Abstract
A flow cytometry-adapted fluorescent antibody to membrane antigen (FAMA) assay to detect IgG antibodies against varicella-zoster virus (VZV) was developed and tested in 62 serum samples, showing 90.32% accuracy obtained from a receiver operating characteristic (ROC) curve with a 0.9125 (95% confidence interval [CI], 0.829 to 1.00) area below the curve compared to the result with standard FAMA.
TEXT
Fluorescent antibody to membrane antigen (FAMA) is considered to be the gold standard for assessing immunity to varicella and detects seroconversion after vaccination or natural disease. Other assays have shown inferior performances. The enzyme-linked immunosorbent assay (ELISA), the most accessible, is not reliable at evaluating individual protection against varicella (3, 5). Among the other assays, glycoprotein ELISA (gpELISA) (9) and the latex agglutination assay have limited availability (5, 7, 9, 12, 14) and may yield false-positive (2) and false-negative (10) results. Individuals with positive FAMA titers have a less than 3% risk of developing varicella after household exposure, while individuals with negative FAMA titers have a 75% risk (12). The disadvantages of the FAMA assay are its nonautomation, subjective interpretation, limited scale, lengthy execution, and need for specific training (1, 6, 8, 11). There remains a need for a practical and reproducible assay that can determine susceptibility to varicella and confirm seroconversion following vaccination and exposure.
FAMA test is an immunofluorescence assay that uses unfixed varicella-zoster virus (VZV)-infected human embryonic lung fibroblast (HELF) cells incubated with serial 2-fold dilutions of sera. The cells are then washed, incubated, and examined using fluorescence microscopy (16).
We developed a flow cytometry-adapted FAMA assay (flow-FAMA) that uses the same HELF cells (in our case, infected less than 48 h, with a cytopathic effect of less than 90%). Similarly to the standard FAMA assay, we incubated the infected cells in 25 μl of diluted sera for 30 min, washed the cells in phosphate-buffered saline (PBS), and incubated them for 30 min in 25 μl of diluted fluorescein-conjugated anti-human immunoglobulin G. After this second incubation and a second wash, the flow-FAMA assay process diverged from the original assay process. Rather than preparing the cells on a slide to be examined under a microscope, we resuspended the cells in 300 μl of calcium/magnesium-negative PBS and transferred them to flow cytometry tubes. The labeled cells were then analyzed using a FACSCalibur (BD Sciences, San Jose, CA) and BD CellQuest software (Becton Dickinson, Franklin Lakes, NJ). We set a threshold for forward scatter (FSC) at 760 and one for side scatter (SSC) at 550, and we collected 5,000 events for each sample.
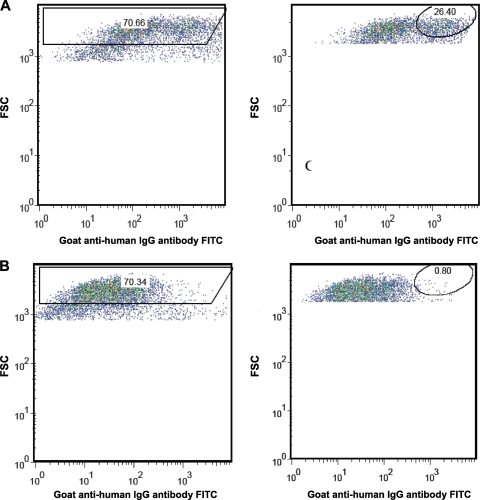
With the intention of assigning a quantitative value that corresponded with humoral immunity, we used FlowJo software 8.8.4 for Mac (Tree Star Inc., Ashland, OR) to create two gates (both fixed throughout the experiments), one that excluded noise and a second that captured events representing humoral immunity (Fig. 1). By calculating the percentage of events appearing within the borders of this second gate, we generated a percent positivity for each sample.
Fig. 1.
FAMA positive-control (A) and negative-control (B) gatings using forward scatter (FSC) and fluorescein isothiocyanate (FITC) axes as templates for analysis. Results are expressed as percentages of positivity inside oval gates (in the right-hand panels).
To evaluate flow-FAMA, we blindly tested two group of samples. Group I (n = 20) (samples A to T in Table 1) were archived sera that had been collected and stored since 1974. Of these 20 samples, 10 were FAMA positive, obtained from subjects with a positive history of the disease and no disease after subsequent exposure, and 10 were FAMA negative, obtained from subjects with no history of varicella or vaccination, who later developed the clinical disease and FAMA seroconversion. To evaluate inter- and intra-assay variation, we tested each sample 5 or 6 times over a period of 4 days: once in triplicate and two or three times individually.
Table 1.
Results obtained with standard FAMA and flow cytometry-adapted FAMA assays
| Sample | Standard FAMA result | Avg flow-FAMA result (%) | No. of runs | Range | SD | CV |
|---|---|---|---|---|---|---|
| Positive control | Positive | 25.08 | 16 | 21.50–27.18 | 1.99 | 7.94 |
| Negative control | Negative | 1.20 | 13 | 0.76–2.50 | 0.51 | 42.51 |
| Positive-control vaccinee | Positive | 11.39 | 2 | 10.18–12.59 | 1.70 | 14.97 |
| A | Positive | 6.09 | 6 | 2.83–8.35 | 2.66 | 43.74 |
| B | Positive | 19.86 | 5 | 17.65–22.36 | 18.53 | 9.33 |
| C | Positive | 19.37 | 5 | 13.26–22.19 | 3.51 | 18.12 |
| D | Positive | 21.49 | 5 | 19.56–23.52 | 1.48 | 6.87 |
| E | Positive | 22.10 | 5 | 20.15–24.36 | 1.66 | 7.52 |
| F | Positive | 14.23 | 5 | 12.71–15.95 | 1.48 | 10.42 |
| G | Positive | 10.96 | 6 | 9.51–13.09 | 1.67 | 15.18 |
| H | Positive | 12.53 | 6 | 9.29–18.29 | 3.57 | 28.49 |
| I | Positive | 15.22 | 6 | 11.52–17.12 | 2.26 | 14.85 |
| J | Positive | 13.73 | 6 | 10.73–15.84 | 1.83 | 13.37 |
| K | Negative | 1.61 | 5 | 1.03–2.35 | 0.48 | 29.85 |
| L | Negative | 1.45 | 5 | 0.77–3.29 | 1.08 | 74.61 |
| M | Negative | 2.83 | 5 | 0.61–6.85 | 2.65 | 93.48 |
| N | Negative | 3.70 | 5 | 1.60–5.07 | 1.62 | 43.81 |
| O | Negative | 2.64 | 5 | 1.33–6.04 | 1.94 | 73.36 |
| P | Negative | 3.59 | 5 | 2.25–4.86 | 1.14 | 31.77 |
| Q | Negative | 4.65 | 6 | 1.88–11.29 | 3.43 | 73.84 |
| R | Negative | 0.76 | 6 | 0.49–0.87 | 0.17 | 21.95 |
| S | Negative | 2.11 | 6 | 1.39–4.85 | 1.36 | 64.25 |
| T | Negative | 0.73 | 6 | 0.28–1.87 | 0.58 | 80.11 |
| 1 | Positive | 1.76 | 3 | 1.44–2.17 | 0.37 | 21.10 |
| 2 | Positive | 4.92 | 3 | 4.78–5.03 | 0.12 | 2.58 |
| 3 | Positive | 7.56 | 3 | 6.47–8.22 | 0.95 | 12.57 |
| 4 | Positive | 13.65 | 3 | 11.72–14.83 | 1.69 | 12.36 |
| 5 | Positive | 4.81 | 3 | 3.54–6.09 | 1.27 | 26.49 |
| 6 | Positive | 6.28 | 3 | 6.01–6.65 | 0.33 | 5.28 |
| 7 | Positive | 4.74 | 3 | 4.22–5.06 | 0.46 | 9.62 |
| 8 | Positive | 14.29 | 3 | 13.48–14.77 | 0.70 | 4.95 |
| 9 | Positive | 6.58 | 3 | 6.13–7.17 | 0.53 | 8.09 |
| 10 | Negative | 3.01 | 3 | 1.71–3.76 | 1.13 | 37.5 |
| 11 | Positive | 11.19 | 3 | 10.47–11.70 | 0.64 | 5.74 |
| 12 | Positive | 10.17 | 3 | 8.46–11.35 | 1.52 | 14.92 |
| 13 | Positive | 10.71 | 3 | 10.09–11.68 | 0.85 | 7.96 |
| 14 | Positive | 5.69 | 3 | 5.39–6.24 | 0.48 | 8.38 |
| 15 | Negative | 12.40 | 3 | 11.89–13.14 | 0.66 | 5.30 |
| 16 | Positive | 7.81 | 3 | 6.21–9.22 | 1.51 | 19.39 |
| 17 | Negative | 5.34 | 3 | 5.07–5.49 | 0.24 | 4.43 |
| 18 | Positive | 9.19 | 3 | 8.66–10.18 | 0.86 | 9.37 |
| 19 | Negative | 4.98 | 3 | 3.47–5.89 | 1.32 | 26.44 |
| 20 | Positive | 11.26 | 3 | 9.63–12.65 | 1.53 | 13.54 |
| 21 | Positive | 6.14 | 3 | 4.97–8.38 | 1.94 | 31.60 |
| 22 | Positive | 9.65 | 3 | 8.83–10.79 | 1.02 | 10.55 |
| 23 | Positive | 13.26 | 3 | 12.15–14.10 | 1.00 | 7.56 |
| 24 | Positive | 12.43 | 3 | 11.6–13.6 | 1.04 | 8.39 |
| 25 | Negative | 2.84 | 3 | 1.93–3.59 | 0.84 | 29.63 |
| 26 | Negative | 0.34 | 3 | 0.11–0.54 | 0.21 | 64.14 |
| 27 | Negative | 0.76 | 3 | 0.71–0.79 | 0.04 | 5.50 |
| 28 | Negative | 0.75 | 3 | 0.59–0.92 | 0.17 | 22.18 |
| 29 | Positive | 9.20 | 3 | 8.16–9.97 | 0.94 | 10.17 |
| 30 | Positive | 9.67 | 3 | 9.21–10.44 | 0.67 | 6.93 |
| 31 | Positive | 11.12 | 3 | 10.23–11.71 | 0.78 | 7.05 |
| 32 | Positive | 7.40 | 3 | 6.69–7.89 | 0.63 | 8.51 |
| 33 | Positive | 4.03 | 3 | 3.51–4.73 | 0.63 | 15.67 |
| 34 | Positive | 7.30 | 3 | 7.12–7.57 | 0.24 | 3.23 |
| 35 | Positive | 8.11 | 3 | 7.37–8.94 | 0.79 | 9.72 |
| 36 | Positive | 11.88 | 3 | 10.55–13.18 | 1.31 | 11.07 |
| 37 | Positive | 7.90 | 3 | 3.90–11.80 | 3.66 | 46.32 |
| 38 | Positive | 10.22 | 3 | 9.48–10.99 | 0.76 | 7.39 |
| 39 | Negative | 10.84 | 3 | 8.88–12.11 | 1.72 | 15.90 |
Group II (n = 39) (samples 1 to 39 in Table 1) were samples that tested as either negative or equivocal using the commercially available ELISA in our hospital. We chose these because they were “problematic” and typically sent to be tested by FAMA. The samples were tested in triplicate (on a single day) and the results averaged (Table 1).
The positive control was a high-titer FAMA-positive serum sample obtained from a subject with a history of varicella and documented immunity after repeated exposure. The negative control was a FAMA-negative serum sample obtained from a subject with no history of varicella infection or vaccination. An alternative positive control, a low-titer FAMA-positive sample obtained from a vaccinee with no history of the disease, was included in the analysis.
Statistical analysis was performed using Minitab version 15.1, SPSS version 16.0, and an Excel 11.2.5 2004 version for Mac. The analysis of interassay and intra-assay variations was based on group I (n = 20). Evaluating the samples run in triplicate from group I, a Friedman test showed no significant intra-assay variation (P = 0.31). Interassay variation was evaluated by analyzing the results of group I samples that were tested across 3 or 4 different days (we averaged the results from the 1 day they were run in triplicate). No significant interassay variation was found between the samples that were measured across either 3 (P = 0.387) or 4 (P = 0.154) days.
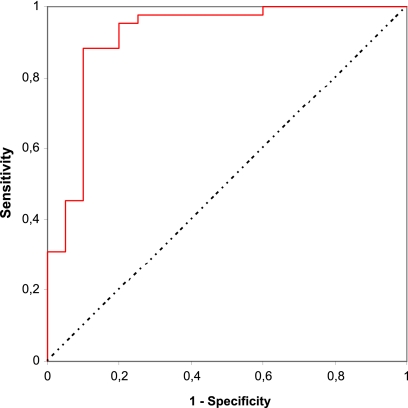
A comparison between the FAMA and flow-FAMA assays was done independently by a statistician using a receiver operating characteristic (ROC) curve, with the average percentages of positivity obtained from the flow-adapted FAMA assay reported as a continuous variable and FAMA results as a categorical variable. The area under the curve was 0.915 (95% confidence interval [CI], 0.829 to 1.00) (Fig. 2). We chose a ≥4.7% positive cutoff between 2 options with the highest accuracy, one that provided higher specificity, and then made a comparison between results of standard FAMA and flow cytometry-adapted FAMA (Table 2) from which we obtained the following results: sensitivity, 95.25%; specificity, 80.0%; prevalence, 67.74%; and accuracy, 90.32%.
Fig. 2.
Receiver operating characteristic (ROC) curve obtained from true positives (Sensitivity) and false positives (1 – Specificity) with different cutoffs of flow-adapted FAMA assay (results expressed as percentages of positivity) compared to standard FAMA (either positive or negative results).
Table 2.
Correlation between standard results obtained with FAMA and flow-adapted FAMA assays using a 4.7%a cutoff obtained from a receiver operating characteristic (ROC) curve
| Flow-adapted FAMA result | Standard FAMA result |
||
|---|---|---|---|
| No. of positive samples | No. of negative samples | Total | |
| Positive | 40 | 4 | 44 |
| Negative | 2 | 16 | 18 |
| Total | 42 | 20 | 62 |
Flow-FAMA result was negative if <4.7% and positive if >4.7%.
Of the 62 samples measured, we encountered 6 discrepancies between the FAMA and flow-FAMA results. The standard FAMA test recorded 2 positive results that were negative by flow-FAMA, obtained from subjects reported as having a history of clinical varicella. Among the four subjects who tested positive by flow cytometry-adapted FAMA but negative by standard FAMA, one had an unknown history regarding varicella or vaccination, another had a negative history of varicella and no vaccination, and two had histories of varicella but no vaccination.
Dealing with samples that were troublesome for commercially available antibody assays that do not reliably detect immunity after vaccination (3, 4, 5, 13, 15), we obtained automated measurements with a simple monochromatic flow cytometry procedure and received results comparable to those of the gold standard FAMA assay. With further efforts toward simplifying the procedure, we believe the assay could become a valuable tool for assessing populations at risk of complications, particularly immunosuppressed subjects, institutionalized individuals, and health care professionals.
Acknowledgments
M. M. Lafer was supported by Brazilian Ministry of Education's CAPES grant 0108-08-1.
Footnotes
Published ahead of print on 25 May 2011.
REFERENCES
- 1. Balfour H. H., Jr., et al. 1988. Laboratory studies of acute varicella and varicella immune status. Diagn. Microbiol. Infect. Dis. 10:149–158 [DOI] [PubMed] [Google Scholar]
- 2. Behrman A., Schmid D. S., Crivaro A., Watson B. 2003. A cluster of primary varicella cases among healthcare workers with false-positive varicella virus titers. Infect. Control Hosp. Epidemiol. 24:202–206 [DOI] [PubMed] [Google Scholar]
- 3. Demmler G., Steinberg S., Blum G., Gershon A. A. 1988. Rapid enzyme-linked immunosorbent assay for detecting antibody to varicella-zoster virus. J. Infect. Dis. 157:211–212 [DOI] [PubMed] [Google Scholar]
- 4. de Ory F., et al. 2006. European seroepidemiology network 2: standardization of assays for seroepidemiology of varicella zoster virus. J. Clin. Virol. 36:111–118 [DOI] [PubMed] [Google Scholar]
- 5. Gershon A. A. 2008. The immunological basis for immunization series. Module 10: varicella-zoster virus. WHO (World Health Organization), Geneva, Switzerland [Google Scholar]
- 6. Gershon A. A., Silverstein S. J. 2009. Varicella-zoster virus, p. 451–473 In Richmann D., Whitley R., Hayden F. (ed.), Clinical virology, 3rd ed. ASM Press, Washington, DC [Google Scholar]
- 7. Gershon A., Steinberg S., LaRussa P. 1994. Detection of antibodies to varicella-zoster virus using a latex agglutination assay. Clin. Diagn. Virol. 2:271–277 [DOI] [PubMed] [Google Scholar]
- 8. Hambleton S., Gershon A. A. 2005. Preventing varicella-zoster disease. Clin. Microbiol. Rev. 18:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuter B., et al. 2004. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr. Infect. Dis. J. 23:132–137 [DOI] [PubMed] [Google Scholar]
- 10. Landry M. L., Ferguson D. 1993. Comparison of latex agglutination test with enzyme-linked immunosorbent assay for detection of antibody to varicella-zoster virus. J. Clin. Microbiol. 31:3031–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maple P. A., et al. 2006. Performance of a time-resolved fluorescence immunoassay for measuring varicella-zoster virus immunoglobulin G levels in adults and comparison with commercial enzyme immunoassays and Merck glycoprotein enzyme immunoassay. Clin. Vaccine Immunol. 13:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michalik D. E., et al. 2008. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J. Infect. Dis. 197:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono E., Lafer M. M., Weckx L. Y., Granato C., de Moraes Pinto M. I. 2004. A simple and cheaper in house varicella zoster virus antibody indirect ELISA. Rev. Inst. Med. Trop. Sao Paulo 46:165–168 [DOI] [PubMed] [Google Scholar]
- 14. Provost P. J., et al. 1991. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 9:111–116 [DOI] [PubMed] [Google Scholar]
- 15. Saiman L., et al. 2001. Persistence of immunity to varicella-zoster virus after vaccination of healthcare workers. Infect. Control Hosp. Epidemiol. 22:279–283 [DOI] [PubMed] [Google Scholar]
- 16. Williams V., Gershon A., Brunell P. 1974. Serologic response to varicella-zoster membrane antigens measured by indirect immunofluorescence. J. Infect. Dis. 130:669–672 [DOI] [PubMed] [Google Scholar]