphocyte proliferative response induced in mice primed and boosted with GK-1 peptidea
| Priming/booster group | Mean ± SD cell proliferation index |
|||||
|---|---|---|---|---|---|---|
| Spleen |
MLN |
PP |
||||
| CD4 | CD19 | CD4 | CD19 | CD4 | CD19 | |
| GK-1/GK-1 | 4.5 ± 0.2a | 5.6 ± 0.92a | 4.7 ± 0.27a | 2.05 ± 0.3a | 1.75 ± 0.72a | 2.25 ± 0.2a |
| BLS-GK-1/GK-1 | 4.5 ± 0.7a | 5 ± 1.03a | 4.25 ± 0.9a | 2.5 ± 0.72a | 2.25 ± 0.52a | 2 ± 0.52a |
| BLS + GK-1/GK-1 | 5.0 ± 1.01a | 7.2 ± 0.25a | 5.0 ± 0.71a | 4.25 ± 0.1b | 2.0 ± 0.48a | 3 ± 0.12a |
Data represent the cell proliferation indexes of CD4 or CD19 lymphocytes of the different lymphoid organs in three different experiments. The stimulation index was calculated by dividing the percentage of lymphocytes which incorporated BrdU from experimental mice by the percentage of cells from nonimmunized PBS-treated mice. Each experiment was performed with lymphocytes pooled from 3 mice. Proliferation was determined by BrdU incorporation into fresh lymphoid PP, MLN, and spleen cells recovered 24 h after boosting with GK-1 and 6 h after BrdU administration. Cells were costained with anti-BrdU (FITC) and rat anti-mouse CD4 (PE) or rat-anti-mouse CD19 (PE). Different letters in each column indicate significant differences (P < 0.05) determined using the Tukey-Kramer multiple-comparisons test.
Fig. 3.
CD4 (A) and CD19 (B) cell proliferation assessed by BrdU incorporation. Fresh lymphoid PP, MLN, and spleen cells from mice orally primed with GK-1, BLS-GK-1, and BLS plus GK-1 and boosted with GK-1 or nonimmunized mice were costained with anti-BrdU (FITC), rat anti-mouse CD4 (PE), or rat anti-mouse CD19 (PE). The percentage of positive cells in each quadrant is shown. Data are representative of three independent experiments.
The groups that exhibited the higher percentage of protection were also those that showed higher local and peripheral activation of CD4 and CD19 cells.
Location of proliferative cells and GK-1 antigen in different lymphoid organs.
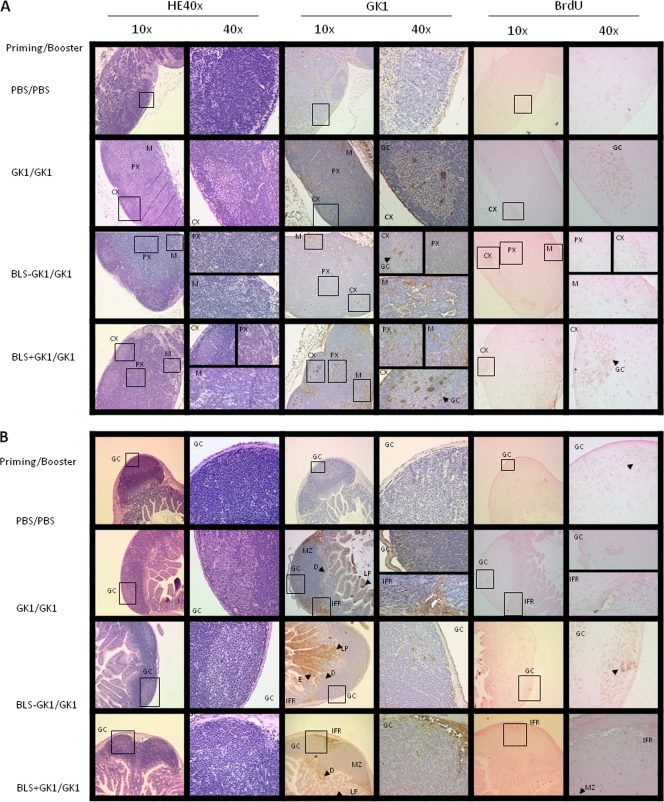
At 24 h after the last boost, mice were fed BrdU, and 4 h later, PP, MLN, and spleen were obtained. The different lymphoid organs were prepared for histological analysis, and the location of GK-1 and the areas of cell proliferation (assessed by BrdU incorporation) were evaluated. As Fig. 4A and B shows, GK-1 was detected in cells within the T and B regions in the PP and MLN, albeit with some differences. In PP (Fig. 4A), GK-1 was located in the germinal center (GC), marginal zone (MZ), interfollicular region (IFR), domo (D), and lamina propria (LP), although the GK-1 signal in GC and LP was very faint. On the other hand, cell proliferation was detected only in GC and IFR. In mesenteric lymph node (Fig. 4B), GK-1 and BrdU were found in regions enriched in B and T cells of the cortex (CX), paracortex (PX), and medulla (M), but BrdU was mainly detected in the GC of the cortex. Similar results were obtained for PP and MLN when mice were primed with BLS-GK-1 and BLS plus GK-1. In spleen, GK-1 was detected at very low levels in the red pulp and within the follicles, and very low proliferation was also detected in the red pulp (data not shown).
Fig. 4.
Photomicrographs of hematoxylin-eosin (HE) and immunohistochemical staining for GK-1 location and BrdU incorporation from sections of MLN (A) and PP (B) of mice primed with GK-1, BLS-GK-1, or GK-1 plus BLS and boosted with GK-1 alone. At 24 h after the last immunization, mice were fed BrdU and the PP, MLN, and spleens were obtained and fixed and embedded in paraffin. E, enterocytes. Data are representative of the images observed in several slides of each experimental group.
Hence, the proliferative immune response took place in the T cells, B cells, and antigen-presenting cell (APC)-enriched region, precisely in the areas in which the GK-1 antigen was identified.
Cytokine release by anti-GK-1 memory cells from different lymphoid organs stimulated in vitro.
To further analyze the type of immune response induced by the different treatments, at 15 days after the last boost, memory cells from spleen, PP, and MLN were stimulated in vitro in the presence of GK-1 for 48 h, and the levels of IFN-γ and IL-4 released into the supernatants were analyzed by ELISA. As shown in Table 3, spleen and PP cells from mice primed with GK-1 alone, as a chimera, or coadministered with BLS produced both IFN-γ and IL-4 cytokines, albeit with some differences. Priming of mice with GK-1 in the presence of BLS resulted in higher production of IFN-γ in the spleen and IL-4 in PP. Moreover, spleen cells released more IFN-γ than PP cells, while PP cells released more IL-4 than spleen cells (Table 3). No significant increases in either of the cytokines were observed in MLN cells (data not shown).
Table 3.
Cytokine production by memory cells specific for GK-1a
| Priming/booster group | Mean ± SD cytokine concn (pg/ml) |
|||
|---|---|---|---|---|
| Spleen |
Peyer's patches |
|||
| IFN-γ | IL-4 | IFN-γ | IL-4 | |
| Nonvaccinated | 302.2 ± 18.7a | 165.6 ± 25.6a | 83.7 ± 14.9a | 31.4 ± 13.9a |
| GK-1/GK-1 | 493.4 ± 53.4b | 195.9 ± 31.5a | 168.9 ± 18.3b | 78.9 ± 12.6a |
| BLS-GK-1/GK-1 | 690.6 ± 45.9c | 289.7 ± 21.5b | 156.2 ± 10.9b | 100.3 ± 17.7a |
| BLS + GK-1/GK-1 | 1,079.0 ± 172.1d | 417.5 ± 51.3c | 216.3 ± 13.2b | 129.1 ± 15.2a,b |
Groups of 9 mice were orally primed with GK-1, BLS-GK-1, or BLS plus GK-1 and boosted with GK-1. At 15 days after the third immunization, lymphocytes were recovered from PP, MLN, and spleen cells, and the lymphocytes from groups of three mice were pooled and placed in 12-well cluster plates. The plates were incubated at 37°C 5% in a CO2 humidified atmosphere supplemented with RPMI 1640 in the presence or absence of BLS or GK-1, according to the immunization protocol. Twelve hours before supernatant collection, ConA was added to nonimmunized and GK-1-immunized cells. Supernatants were collected and stored at −80°C until they used to determine the cytokine levels. Data represent the cytokine concentration secreted by tissue cells. Different letters indicate significant differences at P < 0.05 (Tukey-Kramer multiple-comparisons test).
DISCUSSION
GK-1 is an 18-aa peptide obtained from a cDNA library of T. crassiceps that confers protection through subcutaneous administration against murine cysticercosis (32). GK-1 also exhibits adjuvant properties when it is coadministered with influenza vaccine, owing to the induction of high levels of Th1 cytokines and to the activation of macrophages and dendritic cells (30, 31).
With the aim of analyzing the ability of GK-1 to induce an effective protective immune response against T. crassiceps cysticercosis after oral vaccination, heterologous prime-boost trials were performed. Mice were primed with either GK-1 alone or GK-1 recombinantly bound to BLS or were coadministered GK-1 and BLS. Two boosters with GK-1 alone were administered to determine the specific abilities of GK-1 to induce and maintain an effective memory immune response.
Oral administration of GK-1 alone was able to induce significant protection against cysticercosis in the absence of any adjuvant. This protection was significantly increased when mice were primed with BLS used as an adjuvant or as a carrier. These results emphasize the relevance of BLS in promoting a long-term adaptive immune response in vaccination, as further supported by other experiences. For example, similar results were obtained by replacing an immunogen such as the influenza vaccine (10) by a complex and multiepitope antigen of high molecular weight. The ability of orally administered GK-1 to sustain an effective protective immune response after boosting was an unexpected finding. These exceptional properties of GK-1 could be attributed to its physicochemical properties (32), which make it a molecule easily endocytosed by scavenger receptors located on macrophages and/or dendritic cells, considering their high affinity to negatively charged molecules such as GK-1 (34), thus allowing activation of these professional APCs.
Low peripheral levels of specific IgA and IgG, increased expression of CD69 and CD25 activation markers in CD4 and CD19 lymphocytes from PP and MLN, and increased CD4+ CD69+, CD19+ CD69+, and CD19+ CD25+ cells in spleen accompanied the protection observed in mice primed in the presence of BLS. A similar profile was found in mice immunized only with GK-1, except that no CD4+ CD25+ or CD19+ CD69+ cells were detected in PP. Proliferation of CD4 and CD19 cells was found in the three lymphoid organs analyzed, spleen, MLN, and PP, but to a higher extent in the PP, especially in the particular areas of these organs in which GK-1 was also detected (Fig. 4B). Increased levels of IFN-γ and IL-4 in spleen and PP cells were detected in orally immunized mice, but IFN-γ and IL-4 were present at higher levels in spleen and PP, respectively, in those mice primed with GK-1 and BLS. Thus, the lower level of protection observed in those mice immunized only with GK-1 was accompanied by the absence of CD8+ CD25+ cells in the spleen; the early and late activation markers CD4 and CD19, respectively, in PP; and lower levels of IFN-γ and IL-4 in spleen and PP cells, respectively. These parameters could be relevant in increasing the effectiveness to control parasite growth. It is important to remark that this immune profile was observed in female BALB/cAnN mice, which have the most permissive condition that we have found in the murine model of cysticercosis (6). Whether this immune profile is also observed under more restrictive conditions (male BALB.B or C57BL/6J mice) remains to be tested. However, considering that it has been stated that the biological differences imposed by the sex of the host are a major source of variation, affecting immune responses to vaccination (13), a different immune prolife could probably be induced. Indeed, previous studies have shown that males are more highly protected than females when they are immunized with different immunogens and by different routes of immunization (28, 29), results that suggest greater vaccine effectiveness in males than in females.
On the other hand, it is important to emphasize that the more effective protection in those mice primed with BLS and GK-1 is accompanied by increased levels of both specific IgG and IgA antibodies, a finding that supports their relevance in the higher protection observed in these groups of mice. However, considering the low levels of antibodies, this possibility has to be taken with caution.
With respect to the local and peripheral proliferation observed in the immunohistological studies, it is important to mention that the intestinal mucosa is a site of highly active immune responses, since this mucosa is exposed to a myriad of antigens derived from commensal bacteria and food. Thus, the intestinal mucosa shows increased levels of immunological tolerance to preserve the functional integrity of this organ (16, 21). When exogenous antigens such as GK-1 reach the mucosa, they can get inside the LP, as shown in Fig. 4B. LP is the tissue below the epithelial layer that contains most of the cells involved in the innate and adaptive immune responses (2, 26). Also, antigens can be transported through the M cells present in the epithelial layer above the PP (17). When the antigens enter the LP, antigen-presenting cells such as DCs and macrophages capture them and travel to the MLN, where they induce T and B cell responses, as illustrated in Fig. 4A. In contrast, when the antigen enters the PP, T and B cells are stimulated in this lymphoid organ (Fig. 4B) and travel to the MLN (Fig. 4A). From the MLN, the activated T and B cells enter the general circulation and are distributed to both the different peripheral lymphoid organs and the LP of the intestinal mucosa and other mucosae (22) (Fig. 4B). Consequently, immunization through the intestinal mucosa can generate peripheral and mucosal immunity. This fact would be particularly relevant to prevent porcine T. solium cysticercosis, since the infection is orally acquired by the ingestion of eggs, and thereafter, the oncospheres become cysticerci that are established in the muscles and central nervous system of the pigs. Since GK-1 is expressed in both stages of the parasite (25), mucosal and peripheral immunity could affect oncospheres as well as early established cysticerci.
Altogether, these results portray the potent immunogenic properties of orally administered GK-1 and reinforce the usefulness of BLS as an adjuvant and adequate vaccine delivery system for oral vaccines.
ACKNOWLEDGMENTS
We thank Isabel Pérez Montfort for English corrections, Georgina Díaz and Gerardo Arrellín for assistance with animal care, and Oscar Ramírez-Pliego, Marisela Hernández, and Judith Ruiz Reyes for technical assistance.
This work was supported by PROMEP-UAEMOR-PTC-87 (103.5/03/2530) and CONACYT (S52505-Z, CB-62471, CB-82771, and CB-104915).
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Assana E., et al. 2010. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int. J. Parasitol. 40:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avila G., et al. 2002. Inflammatory responses in the intestinal mucosa of gerbils and hamsters experimentally infected with the adult stage of Taenia solium. Int. J. Parasitol. 32:1301–1308 [DOI] [PubMed] [Google Scholar]
- 3. Berguer P. M., Mundiñano J., Piazzon I., Goldbaum F. A. 2006. A polymeric bacterial protein activates dendritic cells via TLR4. J. Immunol. 176:2366–2372 [DOI] [PubMed] [Google Scholar]
- 4. de Aluja A., et al. 2005. Therapeutic capacity of the synthetic peptide-based vaccine against Taenia solium cysticercosis in pigs. Vaccine 23:4062–4069 [DOI] [PubMed] [Google Scholar]
- 5. Flisser A., et al. 2004. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect. Immun. 72:5292–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fragoso G., Meneses G., Sciutto E., Fleury A., Larralde C. 2008. Preferential growth of Taenia crassiceps cysticerci in female mice holds across several laboratory mice strains and parasite lines. J. Parasitol. 94:551–553 [DOI] [PubMed] [Google Scholar]
- 7. Freeman R. S. 1962. Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda). Can. J. Zool. 40:969–990 [Google Scholar]
- 8. González A. E., et al. 2005. Vaccination of pigs to control human neurocysticercosis. Am. J. Trop. Med. Hyg. 72:837–839 [PubMed] [Google Scholar]
- 9. Guo Y. J., et al. 2004. Protection of pigs against Taenia solium cysticercosis using recombinant antigen or in combination with DNA vaccine. Vaccine 22:3841–3847 [DOI] [PubMed] [Google Scholar]
- 10. Guy B., Fourage S., Hessler C., Sanchez V., Millet M. J. 1998. Effects of the nature of adjuvant and site of parenteral immunization on the serum and mucosal immune responses induced by a nasal boost with a vaccine alone. Clin. Diagn. Lab. Immunol. 5:732–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernández M., et al. 2007. A new highly effective anticysticercosis vaccine expressed in transgenic papaya. Vaccine 25:4252–4260 [DOI] [PubMed] [Google Scholar]
- 12. Huerta M., et al. 2001. Synthetic peptide vaccine against Taenia solium pig cysticercosis: successful vaccination in a controlled field trial in rural Mexico. Vaccine 20:262–266 [DOI] [PubMed] [Google Scholar]
- 12a. Institute of Laboratory Animal Resources, National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 13. Klein S. L., Jedlicka A., Pekosz A. 2010. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 10:338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laplagne D. A., et al. 2004. Engineering of a polymeric bacterial protein as a scaffold for the multiple display of peptides. Proteins 57:820–828 [DOI] [PubMed] [Google Scholar]
- 15. Lightowlers M. W. 1999. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int. J. Parasitol. 29:811–817 [DOI] [PubMed] [Google Scholar]
- 16. Macpherson A. J., Smith K. 2006. Mesenteric lymph nodes at the center of immune anatomy. J. Exp. Med. 203:497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Man A. L., Prieto-Garcia M. E., Nicoletti C. 2004. Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys? Immunology 113:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manoutcharian K., et al. 2004. Recombinant bacteriophage-based multiepitope vaccine against Taenia solium pig cysticercosis. Vet. Immunol. Immunopathol. 99:11–24 [DOI] [PubMed] [Google Scholar]
- 19. Molinari J. L., et al. 1997. Field trial for reducing porcine Taenia solium cysticercosis in Mexico by systematic vaccination of pigs. Vet. Parasitol. 69:55–63 [DOI] [PubMed] [Google Scholar]
- 20. Morales J., et al. 2008. Inexpensive anticysticercosis vaccine: S3Pvac expressed in heat inactivated M13 filamentous phage proves effective against naturally acquired Taenia solium porcine cysticercosis. Vaccine 26:2899–2905 [DOI] [PubMed] [Google Scholar]
- 21. Mowat A. M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341 [DOI] [PubMed] [Google Scholar]
- 22. Nagler-Anderson C. 2001. Man the barrier! Strategic defenses in the intestinal mucosa. Nat. Rev. Immunol. 1:59–67 [DOI] [PubMed] [Google Scholar]
- 23. Nascimento E., Costa J. O., Guimarães M. P., Tavares C. A. 1995. Effective immune protection of pigs against cysticercosis. Vet. Immunol. Immunopathol. 45:127–137 [DOI] [PubMed] [Google Scholar]
- 24. Plancarte A., Flisser A., Gauci C. G., Lightowlers M. W. 1999. Vaccination against Taenia solium cysticercosis in pigs using native and recombinant oncosphere antigens. Int. J. Parasitol. 29:643–647 [DOI] [PubMed] [Google Scholar]
- 25. Rassy D., et al. 2010. Characterization of S3Pvac anti-cysticercosis vaccine components: implications for the development of an anti-cestodiasis vaccine. PLoS One 23:e11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rescigno M., Matteoli G. 2008. Lamina propria dendritic cells: for whom the bell TOLLs? Eur. J. Immunol. 38:1483–1486 [DOI] [PubMed] [Google Scholar]
- 27. Rosas G., et al. 1998. Taenia crassiceps cysticercosis: humoral immune response and protection elicited by DNA immunization. J. Parasitol. 84:516–523 [PubMed] [Google Scholar]
- 28. Rosas G., et al. 2006. Brucella spp. lumazine synthase: a novel adjuvant and antigen delivery system to effectively induce oral immunity. Microbes Infect. 8:1277–1286 [DOI] [PubMed] [Google Scholar]
- 29. Sciutto E., et al. 1990. Cysticercosis vaccine: cross protecting immunity with T. solium antigens against experimental murine T. crassiceps cysticercosis. Parasite Immunol. 12:687–696 [DOI] [PubMed] [Google Scholar]
- 30. Segura-Velázquez R., et al. 2006. A novel synthetic adjuvant effectively enhances the immunogenicity of the influenza vaccine. Vaccine 24:1073–1080 [DOI] [PubMed] [Google Scholar]
- 31. Segura-Velázquez R., Fragoso G., Sciutto E., Sarukhan A. 2009. Towards identification of the mechanisms of action of parasite-derived peptide GK-1 on the immunogenicity of an influenza vaccine. Clin. Vaccine Immunol. 16:1338–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toledo A., et al. 1999. Towards a Taenia solium cysticercosis vaccine: an epitope shared by Taenia crassiceps and Taenia solium protects mice against experimental cysticercosis. Infect. Immun. 67:2522–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toledo A., et al. 2001. Two epitopes shared by Taenia crassiceps and Taenia solium confer protection against murine T. crassiceps cysticercosis along with a prominent T1 response. Infect. Immun. 69:1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamada Y., Doi T., Hamakubo T., Kodama T. 1998. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell. Mol. Life Sci. 54:628–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zylberman V., et al. 2004. High order quaternary arrangement confers increased structural stability to Brucella sp. lumazine synthase. J. Biol. Chem. 279:8093–8101 [DOI] [PubMed] [Google Scholar]