Abstract
A nonspecific binding of antibodies to diphtheria toxin, especially in adult serum samples, was observed in our diphtheria-tetanus-pertussis multiplex immunoassay (DTaP4 MIA). This can be significantly reduced by the use of diphtheria toxoid, achieving a good correlation with the Vero cell neutralization test and the toxin binding inhibition assay.
TEXT
To assess vaccine-related immunogenicity in large-scale immunosurveillance and vaccine studies, several rapid and simple bead-based fluorescent multiplex immunoassays (MIA; Luminex technology) have been developed (2, 3, 5, 7, 8, 11, 12). Besides a good correlation with the enzyme-linked immunosorbent assay (ELISA), additional advantages such as increased sensitivity and sample throughput, small sample volumes and antigen quantities make the MIA a fast alternative to the ELISA. Recently, we reported the development and successful application of a MIA for the simultaneous determination of serum (and plasma) antibodies to diphtheria, tetanus, and Bordetella pertussis (DTaP4) (1, 6, 13, 15).
However, in a recent external quality assurance (EQA) study for diphtheria serology (4), organized by the diphtheria surveillance network (DIPNET), we observed that a considerable number of serum samples from the EQA panel had a higher anti-diphtheria toxin (Dtx) response in our DTaP4 MIA than in the reference assay, the Vero cell neutralization test (NT), and our ELISA-based toxin binding inhibition assay (ToBI). In contrast, the ToBI showed a high correlation (R = 0.92) with the NT reference assay in this study. Similar discrepancies between MIA and ToBI for samples from another large serosurveillance study were found. Here we describe improvements applied to the DTaP4 MIA in order to increase the specificity of the anti-diphtheria response.
Serum samples were derived from the DIPNET EQA serum panel (n = 141), which were obtained from blood donors recruited in Rome, Italy (4), and from a subset of samples (n = 96) of the second cross-sectional population-based serosurveillance study in the Netherlands (14). The DIPNET NT was performed as described by Di Giovine et al. (4). The ToBI and DTaP4 MIA were performed as previously described (6, 15), and competitive MIA experiments were performed by comparing homologous inhibition with noninhibited measurements.
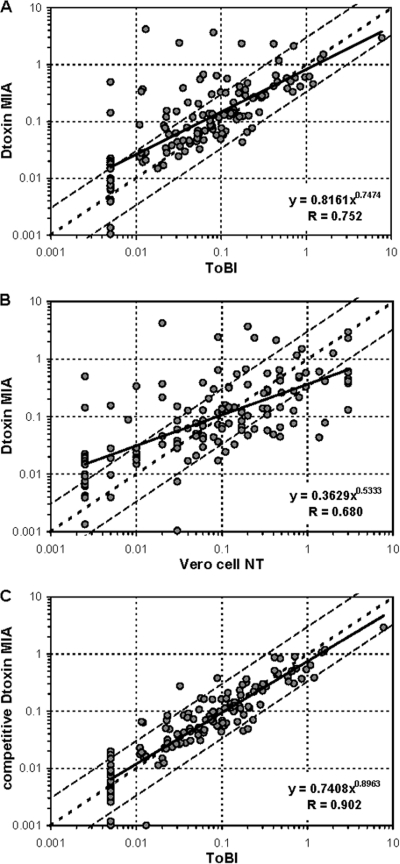
In the DIPNET EQA panel, 33/141 samples showed a ≥3-fold increase in anti-Dtx concentrations with the MIA compared to that shown with the ToBI (R = 0.752) (Fig. 1A), and 32/141 samples showed this increase with the MIA compared to that shown with the NT (R = 0.680) (Fig. 1B). A similar result for a subset of samples from the serosurveillance study was also found (16/96 samples with ≥3-fold increase; R = 0.678) (data not shown), mainly in individuals older than 20 years of age. These results were in contrast to those obtained using serum panels from vaccine studies and routine diagnostic samples, which yielded a good correlation (R ranging between 0.948 and 0.961) (15). Remarkably, as previously reported for the DTaP4 MIA (15), comparable correlations between ToBI and MIA were confirmed for antibody levels against tetanus toxin, both for the EQA sera as well as for the samples from the serosurveillance study (R = 0.964 and 0.967, respectively).
Fig. 1.
Comparison of serum antibody concentrations (IU/ml) for the DIPNET EQA panel (n = 141) as measured by the diphtheria toxin (Dtoxin) MIA and ToBI (A), the diphtheria toxin MIA and Vero cell NT (B), or the competitive diphtheria toxin MIA and ToBI (C). The regression line is indicated as a solid line, the line of identity is dotted, and 3-fold deviations are indicated as interrupted lines. Vertical and horizontal dotted lines indicate the cutoffs to determine negative (<0.01 IU/ml), intermediate (0.01 to 0.09 IU/ml), positive (≥0.1 IU/ml) sera.
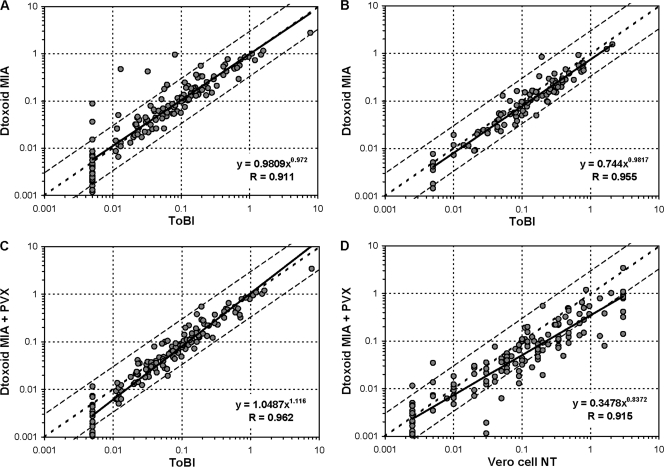
Homologous inhibition experiments revealed that for a number of samples, the anti-Dtx response consisted partly of a nonspecific binding to toxin. The competitive Dtx MIA results for the EQA panel substantially improved the correlation with the ToBI (R = 0.902) (Fig. 1C), which was confirmed by samples from the serosurveillance study (data not shown). Since a competitive MIA is laborious, antigen-consuming, and less reproducible, different approaches to improve the specificity of the Dtx MIA were explored. Simple changes to the sample buffer (introduction of Brij-35 [Sigma-Aldrich, St. Louis, MO] and antibody-depleted human serum [Valley Biomedicals, Winchester, VA] in different concentrations) did not adequately improve the results. Altering the antigen presentation by coupling poly-l-lysine-conjugated (3) diphtheria toxin to the beads did not affect the specificity and even reduced the specific response. Conjugation of the vaccine antigen diphtheria toxoid (Netherlands Vaccine Institute, Bilthoven, Netherlands) to the beads considerably improved the overall correlation of the MIA with the ToBI for both panels (R of 0.911 and 0.955, respectively) (Fig. 2 A and B). Retesting the original serum panels used in the MIA setup with these toxoid-conjugated beads also resulted in an improved correlation with ToBI (R = 0.98) and a shift of the regression line toward the line of identity. For only a small number of samples of the EQA panel, some nonspecific binding remained (9/141 samples with a ≥3-fold increase in MIA versus ToBI and in MIA versus NT), while in the subset of samples from the serosurveillance study, this number turned out to be negligible (1/96 samples). Homologous inhibition using the diphtheria toxoid could only partially resolve this remaining overestimation, indicating that this binding was not specific for toxin or toxoid.
Fig. 2.
Comparison of serum antibody concentrations (IU/ml) as measured by the diphtheria toxoid MIA and ToBI for the DIPNET EQA panel (n = 141) (A) and for a sample subset of the serosurveillance study (n = 96) (B) and by the diphtheria toxoid MIA plus PVX and ToBI (C) and the diphtheria toxoid MIA plus PVX and Vero cell NT (D) for the DIPNET EQA panel (n = 141). The regression line is indicated as a solid line, the line of identity is dotted, and 3-fold deviations are indicated as interrupted lines. Vertical and horizontal dotted lines indicate the cutoffs to determine negative (<0.01 IU/ml), intermediate (0.01 to 0.09 IU/ml), positive (≥0.1 IU/ml) sera.
An intrinsic problem of the Luminex technology for serological assays has been reported by Waterboer et al. (17), who found that human sera might contain (heterophile) antibodies (9) that directly bind to Luminex beads, resulting in a nonspecific background. The proportion of these “bead binders” seemed to depend on the origin of the sera. They found that serum preincubation with “background inhibitors” polyvinyl alcohol (PVA) (P8136: Sigma-Aldrich, St. Louis, MI) and polyvinylpyrrolidone (PVP) (PVP-360; Sigma-Aldrich) and a proprietary reagent, Super ChemiBlock (CBS-K; Chemicon International Inc.), could significantly reduce this nonspecific background. Addition of PVX (0.5% [wt/vol] PVA plus 0.8% [wt/vol] PVP) to the sample buffer of the diphtheria toxoid MIA suppressed the remaining nonspecific binding of the EQA samples in our case (MIA versus ToBI, R = 0.962; MIA versus NT, R = 0.915) (Fig. 2C and D), while a combination of PVX plus 2.5% (wt/vol) CBS-K did not further improve the correlation. The resulting correlation between the diphtheria toxoid MIA and NT was similar to that of the ToBI and NT in the EQA panel (R = 0.92) (4). Besides this good quantitative agreement, the improved MIA also showed a high qualitative agreement with the NT and ToBI, since no negative sera were identified as positive and vice versa (Table 1).
Table 1.
Qualitative agreement between the diphtheria toxoid MIA plus PVX and the ToBI and Vero cell NTa
| Result | % diagnostic agreement | No. of samples with indicated MIA result |
||
|---|---|---|---|---|
| Positive | Equivocal | Negative | ||
| ToBI | 89 | |||
| Positive | 47 | 7 | 0 | |
| Equivocal | 1 | 52 | 7 | |
| Negative | 0 | 1 | 26 | |
| NT | 76 | |||
| Positive | 49 | 22 | 0 | |
| Equivocal | 0 | 34 | 9 | |
| Negative | 0 | 3 | 24 | |
Diphtheria antitoxin levels in individual serum samples were classified as positive, i.e., having the full protective level of circulating antitoxin (≥0.1 IU/ml); equivocal, having partial protective levels of antitoxin (0.01 to 0.09 IU/ml); or negative, providing no protection (<0.01 IU/ml) (4).
Importantly, this nonspecific background was not observed for the other antigens of the DTaP4 MIA, both in the panels described here and in other studies (1, 6, 13). In fact, addition of PVX to the sample buffer of the MIA only marginally improved the correlation with the ToBI for tetanus (EQA panel), and no effect of PVX on the pertussis MIA was found. Nonspecific binding in the DTaP4 MIA seemed restricted to the diphtheria toxin response, especially in adult sera. The observation that this nonspecific binding is found mainly in adults might be caused by a difference in antibody avidity between adult sera and vaccinees. This nonspecific binding is most likely caused by changes in the structure of the toxin when conjugated to Luminex beads. Due to the covalent conjugation, some conformational changes in the structure of diphtheria toxin might occur, which make it more susceptible for nonspecific binding of antibodies. The significant reduction in nonspecific binding after conjugation of diphtheria toxoid supports the idea that formaldehyde inactivation of diphtheria toxin improves the stability of the tertiary structure (10), making it less susceptible for nonspecific binding. In addition, the use of toxoid rather than toxin further improved the conjugation reproducibility of the DTaP4 MIA. However, the optimized coverage of the bead surface with toxoid could not fully prevent “bead binding” in some specific samples.
In conclusion, the specificity of the previously described DTaP4 multiplex immunoassay for the quantitation of anti-diphtheria antibodies could be improved by conjugation of diphtheria toxoid to the beads, rather than that of toxin. Furthermore, addition of PVX to the sample buffer to reduce possible bead binding is strongly recommended.
Acknowledgments
We thank Corine Nellestijn (Laboratory for Infectious Diseases and Screening, National Institute of Public Health and the Environment, Bilthoven, Netherlands) for analytical support and Bernard Metz (Netherlands Vaccine Institute, Bilthoven, Netherlands) for kindly providing diphtheria toxoid.
Footnotes
Published ahead of print on 25 May 2011.
REFERENCES
- 1. de Greeff S. C., et al. 2010. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS One 5:e14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Voer R. M., Schepp R. M., Versteegh F. G., van der Klis F. R., Berbers G. A. 2009. Simultaneous detection of Haemophilus influenzae type b polysaccharide-specific antibodies and Neisseria meningitidis serogroup A, C, Y, and W-135 polysaccharide-specific antibodies in a fluorescent-bead-based multiplex immunoassay. Clin. Vaccine Immunol. 16:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Voer R. M., et al. 2008. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin. Vaccine Immunol. 15:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Giovine P., et al. 2010. External quality assessment for the determination of diphtheria antitoxin in human serum. Clin. Vaccine Immunol. 17:1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elberse K. E., Tcherniaeva I., Berbers G. A., Schouls L. M. 2010. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. 17:674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hendrikx L. H., Berbers G. A., Veenhoven R. H., Sanders E. A., Buisman A. M. 2009. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine 27:6530–6536 [DOI] [PubMed] [Google Scholar]
- 7. Lal G., Balmer P., Joseph H., Dawson M., Borrow R. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lal G., et al. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296:135–147 [DOI] [PubMed] [Google Scholar]
- 9. Martins T. B., Pasi B. M., Litwin C. M., Hill H. R. 2004. Heterophile antibody interference in a multiplexed fluorescent microsphere immunoassay for quantitation of cytokines in human serum. Clin. Diagn. Lab. Immunol. 11:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metz B., et al. 2007. Quality control of routine, experimental and real-time aged diphtheria toxoids by in vitro analytical techniques. Vaccine 25:6863–6871 [DOI] [PubMed] [Google Scholar]
- 11. Pickering J. W., et al. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589–596 [DOI] [PubMed] [Google Scholar]
- 12. Reder S., Riffelmann M., Becker C., Wirsing von Konig C. H. 2008. Measuring immunoglobulin g antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin. Vaccine Immunol. 15:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steens A., et al. 2010. High tetanus antitoxin antibody concentrations in the Netherlands: a seroepidemiological study. Vaccine 28:7803–7809 [DOI] [PubMed] [Google Scholar]
- 14. van der Klis F. R., Mollema L., Berbers G. A., de Melker H. E., Coutinho R. A. 2009. Second national serum bank for population-based seroprevalence studies in the Netherlands. Neth. J. Med. 67:301–308 [PubMed] [Google Scholar]
- 15. van Gageldonk P. G., van Schaijk F. G., van der Klis F. R., Berbers G. A. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 335:79–89 [DOI] [PubMed] [Google Scholar]
- 16. Reference deleted.
- 17. Waterboer T., Sehr P., Pawlita M. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309:200–204 [DOI] [PubMed] [Google Scholar]