Abstract
We observed by flow cytometry that the frequency of both gastric infiltrating Tregs and PD-1-positive CD4 T cells is correlated with the density of Helicobacter pylori, suggesting that cellular immunity against this pathogen is inhibited.
TEXT
Helicobacter pylori, a common human pathogen, infects about 50% of the world's population (9, 10). Many studies have confirmed that the pathogenesis of H. pylori infection is probably due largely to the host immune system, particularly the cellular immune response. However, the mechanism by which H. pylori negatively regulates the immune response against the bacteria is not completely understood.
In recent years, regulatory T cells (Tregs) and related negative regulatory molecules have been a focus of research on the immune mechanisms of diseases. Evidence indicates that removal or reduction of Tregs can enhance immune responses against infectious microbes (1, 2, 5, 6, 8, 11–13, 16). Expression of programmed cell death 1 (PD-1, also called CD279), a negative regulatory molecule in the CD28/B7 family, is induced on CD4+ T cells, CD8+ T cells, natural killer T cells, B cells, and activated monocytes during the course of many diseases. B and T lymphocyte attenuator (BTLA), a newly discovered member of the CD28 family of costimulatory molecules, may have inhibitory function similar to that of PD-1.
The PD-1 signaling pathway is involved in chronic bacterial infection and may be a potential therapeutic target. However, the role of BTLA in H. pylori infection has not been investigated. In this study, we analyzed the frequency of gastric infiltrating CD4+ CD25high Tregs in patients with H. pylori infection, as well as the expression of PD-1 and BTLA on gastric infiltrating CD4+ T cells, and their relationships with H. pylori-related clinical markers.
Biopsy specimens of the gastric antrum were obtained from patients undergoing gastric endoscopy for dyspepsia at China Medical University Hospital. There were 38 H. pylori-infected subjects enrolled in this study. Peripheral blood samples from the same patients served as the control. The presence of H. pylori infection was determined by histopathologic examination (including Giemsa staining) and/or by a positive result for a rapid urease test performed on at least one additional biopsy sample. The density of H. pylori was analyzed as suggested by the updated Sydney system (4): 0, absent; 1, mild; 2, moderate; and 3, marked. The degree of inflammation present in the histological specimens was also classified according to the updated Sydney system. Biopsy specimens of the gastric antrum were collected into sterile collection medium (calcium- and magnesium-free Hanks' balanced salt solution [HBSS] with 5% fetal calf serum and penicillin plus streptomycin). The collected tissues were immediately placed in ice-cold RPMI 1640 complete medium (Gibco BRL, Gaithersburg, MD) which contained 10% fetal calf serum supplemented with penicillin (50 IU/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), and sodium pyruvate (1 mM). Gastric infiltrating lymphocytes were isolated using a modified protocol according to a reported technique (15).
Flow cytometry analysis was performed using a technique described previously (14). The CD4+ T cells were gated from the forward scatter (FSC) low versus CD4+ T cells, which correspond to the lymphocyte population according to our previous data (15). All data were analyzed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL). Mann-Whitney U tests were used for comparison between groups. Pearson correlation tests were performed for correlation analysis. All tests were two tailed, and P values of <0.05 were considered significant.
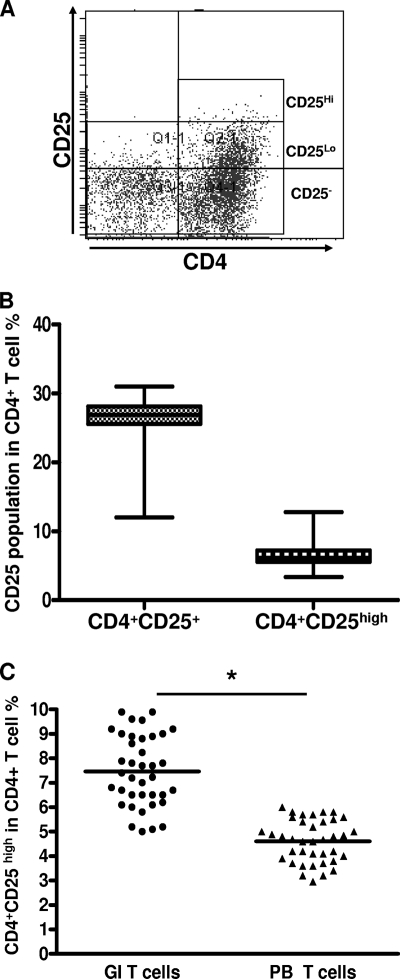
Initially, we determined the percentage of CD25+ T cells in the total CD4+ T-cell population in each of the 38 patients with H. pylori infection (Fig. 1 A). The CD4+ CD25high population represented 3.37 to 12.78% of CD4+ T cells, and the CD4+ CD25+ T cell population represented 12.00 to 31.02% of CD4+ T cells in gastric infiltrating lymphocytes (Fig. 1B). These data indicate that the frequency of CD4+ CD25high Tregs in gastric infiltrating lymphocytes (mean ± standard deviation [SD], 7.38% ± 2.02%) was significantly higher than that in peripheral blood lymphocytes (mean ± SD, 4.58% ± 1.04%) (P = 0.008) (Fig. 1C).
Fig. 1.
Frequency of gastric infiltrating/peripheral blood CD4+ CD25+ regulatory T cells (Tregs) in patients with H. pylori infection. (A) Gastric infiltrating CD4+ T cells were separated into CD25high, CD25low, and CD25− T-cell subsets, as defined by the fluorescence intensity of CD25 obtained using isotypic control antibody. (B) Frequency of gastric infiltrating CD4+ CD25high and total gastric infiltrating CD4+ CD25+ T cells in patients with H. pylori infection. Data are expressed as box plots in which the horizontal lines represent the 25th, 50th, and 75th percentiles of the measured frequencies of Tregs. (C) Percentages of gastric infiltrating (GI)/peripheral blood (PB) CD4+ CD25high Tregs in patients with H. pylori infection. The horizontal bars indicate the median percentages of Tregs. The individual frequency for each subject included in the analysis is shown. Significant of differences were calculated using the Mann-Whitney U test. *, P < 0.05.
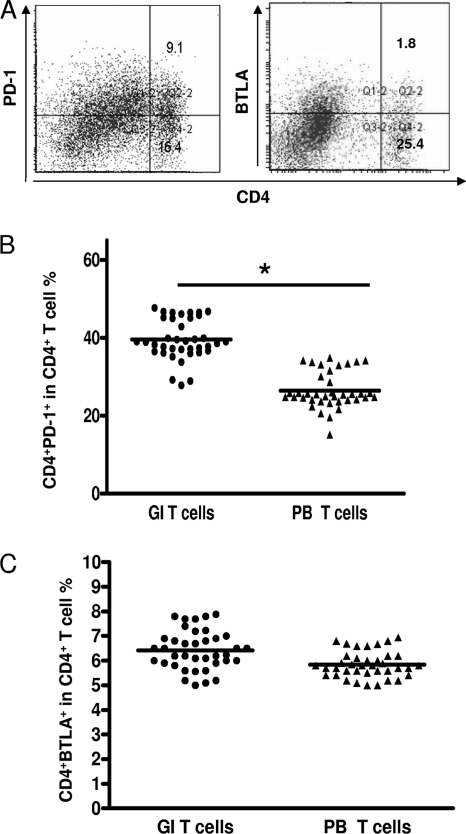
PD-1 and BTLA are important negative-immune-regulatory molecules. The percentage of CD4+ PD-1+ T cells in gastric infiltrating lymphocytes (Fig. 2 A) (mean ± SD, 39.62% ± 10.09%) was significantly higher than that in peripheral blood lymphocyte controls (mean ± SD, 26.64% ± 10.47%) (P = 0.0012) (Fig. 2B). However, the percentage of CD4+ BTLA+ T cells in gastric infiltrating lymphocytes (Fig. 2A) (mean ± SD, 6.68% ± 0.18%) did not differ significantly from that in peripheral blood lymphocytes (mean ± SD, 6.2750% ± 0.3709%) (P = 0.085) (Fig. 2C).
Fig. 2.
PD-1 and BTLA expression in gastric infiltrating/peripheral blood CD4+ T cells in patients with H. pylori infection. (A) Surface expression of PD-1 and BTLA on gastric infiltrating CD4+ T cells in one patient with H. pylori infection. (B and C) Percentages of CD4+ PD-1+ and CD4+ BTLA+ gastric infiltrating (GI)/peripheral blood (PB) T cells in patients with H. pylori infection. Horizontal bars indicate the median percentages of CD4+ PD-1+ T cells and CD4+ BTLA+ T cells. The individual frequency for each subject included in the analysis is shown. Significance was calculated using the Mann-Whitney U test. *, P < 0.05.
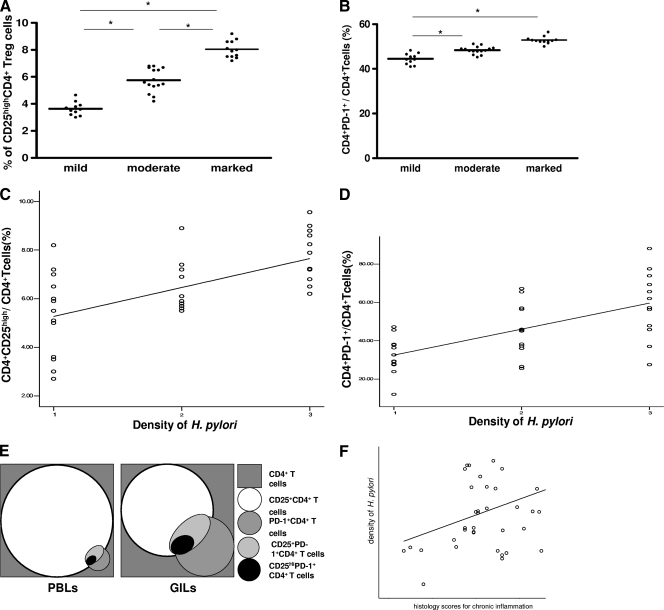
We divided the H. pylori-positive gastric biopsy samples into three groups based on their histology scores for chronic inflammation (marked, moderate, and mild). Patients with marked inflammation had a higher mean percentage of gastric infiltrating CD4+ CD25high Tregs than patients with moderate degree of inflammation (7.84% ± 1.07% versus 5.76% ± 1.23%, respectively; P = 0.0043). There were also significant differences in CD4+ CD25high Treg populations between the marked or moderate group and the mild group (Fig. 3 A). Although the level of PD-1 expression on gastric infiltrating CD4+ T cells in patients with marked inflammation was higher than that in patients with moderate inflammation, there was no significant difference in the number of gastric infiltrating CD4+ T cells expressing PD-1 between patients with marked inflammation and those with moderate inflammation (52.07% ± 11.01% versus 48.25% ± 6.77%, respectively; P = 0.0552). There were significant differences in percentage of gastric infiltrating CD4+ PD-1+ T cells between patients with marked inflammation or moderate inflammation and patients with mild inflammation (Fig. 3B).
Fig. 3.
Association between inflammation degree or density of H. pylori and gastric infiltrating CD4+ CD25high Treg frequency or gastric infiltrating CD4+ PD-1+ T cells in patients with H. pylori infection. Gastric infiltrating lymphocytes were divided into three groups based on inflammation degree (A and B). Significance of differences between inflammation degree and gastric infiltrating CD4+ CD25high Treg frequency or gastric infiltrating CD4+ PD-1+ T cells was analyzed using nonparametric Mann-Whitney U tests. The correlation between density of H. pylori and gastric infiltrating CD4+ CD25high Treg frequency (C) or gastric infiltrating CD4+ PD-1+ T-cell populations (D) was analyzed using Spearman correlation analysis. (E) Correlation of gastric infiltrating (GI)/peripheral blood (PB) T cells in patients with H. pylori infection. The correlation between density of H. pylori and histology scores for chronic inflammation (F) was also analyzed using Spearman correlation analysis. *, P < 0.05.
We measured the density of H. pylori in each patient in order to investigate whether the increase in gastric infiltrating CD4+ CD25high Tregs and gastric infiltrating CD4+ PD-1+ T cells correlated with density of H. pylori. Spearman analysis showed that the frequencies of gastric infiltrating CD4+ CD25high Tregs (r = 0.524; P = 0.0282) and gastric infiltrating CD4+ PD-1+ T cells (r = 0.431; P = 0.016) were positively correlated with density of H. pylori (Fig. 3C and D). These results suggest that an increased frequency of gastric infiltrating CD4+ CD25high Tregs and gastric infiltrating CD4+ PD-1+ T cells may be associated with a weaker immune response, leading to poor bacterial clearance in patients with H. pylori infection. We show PD1/CD25 costaining and proportions of CD25high/PD-1 double and single positive T cells to clarify the relationship in Fig. 3E. In addition, there was no correlation between histology scores for chronic inflammation and density of H. pylori in patients (P = 0.096) (Fig. 3F).
A recent study found that expression of the PD-L1 on gastric epithelial cells after H. pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells (3). In addition, H. pylori-specific CD4+ T cells were shown to accumulate in the human H. pylori-infected gastric mucosa (7). Further studies are needed to clarify the relationship between PD-1 expression and high-level expression of CD25 on gastric infiltrating CD4+ T cells. Our findings demonstrate that the frequency of gastric infiltrating CD4+ CD25high Tregs and PD-1 expression levels on CD4+ T cells were significantly increased in patients with H. pylori infection and that these variables were positively correlated with the degree of inflammation and the density of H. pylori. Tregs and PD-1, as negative regulators of immunity, may not only contribute to the persistence of chronic H. pylori infection but also be potential therapeutic targets for the treatment of chronic H. pylori gastritis patients.
Acknowledgments
This work was supported by grants from the National Science Council, Taiwan (NSC 98-2320-B-039-012-MY3), National Taiwan University, Taiwan (100F008-406), and China Medical University, Taiwan (DMR-100-012, DMR-100-018, and CMU99-NTU-05).
We declare no conflicts.
Footnotes
Published ahead of print on 11 May 2011.
REFERENCES
- 1. Aseffa A., et al. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 169:3232–3241 [DOI] [PubMed] [Google Scholar]
- 2. Belkaid Y., Piccirillo C. A., Mendez S., Shevach E. M., Sacks D. L. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502–507 [DOI] [PubMed] [Google Scholar]
- 3. Beswick E. J., Pinchuk I. V., Das S., Powell D. W., Reyes V. E. 2007. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect. Immun. 75:4334–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dixon M. F., Genta R. M., Yardley J. H., Correa P. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161–1181 [DOI] [PubMed] [Google Scholar]
- 5. Hori S., Carvalho T. L., Demengeot J. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291 [DOI] [PubMed] [Google Scholar]
- 6. Iwashiro M., et al. 2001. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. U. S. A. 98:9226–9230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lundgren A., Trollmo C., Edebo A., Svennerholm A. M., Lundin B. S. 2005. Helicobacter pylori-specific CD4+ T cells home to and accumulate in the human Helicobacter pylori-infected gastric mucosa. Infect. Immun. 73:5612–5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montagnoli C., et al. 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169:6298–6308 [DOI] [PubMed] [Google Scholar]
- 9. Parsonnet J. 1997. Molecular mechanisms for inflammation-promoted pathogenesis of cancer—the Sixteenth International Symposium of the Sapporo Cancer Seminar. Cancer Res. 57:3620–3624 [PubMed] [Google Scholar]
- 10. Parsonnet J., et al. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127–1131 [DOI] [PubMed] [Google Scholar]
- 11. Raghavan S., Suri-Payer E., Holmgren J. 2004. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scand. J. Immunol. 60:82–88 [DOI] [PubMed] [Google Scholar]
- 12. Shevach E. M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423–449 [DOI] [PubMed] [Google Scholar]
- 13. Singh B., et al. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200 [DOI] [PubMed] [Google Scholar]
- 14. Wu Y. Y., et al. 2010. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin. Exp. Immunol. 161:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Y. Y., et al. 2007. Upregulation of CCL20 and recruitment of CCR6+ gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Infect. Immun. 75:4357–4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu D., et al. 2003. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 170:394–399 [DOI] [PubMed] [Google Scholar]