Abstract
Influenza viruses remain a major threat to global health due to their ability to undergo change through antigenic drift and antigenic shift. We postulated that avian IgY antibodies represent a low-cost, effective, and well-tolerated approach that can easily be scaled up to produce enormous quantities of protective antibodies. These IgY antibodies can be administered passively in humans (orally and intranasally) and can be used quickly and safely to help in the fight against an influenza pandemic. In this study, we raised IgY antibodies against H1N1, H3N2, and H5N1 influenza viruses. We demonstrated that, using whole inactivated viruses alone and in combination to immunize hens, we were able to induce a high level of anti-influenza virus IgY in the sera and eggs, which lasted for at least 2 months after two immunizations. Furthermore, we found that by use of in vitro assays to test for the ability of IgY to inhibit hemagglutination (HI test) and virus infectivity (serum neutralization test), IgYs inhibited the homologous as well as in some cases heterologous clades and strains of viruses. Using an in vivo mouse model system, we found that, when administered intranasally 1 h prior to infection, IgY to H5N1 protected 100% of the mice against lethal challenge with H5N1. Of particular interest was the finding that IgY to H5N1 cross-protected against A/Puerto Rico/8/34 (H1N1) both in vitro and in vivo. Based on our results, we conclude that anti-influenza virus IgY can be used to help prevent influenza virus infection.
INTRODUCTION
Influenza viruses have been the cause of the most devastating infectious disease pandemics in the world. The Spanish flu of 1918 killed over 50 million people, with an overall mortality rate of 2.5% in the United States. Thus far (April 2011 statistics), the current highly pathogenic avian influenza (HPAI) virus strain H5N1 has infected 486 people, with a 60% mortality rate. Seasonal influenza outbreaks have also had powerful impacts on human health, with 20,000 to 40,000 people (mainly the elderly) dying each year in the United States alone. Recently, the world has experienced an outbreak of a new pandemic strain, H1N1 09, which originated in Mexico and then spread worldwide very rapidly. Over the first 6 months of the pandemic, 340,000 people became infected, with over 4,000 deaths (1.2% mortality rate of infected people), many of them young children. Until March 2010, nearly 16,000 deaths were attributed to this new strain of H1N1.
The means of control for influenza are based on antiviral drugs (neuraminidase and ion channel inhibitors) and killed or attenuated vaccines. However, both drug resistance and vaccine development and production problems pose difficulties for influenza control programs. For example, the very stable antiviral drug oseltamivir (Tamiflu) has been found in watercourses in Japan where ducks that harbor influenza A viruses come into direct contact with the drug, potentially leading to the selection of oseltamivir-resistant strains of influenza virus (2). In addition, vaccine production can be problematic, due to the difficulty of working with highly pathogenic avian influenza (HPAI) viruses, the relatively low immunogenicity of some strains, and the need to protect against the large number of strains of H5N1, H7N1, H9N3, etc., circulating in the environment. The influenza vaccine production problem has been observed in the current pandemic, where even after 6 months only a limited supply of commercial vaccine was available. Due to these problems, it is crucial to develop a new means of influenza therapy that can quickly provide protection against a wide range of influenza viruses, particularly within the first few months of the start of a pandemic.
In the present study, we tested the potential use of chicken IgY as a means of providing passive immunoprophylaxis against influenza viruses. The advantages of using IgY rather than serum or monoclonal antibodies are that it is easy and cheap to produce (5) and often recognizes highly conserved epitopes not normally seen by the mammalian immune system. Furthermore, it can be administered orally (11) and is well tolerated in humans, and one can utilize commercial laying hens that are globally available for each individual country to be able to produce and stockpile. In addition, IgY antibody can be purified, stored for lengthy periods of time even at room temperature, and formulated to provide rapid passive protection in hospitals, airplanes, and workplaces.
In order to test this hypothesis, laying hens were immunized with purified and inactivated influenza virus strains H1N1, H3N2, and H5N1 in Freund's adjuvant. Enriched IgY preparations were produced from egg yolks collected at the appropriate time period and tested for their ability to protect in vitro by hemagglutination inhibition (HI) and serum neutralization (SN) tests, as well as in vivo in a mouse model system by intranasal administration. The results of our studies demonstrate that these IgY antibodies can be used as an effective means of immunoprophylaxis for the prevention of both seasonal and pandemic influenza and that they can even cross-protect against influenza viruses of different clades and strains.
MATERIALS AND METHODS
Ethics statement.
All of the procedures used in the trial were approved by the animal ethics committees of the University of New England, Armidale, Australia and the St. Jude Children's Hospital, Memphis, TN, and by the regional ethics committee of Uppsala, Sweden.
Influenza viruses.
Purified commercial strains of H1N1 and H3N2 viruses (A/New Caledonia/20/99 and A/Hiroshima/52/2005, respectively) were kindly provided by CSL Ltd., Melbourne, Australia. The H1N1 strain used to challenge mice was A/Puerto Rico/8/34 (A/PR/8/34, or PR8). The purified Vietnamese strain of H5N1 virus (A/Vietnam/1194/04) was purchased from the NIBSC, England, and the Swedish strain of H5N1 virus (A/tufted duck/Sweden/V789/06 [SVA 789/06]) was isolated at the Swedish National Veterinary Institute. The Swedish strain was passaged in eggs, inactivated using β-propiolactone, and formulated individually and in combination with the other 3 viral strains in complete and incomplete Freund's adjuvant. Complete viral inactivation was confirmed by passage in embryonated eggs, and strain purity was confirmed by PCR.
Immunization of laying hens.
Whole inactivated H1N1, H3N2, and H5N1 viruses were suspended in phosphate-buffered saline (PBS), and the hemagglutinin (HA) protein concentrations were determined using the single radial immunodiffusion (SRiD) assay. The viral suspensions were then diluted to the appropriate concentration such that 0.5 ml contained the desired amount of viral protein. An equal volume of Freund's adjuvant was added, and the suspension was mixed by pushing it up and down in a 19-gauge needle attached to a 5-ml syringe until the emulsion was stable. Commercial light-breed laying hens maintained in biological containment level 1 (BCL1) or BCL2 housing, for seasonal or H5N1 strains, respectively, were immunized twice by injecting 1.0 ml of the emulsion into the breast muscle of each of the influenza virus strains emulsified in Freund's complete (first immunization) or incomplete (second immunization, administered 4 weeks after the first) adjuvant. The hens were bled on day 1 of the experiment (negative-control serum), as well as 1 week and 2 months after the second immunization. Eggs were collected commencing 2 weeks postimmunization and were stored at 4°C until sufficient numbers were obtained.
IgY antiviral reactivity.
Sera from all of the individual hens as well as pools of 5 to 10 egg yolks from each group were tested for the relative level of reactivity of the IgY against the viral antigen used for immunization by enzyme-linked immunosorbent assay (ELISA). Briefly, ELISA plates were coated with viral antigen at a concentration of 1 μg per well, washed with PBS plus 0.3% Tween 20, and blocked with PBS plus 3% milk powder. The plates were then incubated at 37°C for 2 h with yolk IgY diluted 1:500, washed, and then incubated with the conjugate (rabbit anti-chicken IgY-horseradish peroxidase) for 2 h at 37°C. The plates were then washed, and substrate was added. Finally, the plates were scanned and read at 405 nm.
IgY extraction and concentration.
Ten eggs from each group were collected at the time when the IgY antiviral reactivity was high, as determined by ELISA. They were cracked open, and the yolks were collected, rinsed with PBS, and then punctured using a scalpel blade. The yolk contents from each group (hens immunized with H1N1, H3N2, or H5N1 alone or hens immunized with H1N1 and H3N2 together) were pooled, and 9 volumes of super Q water was added. The solutions were then mixed thoroughly and placed at 4°C overnight. The following day, they were centrifuged at 1,000 × g for 30 min at room temperature, and the upper water layer was siphoned from the top, filtered through a 0.45- or 0.2-μm filter, and stored at 4°C.
The total water-extracted protein concentration was determined by the Bradford assay using a kit purchased from Sigma. The total IgY concentration was determined by ELISA using a kit purchased from Hycult Biotechnology b.v., Uden, Netherlands, and the test was performed according to the manufacturer's instructions. The absorbance was measured at 450 nm. Samples were compared to a standard curve created using a four-parameter fit by Softmax Pro (Molecular Devices, CA).
In vitro HI and serum neutralization assays.
The hemagglutinin inhibition (HI) assay was carried out as previously described (4). Briefly, a serial dilution of the water-extracted IgY antibodies was added to 4 hemagglutinating units of virus in a microtiter plate, the mixture was incubated for 45 min, and 0.5% chicken erythrocytes were added. The plate was then gently rocked, and after 30 min at room temperature, the agglutination was visualized. The reciprocal of the IgY dilution that inhibited hemagglutination was recorded for the various samples tested.
The serum neutralization (SN) assay was carried out as follows. Briefly, IgY was filter sterilized, inactivated at 56°C, and serially diluted from 1/2 to 1/256 in a microtiter plate. Then, 100 50% tissue culture infective doses (TCID50) of H1N1, H3N2, or H5N1 virus was added, and the plates were incubated for 1 h at room temperature. An MDCK cell suspension in Eagle's minimum essential medium (EMEM) containing 10% fetal bovine serum (FBS) was then added to the wells and incubated at 37°C for 3 to 5 days. Regarding H1N1, the MDCK cells were cultivated to confluence in a separate plate, the cell culture medium was discarded, and the different serum dilutions were added to the cells. After a 1-h adsorption at room temperature, EMEM containing 2.5 μg of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Worthington, NJ) was added. Positive (virus only), negative (cells alone), and normal chicken serum/IgY controls were used. The titer is expressed as the reciprocal of the highest dilution that gives 50% neutralization of the virus.
In vivo challenge trials in mice.
Mice of BALB/c and C.B-17 strains were obtained from Bomholtsgaard, Denmark, and were bred and maintained at the animal facilities of SVA. Mice of age 8 to 10 weeks were used. Challenge trials in mice were carried out as follows. Groups of anesthetized BALB/c or C.B-17 mice (4 to 6 weeks old, mean weight of 18 to 20 g) were treated by intranasal administration of either a mixture of 50 μl of water-extracted IgY plus 50 μl of 103 TCID50 H5N1 virus or 30 to 100 μl of IgY by itself. The mice that received only the IgY intranasally were then challenged 1 h later intranasally with either H1N1 PR8 virus (104, 106, or 108 TCID50 in a volume of 50 μl) or H5N1 virus (103 TCID50 in a volume of 50 μl). Control mice were given either IgY that had no antiviral titer or PBS. Mice were then monitored daily for weight gain and mortality for a period of 8 to 16 days.
A postmortem on all of the H1N1 PR8-challenged mice was carried out at the termination of the trial. The mice were sacrificed by inhalation of CO2, and a blood sample from the heart for serology was taken immediately after death. At necropsy, the organs were examined for gross changes, and samples and tissue specimens for PCR and histopathology were taken from the lungs and the nasal mucosa. The instruments were thoroughly heated between sampling sites and animals to avoid contamination. Samples for PCR analysis consisted of pieces of different lung lobes. After the surface of the bones was disinfected, the nasal mucosa was cut open using scissors and sampled by thorough swabbing. For histology, tissue specimens were fixed in 10% neutral buffered formalin overnight, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. The nasal tissues were decalcified for 24 h before the histological preparation. A Nikon E600 microscope (Tokyo) and a Nikon DXM1200 (Tokyo) were used for the histological examinations and photography.
The qualitative determination of H1N1 PR8 influenza virus nucleic acid in the respiratory tract, lung, brain, and feces was performed using an AgPath-ID one-step real-time transcriptase PCR (RT-PCR) kit (Applied Biosystems). Tissue samples were homogenized in a Tissue Lyser (Qiagen), and extraction of virus nucleic acid was done with a Nordiag Magnatrix 8000+ extraction robot using a Viral NA kit (Nordiag). The following PCR primers were used: PaninflAForward, GGGTAGATAATCACTCACTGAGTG; PaninflAReverse, CTCTGATYTCAGTNGCATTCTG; and pan-influenza virus NP, 6-FAM-ATGGCGTCTCAAGGCACCAAACG-BHQ-1 (fluorescence-labeled probe with black hole quencher).
Positive virus controls and negative Tris-EDTA (TE) buffer controls were used in the real-time RT-PCR.
Statistical analysis.
The ELISA was analyzed by comparing the average optical density at 405 nm (OD405) values of the groups using the Student t test. The weight differences between the groups of mice treated with IgY antibodies were analyzed using analysis of variance (ANOVA).
RESULTS
Immunization of laying hens with influenza virus antigens.
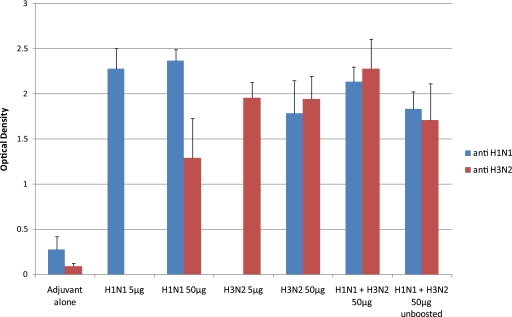
Light-breed laying hens were immunized twice intramuscularly and were bled on the day of the first immunization as well as 1 week and 2 months after the second immunization. Eggs were collected commencing 3 weeks after the first immunization and continuing weekly until 2 weeks after the second immunization. ELISA was carried out with the sera in order to assess the immune response of the individual hens, and the results are summarized in Fig. 1. As can be seen, the average levels of reactivity were high, with an excellent consistency in immune response between hens as reflected by low standard deviations. Furthermore, there was no significant difference in antibody response (based on the OD405 reading) between the serum samples from hens immunized with 5 or 50 μg of either H1N1 or H3N2 viral antigen. Serum IgG produced in hens sham immunized with adjuvant alone was used as a negative control and had very low titers, with OD405 values in the range of 0.1 to 0.3. In addition, 100% of the hens immunized with viral antigen showed strong reactivity with the homologous viral antigens. Interestingly, sera produced against either H1N1 or H3N2 virus also reacted strongly with the heterologous strain. We also found that the hens that received a mixture of H1N1 and H3N2 viral antigens responded strongly to vaccination, reaching a level of response similar to that obtained by using each antigen separately (although it was significantly slightly lower than that for H1N1 alone and significantly higher than that for H3N2 alone). Finally, we found that even after a single immunization with 50 μg of the mixture of H1N1 and H3N2 viruses (Fig. 1, unboosted), there was a very significant antibody response against both viruses induced in all of the immunized hens.
Fig. 1.
Serology on serum samples taken from hens immunized with H1N1 and H3N2 influenza viruses. This graph shows the ELISA results measured as the average OD405 ± standard deviation of individual hen serum samples from the groups immunized with a single viral strain, taken 2 weeks after the second immunization. The graph also shows the ELISA results from the groups immunized with H1N1 and H3N2 viruses in combination, using serum samples taken after 2 immunizations (H1N1 + H3N2 50 μg) or those taken after one immunization (H1N1 + H3N2 50 μg unboosted). The number of samples for each group was as follows: negative-control adjuvant alone, 16 serum samples; H1N1 5 μg and 50 μg, 15 serum samples each; H3N2 5 μg, 9 serum samples; H3N2 50 μg, 12 serum samples; H1N1 + H3N2 50 μg, 10 to 12 serum samples; H1N1 + H3N2 50 μg unboosted, 4 serum samples.
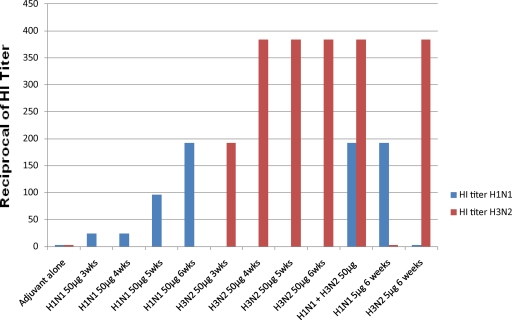
The hemagglutinin inhibition (HI) test was used to measure the agglutinating titer of the egg IgY (present in water-extracted egg yolks) from immunized hens at various time points postimmunization, and the results are summarized in Fig. 2. As can be seen, the HI titer in the groups of egg yolks tested using the homologous viral strain started to increase at 3 weeks after the first injection for both H1N1 and H3N2 and reached a high level 2 weeks after the second injection, i.e., 1:192 for H1N1 and ≥1:384 for H3N2. If one takes into account the IgY concentrations, it is apparent that both preparations induced a very strong immune response in the hens even when used at a dosage level of 5 μg per immunization. As seen in the ELISA results, the combined viral antigens worked equally well and induced titers that were the same as those seen when either antigen was used alone. Finally, there was no significant level of cross-strain inhibition between IgYs to H1N1 and H3N2 in spite of the cross-reactivity seen by ELISA.
Fig. 2.
Hemagglutinin inhibition titers in eggs from hens immunized with H1N1 and H3N2 viruses. This graph shows the HI titers of egg yolks (pools of 5 egg yolks per group) from the various vaccinated groups of hens. The first 8 groups of egg yolks were from eggs taken at various times after the first immunization. The rest of the groups were from eggs taken at 6 weeks after the first immunization. The results are plotted as the reciprocal of the HI titer.
For testing the immunogenicity of the H5N1 influenza virus, groups of 3 light-breed, commercial laying hens were immunized twice intramuscularly with 5, 10, or 50 μg of inactivated H5N1 virus (A/Vietnam/1194/04) as described in Materials and Methods. Serum samples from each of the individual hens were taken 2 weeks postboosting and were tested for antibody titer to H5N1. As we had found for H1N1 and H3N2, all of the hens immunized with 5, 10, or 50 μg of viral antigen had high and consistent antibody titers, with 6/9 having titers of >1:256 based on a serum neutralization test (the other 3 had titers of 1:64 or 1:128, similarly to the positive-control serum, which had a titer of 1:64) and with all of the negative-control prebleed sera having background SN titers of <1:4 (data not shown). We therefore pooled the serum and yolk samples from each immunized group to test for cross-reactivity against different viral strains by HI (Table 1).
Table 1.
HI titers of serum IgG and egg yolk IgY raised against H5N1 viral strain (A/Vietnam/1194/04)
| Sample type and group | Total IgY concn (mg/ml)a | HI titer to strain: |
||
|---|---|---|---|---|
| H5N1 SVA 789/06 | H1N1 A/PR/8/34 | H5N1 (adjusted)b | ||
| Hen serum | ||||
| Adjuvant control | <1:8 | |||
| H5N1 5 μg | 8.0 | 1:1,024 | 1:3,673 | |
| H5N1 10 μg | 13.0 | 1:1,024 | 1:2,260 | |
| H5N1 50 μg | 28.7 | 1:4,096 | 1:4,096 | |
| Egg yolk | ||||
| Adjuvant control | 0.4 | <1:8 | 1:2 | |
| H5N1 5 μg | 0.43 | 1:64 | 1:4,271 | |
| H5N1 10 μg | 0.61 | 1:32 | 1:1,505 | |
| H5N1 50 μg | 0.83 | 1:128 | 1:64 | 1:4,426 |
These concentrations were determined in serum and yolk samples taken 2 to 3 weeks postboosting.
Adjusted for IgY concentration. The HI titer to H5N1 is adjusted for antibody concentration by normalizing all of the results to the IgG concentration in the serum sample from hens immunized with H5N1 at 50 μg (28.7 mg/ml).
As can be seen, the HI titer was very high in the hens immunized with the different amounts of viral antigen, with the sample from hens immunized with 50 μg having the highest HI titer in both the pooled serum and yolk samples. However, taking into account the IgY concentration found in the serum and yolk (there is a 10-fold dilution in the egg yolk water extraction process) at the various dosage levels, the anti-H5N1 titers were high at all 3 dosage levels, indicating that even 5 μg of antigen is sufficient to induce a good immune response, as seen using H1N1 and H3N2 viruses (see the results that were adjusted for antibody concentration by normalizing all of the results to the total IgG concentration in the serum sample from hens immunized with H5N1 at 50 μg). Finally, samples taken from the same hens 2 months postboosting had a titer that was the same as that seen 1 to 2 weeks postboosting (data not shown).
In terms of the type specificity of these antibodies, egg IgY was tested from hens that were immunized with 50 μg of H5N1 or sham immunized with adjuvant alone, and titers against both the H5N1 (SVA 789/06) Swedish strain and the H1N1 (PR8) strain of virus were tested by HI (Table 1). Our results showed that there was a strong HI titer against the heterologous PR8 strain of H1N1, which was about half of the titer seen for the H5N1 viral strain used, whereas control IgY from hens immunized with adjuvant alone had no significant HI titer against either strain.
In vitro virus neutralization.
The serum neutralization (SN) test was used to determine the in vitro neutralizing capabilities of the serum and yolk samples from hens immunized with H1N1 and H5N1 viruses. Pooled yolk samples were used in this test, since as shown in Fig. 1, there was very low variability between samples. The results from these experiments are summarized in Table 2, where it can be seen that while the H1N1 IgY raised against the A/New Caledonia/20/99 strain of H1N1 was effective in inhibiting the Puerto Rican strain of H1N1 (A/PR/8/34), it did not show any inhibitory effect on the H5N1 strain of virus.
Table 2.
SN titers of egg yolk IgY raised against H1N1, H3N2, and H5N1 viruses on heterologous viral strains and types
| Groupa | Titer |
|
|---|---|---|
| A/PR/8/34 (H1N1) | SVA 789/06 (H5N1) | |
| Adjuvant control | <1:4 | <1:4 |
| H1N1 5 μg | 1:32 | <1:4 |
| H1N1 50 μg | 1:4 | <1:4 |
| H3N2 5 μg | <1:4 | <1:4 |
| H3N2 50 μg | <1:4 | <1:4 |
| H1N1 + H3N2 50 μg | 1:8 | <1:4 |
| H5N1 50 μg | 1:32 | ≥1:256 |
| H1N1 pos control | 1:16 | <1:4 |
| H3N2 pos control | <1:4 | <1:4 |
| H5N1 pos control | 1:4 | 1:64 |
The following positive-control serum antibodies (pos control) were used in the assay: duck antibodies to H1N1 (A/Dk/Alb/35/76), turkey antiserum to H3N2 (A/Tky/Eng/69), and chicken antiserum to H5N1 (A/Ck/Scot/59).
We found that the pooled yolk sample from hens immunized with 5 μg of H1N1 virus had a higher SN titer against a heterologous strain of H1N1 than the yolk sample from hens immunized with 50 μg. This result is in contrast to the HI results using the same yolk samples, where similar titers were observed at both dosage levels when tested against the homologous H1N1 viral strain (Fig. 2). Finally, the negative-control IgY from sham-immunized hens (adjuvant control) as well as IgY raised against the H3N2 virus (which had a high HI titer against the H3N2 strain, A/Hiroshima/52/2005, used for immunization [data not shown]) showed no significant SN titer against either the H1N1 or the H5N1 virus.
The results using IgY raised against the Vietnamese strain of H5N1 showed that it was highly effective in neutralizing the Swedish strain of H5N1, while IgY to the H1N1 virus had no effect on H5N1 (Table 2). Unexpectedly, IgY to H5N1 also neutralized the H1N1 PR8 strain. Positive-control serum to H5N1 also was found to slightly cross-react with this strain of H1N1, while the positive-control duck serum to H1N1 did not inhibit H5N1 (Table 2). Finally, positive-control serum to H3N2 did not inhibit either the H1N1 or the H5N1 virus.
In vivo protection in a mouse model system.
Mouse trials were next performed in order to test anti-influenza virus IgY in H1N1- and H5N1-infected mice. In the first trial, with homologous challenge infection, IgY to H5N1 (A/Vietnam/1194/04) and H5N1 challenge virus (A/Vietnam/1203/04, 103 TCID50) were mixed together and administered intranasally to groups of 5 BALB/c mice. It was found that whereas 5 out of the 5 mice that were used as controls and received the mixture with only PBS all died by day 10, the mice that received the mixture with IgY to H5N1 all survived, with good weights both during and at the end of the experiment (data not shown). In the next experiment, the IgY was first administered intranasally to mice and the challenge infection was given 1 h postimmunization. As shown in Table 3, the mice given IgY to the H5N1 virus were all protected throughout the experiment and survived with good weight gain until day 16 when the experiment was terminated. In contrast, 5 out of the 5 control mice all were euthanized or died by day 10 and lost very large amounts of weight commencing day 3 postchallenge. Finally, IgY administered either 1 day prior to or 1 day after lethal challenge with H5N1 provided only partial protection (40% and 20% survival, respectively [data not shown]). These results indicate that complete protection requires the IgY to be administered shortly before challenge.
Table 3.
In vivo mouse trial using IgY to H5N1 and protection based on weight gain and mortalitya
| Group | Weight gain or loss (%) | Day measured | Mortality |
|---|---|---|---|
| PBS control | −30.1 | 7 | Euthanized on day 7 |
| −13.8 | 7 | Died on day 10 | |
| −22.3 | 7 | Euthanized on day 7 | |
| −11.1 | 3 | Died on day 7 | |
| −6.7 | 3 | Died on day 6 | |
| H5N1 IgY | +0.1 | 7 | Survived to day 16 |
| +3.6 | 7 | Survived to day 16 | |
| +2.5 | 7 | Survived to day 16 | |
| +6.9 | 7 | Survived to day 16 | |
| +2.5 | 7 | Survived to day 16 |
Results from individual BALB/c mice given either PBS or IgY to H5N1 and then challenged 1 h later.
We next wished to establish a good model system to be able to test the ability of the IgYs against H1N1 and H5N1 viruses to block or inhibit the pathological effect of challenge infections with H1N1. A variety of mouse breeds were initially tested in order to choose the one that is highly susceptible to infection with the Puerto Rican strain of H1N1 (A/PR/8/34). We found that both C.B-17 and BALB/c mice were highly susceptible to infection, with clear pathogenicity of virus seen, as measured by weight loss of 5 to 25% at a challenge dosage level of 104 TCID50 (10 times the challenge dose used for H5N1, due to its lower pathogenicity), similar to that seen using H5N1 to challenge BALB/c mice.
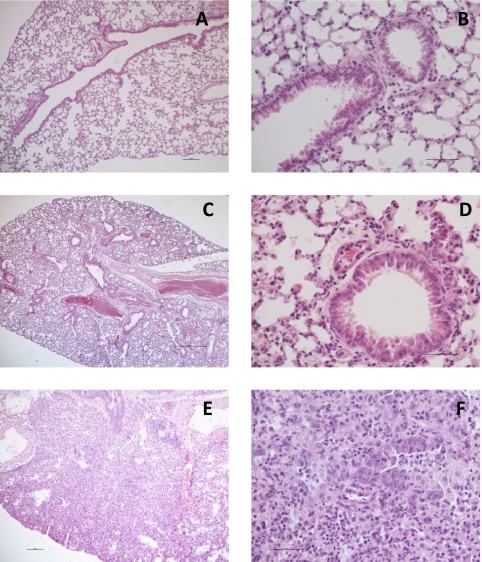
Postmortem histopathology and PCR tests were performed on the challenged mice, and the results are shown in Fig. 3. As can be seen, the C.B-17 control mice challenged with 104 TCID50 PR8 virus had severe lesions in the lungs (Fig. 3E and F) and tested positive by PCR. Based on these results, we concluded that BALB/c or C.B-17 mice with a challenge dose of 104 TCID50 of virus or higher can be used as a model system in which to test the IgY antibodies.
Fig. 3.
Histopathology on different mouse strains challenged with H1N1 PR8 virus with or without pretreatment with IgY to H5N1. (A and B) C.B-17 uninfected control; normal lungs. (C and D) C.B-17 mouse treated with IgY against H5N1 and challenged with 104 TCID50 PR8. (A and C) The lungs do not show any inflammatory lesions. (B and D) Intact bronchiolar mucosa and alveoli are visible. (E and F) C.B-17 mouse treated with control IgY and challenged with 104 TCID50 PR8. (E) Severe pulmonary inflammation. (F) The bronchiolar mucosa is damaged and infiltrated with leukocytes, predominantly polymorphs and macrophages. Leukocytes in the lumen and the peribronchial tissues are also visible. Magnifications, ×60 (A), ×240 (B), ×24 (C), ×240 (D), ×60 (E), and ×240 (F).
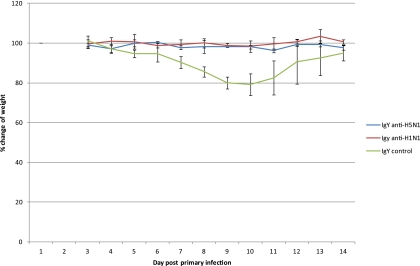
In order to test the prophylactic effect of the IgY antibodies against H1N1, BALB/c mice in groups of 4 were intranasally immunized with 100 μl (80 μg) of the antibodies to the New Caledonian strain of H1N1, and 1 h later they were challenged with the H1N1 PR8 strain (A/PR/8/34) in 50 μl. Our results (Fig. 4) showed that starting from 6 days postchallenge there was a reduction in weight of about 20% in 4/4 mice in the control group given IgY extracted from control eggs, while IgY to H1N1 protected all 4 of the mice against weight loss throughout the experiment at an H1N1 challenge dosage level of 104 TCID50 (significant at a P value of ≤0.05). Furthermore, based on the PCR test, the two mice given the IgY to H1N1 at a challenge dosage level of 104 TCID50 had no detectable amount of virus in their lungs and airways, while the two controls were positive in both the lungs and the airways. These results demonstrate the partial cross-protective effect of the IgY to one strain of H1N1 against severe challenge infection with a second strain of H1N1 virus in vivo.
Fig. 4.
Studies on mice immunized intranasally with IgY to H1N1 or H5N1 and challenged with a sublethal dose of H1N1 virus. This graph shows the average percent weight gain or loss ± standard deviation in BALB/c mice treated with IgY to either H1N1 or H5N1 virus and challenged with 104 TCID50 of the H1N1 PR8 virus over a period of 14 days postinfection. All of the points shown for the IgY control group between 6 and 11 days corresponded with a significant level of weight loss (P < 0.05 or P < 0.01).
Tests were then performed to determine if antibodies to H5N1 could cross-protect against H1N1 in the mouse model system. We found that at challenge dosage levels of 104 and 106 TCID50 H1N1 virus in BALB/c mice, IgY to H5N1 protected mice against weight loss throughout the trial (Fig. 4 shows weights at the 104 dosage level, in which 4 out of 4 mice had no significant weight loss), which was statistically significant (P ≤ 0.05 for 104 TCID50 and P ≤ 0.001 for 106 TCID50), and, as shown in C.B-17 mice, reduced the pathology in the lungs and nasal passage (Fig. 3C and D).
PCR data confirmed the protective effect of IgY (Table 4), where we found that in cases where the histopathology was negative, both the lungs and the airways of BALB/c or C.B-17 mice immunized with IgY to H5N1 were H1N1 virus negative. In cases in which the histopathology was mild in mice treated with IgY to H5N1 (i.e., partial protection), we found the presence of virus in the airways but not in the lungs. In the case of mice sham immunized with either PBS or control IgY, we always found severe and extensive histopathology, with PCR results showing the presence of virus in both the lungs and the airways. These results demonstrate the ability of IgY to H5N1 to cross-protect mice against H1N1 at a relatively high challenge dosage level in vivo.
Table 4.
In vivo mouse trial showing a correlation between histopathology and PCR resultsa
| Mouse no. | Treatment group | Challenge dose (TCID50) | PCR result |
Histopathology result | |
|---|---|---|---|---|---|
| Lungs | Airways | ||||
| 1 | IgY to H5N1 | 104 | 0 | 0 | 0 |
| 2 | IgY to H5N1 | 106 | 0 | 0 | 0 |
| 3 | IgY to H5N1 | 106 | 0 | Positive | + |
| 4 | Control IgY | 104 | Positive | Positive | +++++ |
| 5 | Control IgY | 106 | Positive | Positive | +++++ |
| 6 | PBS | 104 | Positive | Positive | +++++ |
| 7 | PBS | 106 | Positive | Positive | +++++ |
Typical results for mice from the different groups used in the trial are represented. 0, no detectable virus by PCR and no histopathology; +, mild histopathology with perivascular infiltrates; +++++, severe histopathology with bronchopneumonia.
DISCUSSION
As a potential means of complementary defense against influenza viruses, we have been working toward the use and application of IgY in the control of both seasonal (H1N1 and H3N2) and zoonotic (H5N1) influenza viruses. We have shown in this study that the three major strains of influenza virus we have tested (H1N1, H3N2, and H5N1) are highly immunogenic in hens (when tested alone or in combination) and can induce a very-high-titer, long-lasting humoral immune response of at least 2 months without the need for additional boosts. In other studies, it was shown that hens maintain a high antibody titer against a variety of antigens used for immunization for atleast 3 to 4 months (16). Furthermore, in this study we tested commercially available viral strains used in the production of seasonal influenza vaccines for the U.S. market. Thus, we have demonstrated the feasibility of using this approach to produce very large quantities of IgY for the potential prevention of influenza.
The IgY was highly effective in vitro both in the hemagglutination inhibition test and by serum neutralization of live influenza virus. Our results showed that inhibition was observed when the IgY was tested against the homologous virus used for immunization of the hens as well as for heterologous strains. Of particular interest was the finding that IgY raised against H5N1 could neutralize an H1N1 virus but that when IgY to H1N1 was tested against H5N1 it did not show cross-inhibition, while it was able to neutralize a heterologous strain of H1N1. Furthermore, IgY to H5N1 showed a substantial HI titer against H1N1 virus, indicating that the in vitro inhibitory effect may be mediated via cross-reactivity of the hemagglutinin proteins from the two different types of viruses. These results demonstrate that chickens immunized with whole inactivated H5N1 (Vietnamese strain) recognized conserved epitopes present in both virus types (H5N1 and H1N1). In contrast, IgY raised against the H1N1 whole inactivated virus recognized only type-specific protective epitopes that are capable of protecting only against different strains of H1N1 but not against H5N1. Further work is needed to elucidate the molecular basis of this cross-protection.
We established a mouse model system for testing the IgY preparations against H1N1. The PR8 strain was chosen for this study (due to its relatively high pathogenicity in mice compared to that of other strains of H1N1), and we found that it was very pathogenic in both BALB/c and C.B-17 mice.
Our in vivo results also showed that IgY to H5N1 could protect mice against lethal infection with H5N1 when administered intranasally either at the same time as or 1 h prior to infection. In addition, we were able to show that IgY to the Vietnamese strain of H5N1 can cross-protect in vivo against a pathogenic strain of H1N1, confirming our in vitro data. This result gives hope to the possibility of developing a means of immunoprophylaxis for a wide range of influenza viruses using this approach.
IgY antibodies have previously been shown to be useful in providing passive protection against a variety of oral, intestinal, and respiratory pathogens (11, 12). Recently, results have shown that in patients suffering from cystic fibrosis (CF), IgY raised against Pseudomonas aeruginosa (a major cause of chronic lung infection in CF patients) can provide significant protection against colonization of the respiratory tract by this pathogenic organism (6). In addition, this treatment has been shown to be safe in humans, probably due to the fact that there are no Fc receptors for chicken IgY and there are no antibodies produced against IgY in people that are treated (1). These encouraging results indicate that IgY can be used commercially as an oral treatment to prevent respiratory infections, including that caused by influenza viruses.
A few reports have described work using sera raised against avian influenza viruses from a variety of animal sources for passive immunotherapy. One very promising study has shown the protective effect of horse antiserum raised against H5N1 (8). High levels of protection against lethal challenge with the virus was found using the F(ab′)2 fragments of the antisera. In addition, another study showed that sheep antisera raised against a single strain of H5N1 can cross-protect against a variety of H5N1 strains from other clades of viruses (13). However, only in chicken IgY samples did we observe this cross-protection between H5N1 and H1N1 viruses.
In a very recent report by Nguyen et al. (9), it was shown that IgY to H5N1 isolated from eggs available in supermarkets in Vietnam can protect against H5N1 and H5N2 viruses in mice. In addition, they showed that IgY antibodies to A/PR/8/34 (H1N1) can protect mice against a homologous lethal challenge infection. These results support the data we have presented here. However, in that study, no cross-protection was observed using IgY against H5N1 in mice challenged with H1N1. Our results, which showed good cross-protection between H5N1 and H1N1 both in vitro and in vivo, may be explained by the administration of the IgY antibodies at the same time as or 1 h prior to challenge of the mice (compared to 6 h prior to challenge in the study of Nguyen et al.) and the use of a sublethal dosage of H1N1 virus.
It has been reported that there exist highly conserved epitopes in the stem region of the HA molecule that may become a target for a “universal flu vaccine” (3, 7, 10, 14, 15). Humanized monoclonal antibodies raised against these conserved epitopes from a single influenza virus strain were shown to provide strong cross-protection against heterologous strains (13). These results provide hope toward producing either antigens or antibodies that can be used for active or passive immunization against a large variety of influenza viruses. However, the cost of production and administration of monoclonal antibodies for protection against influenza on a global scale would be problematic and perhaps economically prohibitive.
We used a simple water extraction and filtration method to isolate the enriched, sterile IgY from egg yolks. We found that 5 μg of viral antigen can be used to induce a very high HI and SN titer in the serum and yolk of immunized hens. In addition, as little as 80 μg of IgY can be used to prevent infection by 104 TCID50 of H1N1 virus. Since a single egg contains 100 mg of total IgY and each hen produces 150 eggs during the laying period, it is clear that very large quantities of protective IgY can be produced at a low cost. This IgY, raised against a variety of seasonal and pandemic influenza virus strains, can be formulated as a nasal, oral, or aerosol spray to provide rapid protection of individuals and the environment (schools, hospitals, airplanes, etc.) as well as stockpiled on a large scale for global use in the time of a pandemic. Furthermore, in the event that entirely new viral strains appear, IgY can be produced within 6 weeks of vaccinating the hens. Finally, egg IgY is well tolerated in humans and has been used for long-term treatments of oral and intestinal pathogens without any serious side effects (5, 6). Thus, this approach has great potential in helping to control highly pathogenic influenza viruses at the source of infection before they spread worldwide and cause a pandemic.
ACKNOWLEDGMENTS
We thank Emma Ball and Peter Kipnis of CSL Limited, Melbourne, Australia, for providing us with the stocks of H1N1 and H3N2 viruses.
This research was supported by Epizone and by the Swedish Civil Contingencies Agency (MSB).
Footnotes
Published ahead of print on 25 May 2011.
REFERENCES
- 1. Carlander D., Stalberg J., Larsson A. 1999. Chicken antibodies a clinical chemistry perspective. Uppsala J. Med. Sci. 104:179–189 [DOI] [PubMed] [Google Scholar]
- 2. De Jong M. D., et al. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353:2667–2672 [DOI] [PubMed] [Google Scholar]
- 3. Gerhard W., Mozdzanowska K., Zharikova D. 2006. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 12:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann E., Lipatov A. S., Webby R. J., Govorkova E. A., Webster R. G. 2005. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proc. Natl. Acad. Sci. U. S. A. 102:12915–12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karlsson M., Kollberg H., Larsson A. 2004. Chicken IgY: utilizing the evolutionary advantage. Worlds Poult. Sci. J. 60:341–347 [Google Scholar]
- 6. Kollberg H., et al. 2003. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase 1 feasibility study. Pediatr. Pulmonol. 35:433–440 [DOI] [PubMed] [Google Scholar]
- 7. Kubota-Koketsu R., et al. 2009. Broad neutralizing human monoclonal antibodies against influenza virus from vaccinated healthy donors. Biochem. Biophys. Res. Commun. 387:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu J., et al. 2006. Passive immunotherapy for influenza A H5N1 virus infection with equine hyperimmune globulin F(ab′)2 in mice. Respir. Res. 7:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen H. H., et al. 2010. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS One 5:e10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okuno Y., Isegawa Y., Sasao F., Ueda S. 1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67:2552–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reilly M. R., Domingo R., Sandhu J. 1997. Oral delivery of antibodies: future pharmacokinetic trends. Clin. Pharmacokinet. 32:313–323 [DOI] [PubMed] [Google Scholar]
- 12. Shin J.-H., et al. 2002. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin. Diagn. Lab. Immunol. 9:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simmons C. P., et al. 2007. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 4:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sui J., et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Throsby M., et al. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wallach M., et al. 1995. Eimeria maxima gametocyte antigens: potential use in a subunit maternal vaccine against coccidiosis in chickens. Vaccine 13:347–354 [DOI] [PubMed] [Google Scholar]