Abstract
To prevent complications that might follow an infection with varicella-zoster virus (VZV), the live attenuated Oka strain (V-Oka) is administered to children in many developed countries. Three vaccine brands (Varivax from Sanofi Pasteur MSD; Varilrix and Priorix-Tetra, both from Glaxo-Smith-Kline) are licensed in Germany and have been associated with both different degrees of vaccine effectiveness and adverse effects. To identify genetic variants in the vaccines that might contribute to rash-associated syndromes, single nucleotide polymorphism (SNP) profiles of variants from the three vaccines and rash-associated vaccine-type VZV from German vaccinees were quantitatively compared by PCR-based pyrosequencing (PSQ). The Varivax vaccine contained an estimated 3-fold higher diversity of VZV variants, with 20% more wild-type (wt) SNPs than Varilrix and Priorix-Tetra. These minor VZV variants in the vaccines were identified by analyzing cloned full-length open reading frame (ORF) orf62 sequences by chain termination sequencing and PSQ. Some of these sequences amplified from vaccine VZV were very similar or identical to those of the rash-associated vaccine-type VZV from vaccinees and were almost exclusively detected in Varivax. Therefore, minorities of rash-associated VZV variants are present in varicella vaccine formulations, and it can be concluded that the analysis of a core set of four SNPs is required as a minimum for a firm diagnostic differentiation of vaccine-type VZV from wt VZV.
INTRODUCTION
The varicella-zoster virus (VZV) live attenuated Oka strain (V-Oka) is licensed in developed countries for vaccination against varicella. In Germany, routine vaccination of healthy children >11 months of age has been performed since 2004. Since then, varicella morbidity has declined (16). The mixtures of V-Oka strains with different single nucleotide polymorphism (SNP) profiles present in vaccine preparations and the reported genomic variation of vaccine VZV strains have prompted investigations of the linkage of certain genomic variants to episodes of vaccine-induced rash (9, 10).
We aimed at comparing the SNP profiles of the varicella vaccines used in Germany and of vaccine-type VZV from German vaccinees for several reasons. First, three vaccine brands are distributed in Germany (Varivax from Sanofi Pasteur MSD [SPMSD], Leimen, Germany; Varilrix and Priorix-Tetra, both from GlaxoSmithKline [GSK], Munich, Germany) and have been associated with different degrees of vaccine effectiveness (17). In addition, data from the German varicella sentinel (16) indicated that the vaccines might be related to different frequencies of rash-associated syndromes, i.e., mild forms of varicella or zoster (Anette Siedler and Bernhard Ehlers, unpublished data). Second, vaccine-type VZV strains causing rash-associated clinical syndromes in vaccinees have been reported to contain a number of wild-type (wt) SNPs. It has been hypothesized that these vaccine-type, wt SNP-containing VZV strains are minor components of the vaccines (1, 9, 10, 13). To substantiate this hypothesis and to search for genetic correlates of the observed differences among the vaccines, we compared the SNP profiles of (i) VZV from the three vaccine brands, (ii) rash-associated vaccine-type VZV from German vaccinees, and (iii) wt VZV from nonvaccinated and vaccinated individuals suffering from varicella by PCR, quantitative pyrosequencing (PSQ), and chain termination sequencing (CTSQ). In addition, open reading frame (ORF) orf62 sequences were cloned in Escherichia coli and analyzed by the same methods.
MATERIALS AND METHODS
Sample collection, vaccines, and DNA extraction.
Vesicle material from vaccinees with varicella-like rash or herpes zoster was collected as part of the varicella sentinel in Germany (16). Three vaccines from two manufacturers were analyzed: Varivax (monovalent, lots NA28970 and HW08500) from SPMSD (Leimen, Germany) and Varilrix (monovalent, lot A70CA216B) and, with the same VZV component, Priorix-Tetra (tetravalent; lot A71CA024A) from GSK (Munich, Germany). DNA was extracted using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
LD-PCR and sequence analysis.
Long-distance PCR (LD-PCR) was performed as follows: the reaction mixture was set up on ice and contained 50 μl of ExTaq buffer with MgCl2, 400 μM each deoxynucleoside triphosphate (dNTP), 400 nM each primer, and 2.5 units of ExTaq polymerase (TaKaRa Bio Inc., Japan). Thirty cycles of amplification were performed, with each cycle consisting of a denaturing step at 98°C for 20 s, an annealing step at 63°C for 30 s, and an elongation step at 72°C. The duration of the elongation step was 7 min in the first 15 cycles and 10 min plus a 5-s ramp time for each of the last 15 cycles. The final elongation step was performed for 30 min at 72°C. LD-PCR was performed in a nested format using primer pairs LD_a1 and LD_a2 in the 1st and 2nd rounds (Table 1). When no PCR product was obtained (because of very low copy number), the three alternative primer pairs, LD_b1, LD_b2, and LD_b3, were used (Table 1).
Table 1.
Long-distance PCR primer sets
| ORF | Primer namea |
Annealing temp (°C) | Product size (bp) | Sequence (5′ → 3′) |
||
|---|---|---|---|---|---|---|
| Sense primer | Antisense primer | Sense | Antisense | |||
| 62 | LD_a1-s | LD_a1-as | 63 | 3,818 | GCGGGGTCGCCTGATACTT | GAGGACAACAGCTCCACCTTGAC |
| 62 | LD_a2-s | LD_a2-as | 63 | 3,686 | CGCCTGATACTTTGGAGTTAATGG | CCCCTCCTCGCTGTCCCA |
| ORF62 + NCRb | LD_b1-s | LD_b1-as | 60 | 4,565 | AGTCGGGTGTATTGGGACAG | AGTCGGGTGTATTGGGACAG |
| ORF62 + NCR | LD_b2-s | LD_b2-as | 60 | 4,516 | GGGACAGTTACTCCATTAGAGGC | GGGACAGTTACTCCATTAGAGGC |
| ORF62 + NCR | LD_b3-s | LD_b3-as | 60 | 4,436 | CTGGGATAGGGTAATGCAAG | CCTCGAGTCTCGTCCAATCA |
s, sense; as, antisense.
NCR, noncoding region.
PCR products were purified with an Invisorb Spin PCRapid kit (Invitek, Berlin, Germany) and directly sequenced by CTSQ using a BigDye Terminator cycle sequencing kit and an ABI Prism genetic analyzer (Applied Biosystems) according to the manufacturer's instructions. The programs DNAStar Lasergene (version 8) and MacVector (version 10.6) were used to examine the SNPs by comparing the obtained sequences with those of reference strains.
When patient specimens were nearly exhausted, DNA was expanded in toto with a GenomiPhi (version 2) DNA amplification kit (GE Healthcare Life Sciences, Munich, Germany) according to the manufacturer's instructions. The qualitatively and quantitatively correct reamplification of VZV DNA and VZV variant mixtures from specimens with the GenomiPhi kit had been previously controlled using quantitative real-time PCR, conventional PCR, CTSQ, and PSQ (Judit Küchler and Bernhard Ehlers, unpublished data).
Cloning in E. coli.
PCR products spanning the complete orf62 (approximately 4 kb) and short PCR products containing a single SNP (<0.3 kb) were transformed into chemically competent E. coli cells using a TOPO-TA cloning kit (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions. The plasmids containing the 4-kb inserts were extracted with a QIAprep spin miniprep kit (Qiagen, Hilden, Germany) and analyzed by cycle sequencing (see above). The plasmids containing the short inserts were tested with colony PCR and pyrosequencing (see below).
PCR-based pyrosequencing.
PSQ (12) was used for analyzing the allele frequency of VZV SNPs. Thirty-two PSQ assays, each consisting of a primer pair (with one primer biotinylated) for PCR (to generate a product containing the SNP of interest) and a separate sequencing primer, were designed by using the pyrosequencing assay design software (Biotage, Uppsala, Sweden) (Table 2). Each PCR mixture contained 35 μl of AmpliTaq Gold buffer, 2.0 mM MgCl2, 200 μM each dNTP, 1 μM each primer, 1.5 units of AmpliTaq Gold (Applied Biosystems), and DNA extracted either from vaccines (adjusted to 105 VZV genome copies per reaction setup) or from vaccinated individuals. Forty-five cycles of amplification were performed after enzyme activation at 95°C for 12 min. Each cycle consisted of a denaturing step at 95°C for 30 s, an annealing step at an appropriate temperature (Table 2) for 30 s, and an elongation step at 72°C for 60 s. The final elongation step was done for 10 min at 72°C.
Table 2.
PCR plus PSQ primer sets
| ORF | SNP position in genomec | SNP position in ORF | Primer namea |
Annealing temp (°C) | Product size (bp) | Sequence (5′ → >3′)b |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sense primer | Antisense primer | Pyrosequencing primer | PCR sense | PCR antisense | PSQ | |||||
| 5′ noncoding | 560 | 560 | 5′-NCR-s | 5′-NCR-as | seq_5′-NCR-s | 62 | 210 | CACCCAACAGTCTTACGATTGC | 5′-bio-TCCCCTTCCGGACAGTAGTTT | GGAACCTCCCAACTC |
| 1 | 703 | 115 | orf1.1-s | orf1.1-as | seq_orf1.1-s | 58 | 126 | TCGCCATCTGGAGTACTACACCC | 5′-bio-CACCGGCGGTTCTTATTCC | CAGTACGTTGCATAACCT |
| 1 | 763 | 175 | orf1.2-s | orf1.2-as | seq_orf1.2-s | 59 | 79 | ATCGTGGCCTTGGGACATC | 5′-bio-AACAACGCGTCGCCACTC | GCCTTGGGACATCAAC |
| 6 | 5745 | 420 | orf6-s | orf6-as | seq_orf6-s | 62 | 157 | AGTTACCCCGCGCTCTTTTAG | 5′-bio-TTCAAGACCGCAGTCGAAT | TTAAAGATATTTGCGGG |
| 9A | 10900 | 233 | orf9A-s | orf9A-as | seq_orf9A-s | 58 | 99 | GTGTGTAGCCCTTATTTCGTTAGC | 5′-bio-CCTTTTATAGGCAAACGGGTTTA | TGTATTACGCAGCACG |
| 10 | 12779 | 620 | orf10-s | orf10-as | seq_orf10-s | 58 | 121 | CGGCGAAAAGGACGACAATAG | 5′-bio-AGAAGACGCGCCAAACTTG | CAAAACCCTGACCAGA |
| 14 | 19431 | 1 | orf14-s | orf14-as | seqorf14-s | 58 | 191 | TGGGACGATTTTCAGCTTGAT | 5′-bio-TCGCAGTTATCGCAACCCTATG | CGTTTGGTTGGTTTCT |
| 21 | 31732 | 974 | orf21-s | orf21-as | seq_orf21-as | 62 | 166 | 5′-bio-CCGGGAATGCTTGCTAGGA | CACGGGTGTGTTCTGCTACATTAC | GCGCCTCAATATGAAT |
| 31 | 58595 | 1588 | orf31-s | orf31-as | seq_orf31-s | 60 | 121 | TGTCCAGAACTGGGATCAGAT | 5′-bio-TCCCGGACCCATTTAAACTAACTA | TGGGATCAGATACACG |
| 39 | 71252 | 620 | orf39-s | orf39-as | seq_orf39-s | 60 | 80 | CTGTGTCGTGGGGTCCAAGT | 5′-bio-CGTGTCCACCAAACAGTCCATAG | GTCCAGCCGTGTTACT |
| 50 | 87306 | 732 | orf50-s | orf50-as | seq_orf50-as | 60 | 144 | 5′-bio-TTTCCGCGTCCACAAAAATA | TTTTTGGGGTCTTTTGTCATTGT | CTTGGACATTTTGCG |
| 51 | 89734 | 1854 | orf51-s | orf51-as | seq_orf51-s | 62 | 251 | CTACGCTTCCGGTTGGTTTC | 5′-bio-CGTTACAGCGCATAACCTCAAA | GCCCCAAATTTAACCA |
| 52 | 90535 | 43 | orf52-s | orf52-as | seq_orf52-s | 62 | 77 | GCAACGCAGATTACCTTGGTTAG | 5′-bio-CCGGACTGTGTCCAGGATGT | CTTGGTTAGAGAAAGCG |
| 54 | 94167 | 493 | orf54-s | orf54-as | seq_orf54-as | 58 | 81 | 5′-bio-TATGTACCTCGCTTTGAGTTACCA | CATTACTAGTTGCGCCGTATTTT | TACGGGCCACCCGAT |
| 55 | 97748 | 1753 | orf55.1-s | orf55.1-as | seq_orf55.1-as | 60 | 245 | 5′-bio-ATCGAATATGCTTACCGGTTTCT | CAGAAGGCTTTATCGGCAGAAGT | ACCGCTGCTTCGTGT |
| 55 | 97796 | 1801 | orf55.2-s | orf55.2-as | seq_orf55.2-s | 58 | 89 | ACAAAGCCCCCTTACACGAA | 5′-bio-GAAGGCTTTATCGGCAGAAGT | GGGCGGGTGCCGGGA |
| 59 | 101089 | 788 | orf59-s | orf59-as | seq_orf59-s | 58 | 251 | AATACCTTTCGCGTACGGCTCTTT | 5′-bio-AGGACATTTATGTTTTGGCCCATC | TCTAGCTTAACCCCCA |
| 61/62 | 105169 | 684 | orf61/62-s | orf61/62-as | seq_orf61/62-as | 60 | 299 | 5′-bio-CTGGGATAGGGTAATGCAAGTC | TGACGGAGTCCCCTCCTTT | TCCTTTTCTCGTGAGC |
| 62 | 108838 | 296 | orf62.1-s | orf62.1-as | seq_orf62.1-s | 62 | 165 | TTCAACCAGAACCCAGAACG | 5′-bio-GATCGGCTCCTGTTGGTTCTC | GGACGGTCAGGATCT |
| 62 | 108111 | 1023 | orf62.2-s | orf62.2-as | seq_orf62.2-s | 62 | 138 | AGACCCGATTGAGGATGACA | 5′-bio-GACCTGCTGCCTGTAGTTTCACT | TGAGGATGACAGCCC |
| 62 | 107797 | 1337 | orf62.3-s | orf62.3-as | seq_orf62.3-as | 62 | 165 | 5′-bio-CGCCAGAGACAGAAATCATTTTCCT | TAAAGCGGCACGGGTTCAGT | GGGGTTCTGGATCGC |
| 62 | 107599 | 1535 | orf62.4-s | orf62.4-as | seq_orf62.4-as | 62 | 141 | 5′-bio-AACAGGGTGCGGTTTGGAC | TCTAGCCGGATCTCCCAACTC | CGGCACGTAAAGCGG |
| 62 | 107252 | 1882 | orf62.5-s | orf62.5-as | seq_orf62.5-as | 62 | 104 | 5′-bio-CAGAGTCTCCGCAGAGCCTT | TGAGGGTCGGGAGCCTGT | CCTCGGGGTATGCCA |
| 62 | 107136 | 1998 | orf62.6-s | orf62.6-as | seq_orf62.6-as | 62 | 84 | 5′-bio-ACAGACTCCCGACCCTCAGC | TTGCGCGGAGTTCGTAAAC | CGGTGGACACACAGAAA |
| 62 | 106262 | 2872 | orf62.7-s | orf62.7-as | seq_orf62.7-as | 62 | 171 | 5′-bio-CGGGGCCGTCGAGTATCT | TTCTGTGACCGCCGAGTCT | ACAAAGCGGGTCCAT |
| 62 | 105705 | 3429 | orf62.8-s | orf62.8-as | seq_orf62.8-as | 62 | 227 | 5′-bio-ACCTGGGCGAGGGTGTTT | CCCGCCTGGGTTTCTGAC | GGAAGCACGAGTGGT |
| 62 | 105544 | 3590 | orf62.9-s | orf62.9-as | seq_orf62.9-as | 62 | 227 | 5′-bio-ACCTGGGCGAGGGTGTTT | CCCGCCTGGGTTTCTGAC | CCCGTGGTGTCCGAA |
| 62 | 105356 | 3778 | orf62.10-s | orf62.10-as | seq_orf62.10-s | 62 | 290 | AGGGGAGCGACGGAACAC | 5′-bio-GGACAACAGCTCCACCTTGA | ACCGGGGTCATCCTT |
| 62 | 105310 | 3824 | orf62.11-s | orf62.11-as | seq_orf62.11-as | 62 | 114 | 5′-bio-GGGTCATCCTTTGGGGTGAG | AGGACAACAGCTCCACCTTGA | CTCCACCTTGACCGC |
| 62/63 | 109137 | 657 | orf62/63.1-s | orf62/63.1-as | seq_orf62/63.1-s | 62 | 148 | GTGTAGAGCGCTGCATCGG | 5′-bio-GGGGGCACAACACGTTTTAAGTAC | GCATCGGCGGCGTAT |
| 62/63 | 109200 | 720 | orf62/63.2-s | orf62/63.2-as | seq_orf62/63.2-as | 59 | 100 | 5′-bio-AGACGTACCCGAGTTTTCCA | GGGGGCACAACACGTTTT | CTCCCTCACCAACCG |
| 64 | 111650 | 86 | orf64-s | orf64-as | seq_orf64-s | 58 | 148 | CTTTACTGCACACGACACGATACC | 5′-bio-ACAAACTCGCGCACGGTCT | ATACCCCCGCGCACC |
s, sense; as, antisense; NCR, noncoding region.
bio, biotin.
Strain Dumas.
For analysis of single E. coli clones, colony PCR was performed using the PSQ primer pairs with the same reaction setup. Colonies were picked and transferred directly into the pyrosequencing PCR mixtures. Spare material of the colony was streaked on a grid plate for later countercheck.
For PSQ, 30 μl of the PCR products was immobilized, denatured, and washed using a PyroMark Vakuum prep workstation (Biotage, Uppsala, Sweden). The single-stranded DNA was transferred into a 96-well optical reaction plate (PSQ 96 Plate low; Biotage, Uppsala, Sweden) containing an annealing buffer and a specific sequencing primer. The annealing of the primer was done at 80°C for 2 min. After cooling down of the reaction mixture, PSQ was performed at room temperature on the PyroMark ID apparatus (Biotage, Uppsala, Sweden). The resulting pyrograms allowed qualitative and quantitative SNP analysis. Quantification was conducted by PSQ software and is based on the fact that PSQ peak heights are proportional to the frequency of an allele in the sample. Therefore, PSQ peak heights provide an accurate measure of the proportion of VZV wild-type and vaccine-type alleles in a sample. In addition, the results of the analysis are presented in sequence context, confirming that the analysis was made at the correct sites. All PCRs plus PSQ measurements of the vaccines and the patient specimens with vaccine-type VZV were repeated two to four times.
Reference strains.
The VZV reference sequences used were those of strain Dumas (GenBank accession no. NC_001348), strain Oka-P (GenBank accession no. AB097933), Oka-V (GenBank accession no. AB097932), Varilrix (GenBank accession no. DQ008354), and Varivax (GenBank accession no. DQ008355).
RESULTS AND DISCUSSION
As part of the German varicella sentinel (16), specimens from vaccinated individuals with rashes were subjected to VZV PCR and PSQ for differentiation between wt VZV and vaccine-type VZV by analyzing five SNP positions (nt 105544, 105705, 106262, 107252, and 108111; positions were based on the genome sequence of the Dumas strain). Between January 2004 and December 2010, 505 specimens were found by PCR and sequencing to contain the VZV wt and 20 were found to contain a VZV vaccine type. Of the latter, 12 had been clinically diagnosed to originate from a varicella-like rash and 8 originated from herpes zoster (HZ) (Bernhard Ehlers and Annette Siedler, unpublished data). To comprehensively determine the frequency of wt alleles and their fraction in mixed alleles at SNP positions in these vaccine-type VZV isolates, SNPs (n = 32) present in the VZV genome were analyzed with a panel of PCRs and subsequent PSQ. The PCR and PSQ primers used are listed in Table 2. The specimens of nine vaccinees (vaccinees 1 to 9; Fig. 1, 2, and 3) contained enough VZV genome copies for this genome-wide SNP analysis. Those with low copy numbers (vaccinees 1 to 6) had to be expanded in toto as described in the Materials and Methods section to perform all analyses of this study. The remaining 11 specimens had such low copy numbers that not all PCR amplifications were successful. These were not included in the study. For comparison, the SNP profiles of wt VZV from nonvaccinated (n = 4) and vaccinated (n = 5) individuals suffering from varicella were analyzed by the same PSQ approach. Before the patient specimens were analyzed, the SNP profiles of VZV from the three varicella vaccines distributed in Germany (Varivax from SPMSD, Varilrix and Priorix-Tetra from GSK) were quantitatively evaluated.
Fig. 1.
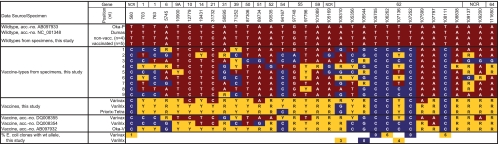
VZV SNPs determined by PCR and PSQ. SNPs (n = 32) from nine patients with vaccine-type VZV, four nonvaccinated and five vaccinated patients with wild-type VZV, and VZV from the three vaccine brands are shown. The corresponding SNPs of Varivax and Varilrix VZV, the original vaccine strain Oka-V, and wt strains Oka-P and Dumas are included for comparison. The last two lines show the percentages of wt markers (positions 560, 105310, 106262, 107136, and 108111) and of vaccine-type markers (positions nt 105544, 105705, and 107252) that were revealed by PSQ of E. coli clones. Red, wt marker; blue, vaccine marker; yellow, mixed marker; NCR, noncoding region.
Fig. 2.
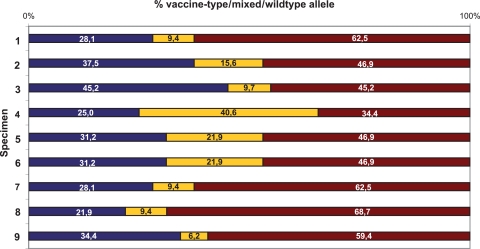
Percentage of markers detected in the nine specimens from vaccine-associated rashes. For each specimen, 32 SNP positions were analyzed by PSQ. Red bars, wt markers; blue bars, vaccine markers; yellow bars, mixed markers.
Fig. 3.
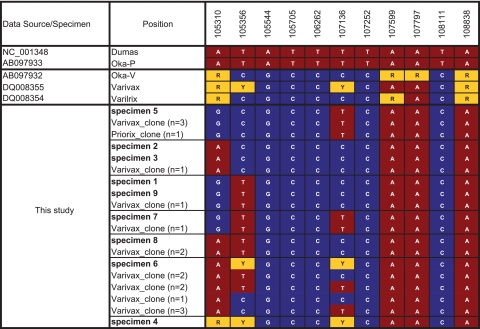
SNPs in the orf62 sequences of nine vaccine-type VZV strains from vaccinees with rashes. The orf62 sequences were amplified with LD-PCR and completely sequenced (chain termination sequencing). Twelve SNP positions are compared with orf62 clone sequences from Varivax VZV (n = 16) and Priorix-Tetra VZV (n = 1). Red boxes, wt markers; blue boxes, vaccine markers; yellow boxes, mixed markers.
Comparison of Varivax, Varilrix, and Priorix-Tetra vaccine VZV by PSQ.
To get more detailed information about the SNP distribution in Varivax, Varilrix, and Priorix-Tetra vaccine VZV, we examined 32 SNP positions by PSQ. All SNP profiles were tabulated and compared to those of the previously published SNPs of Varivax and Varilrix VZV (18), the original vaccine strain Oka-V, as well as wt parent strain Oka-P (7) and the wt Dumas strain (3) (Fig. 1). To firmly discriminate pure wt and pure vaccine-type alleles from mixed alleles in the vaccines, the performance of all PSQ assays with specimens from nine patients infected with wt VZV was assessed. Here, we expected PSQ measurements of 100% wt and 0% vaccine-type alleles at all 32 SNP positions. In fact, only a part of the PSQ assays revealed 100% wt allele. The others revealed background signals in a highly reproducible fashion, falsely indicating the presence of the vaccine-type allele (data not shown). Therefore, we calculated individual limits of detection for the minority allele for all PSQ assays. To achieve this, the median height of the background signals obtained from the nine patients with wt VZV was calculated for 25/32 PSQ assays. For the remaining seven PSQ assays targeting SNPs at positions nt 560, 105310, 105544, 105705, 106262, 107252, and 108111, cloned VZV sequences were available (see below), and their pyrograms were used for calculation of the detection limits. The median of the peak height of the background signal plus 2-fold standard deviation was defined as the individual limit of detection for each assay. The resulting detection limits of up to 12% for the 32 assays partially exceeded those of a previous study aimed at detecting mutant minorities of human cytomegalovirus. In that study, detection limits of 6% had been defined for four PSQ assays (15). In the present study, the detection limits of 19 assays were <6%, but those of 10 assays were >6% and those of 3 assays were >10%. The 13 assays with high detection limits were probably due to the high GC content of the VZV genome, which occasionally hampered the design of optimal PSQ assays. All PSQ values determined in our study which exceeded the detection limit of the respective PSQ assay were considered to represent true alleles (Fig. 1 to 4).
Fig. 4.
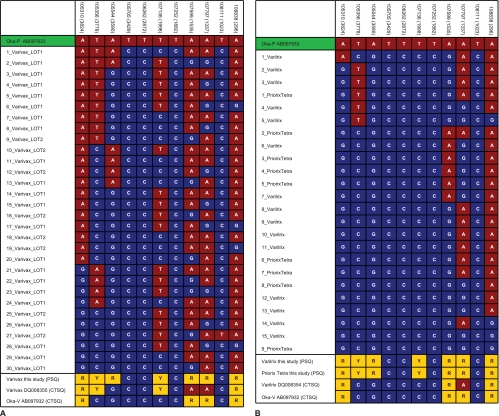
SNPs in the orf62 sequences of individual vaccine VZV genomes from three varicella vaccines. SNPs were determined from individual E. coli clones of complete orf62 sequences from Varivax VZV (A) and Varilrix and Priorix-Tetra VZV (B) with CTSQ. Red boxes, wt markers; blue boxes, vaccine markers; yellow boxes, mixed markers. Note the wt allele at position 108111 in clone 27 of Varivax VZV.
The qualitative SNP profile of Varivax VZV determined here matched only at 20 SNP positions with the Varivax VZV profile published by Tillieux et al. (18). Similarly, the present SNP profiles of Varilrix and Priorix-Tetra VZV matched at only 18 positions the Varilrix VZV profile determined by these authors (18). Using the CTSQ methodology, they detected (with reference to the panel of 32 SNPs investigated in this study) 11 pure wt SNPs in Varivax VZV and 4 in Varilrix VZV. Here, we found only four pure wt SNPs in Varivax VZV and none in Varilrix or Priorix-Tetra VZV. In addition, 9 pure vaccine-type SNPs in Varivax VZV and 14 in Varilrix VZV had been detected by CTSQ. We identified only five pure vaccine-type SNPs in Varivax VZV, six in Varilrix VZV, and seven in Priorix-Tetra VZV (Fig. 1). In addition, some mixed SNPs revealing minority allele fractions of >20% in our study (Varivax VZV, nt 703, 763, 58595, and 107797; Varilrix/Priorix-Tetra VZV, nt 763, 19431, 31732, 71252, 87306, 89734, 105310, and 107797) had not been identified to be mixed in earlier reports of studies using CTSQ (7, 10). Most likely, this indicates differences between vaccine batches (Fig. 1). It has already been noted that Varivax V-Oka has undergone some changes in variant content compared with that of the original Biken vaccine (9) (compare also Varivax VZV accession no. DQ008355 and V-Oka accession no. AB097932 in Fig. 1). This is underlined by the SNP profile of VZV from the Varivax lot analyzed in this study, which differs from both published vaccine VZV profiles (Fig. 1) and therefore indicates additional changes over time. Finally, we also identified mixed SNPs with wt allele fractions below 20%. These minor wt fractions had been missed by CTSQ (7, 10), most likely because the PSQ method is more sensitive and quantifies more accurately than CTSQ (12).
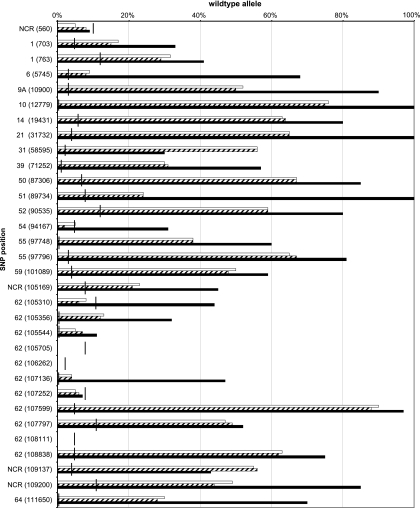
A quantitative examination of all vaccine PSQ measurements was performed, and the percentages of wt alleles are plotted in Fig. 5. The data show nearly identical wt allele fractions in Varilrix and Priorix-Tetra VZV strains. This was theoretically expected for all 32 SNPs since the VZV component in Priorix-Tetra is the same as that in Varilrix. In addition, the wt allele percentages of Varivax VZV measured by PSQ in this study were very similar to the PSQ values reported previously for Varivax VZV (10) (see Fig. S1 in the supplemental material). These data convincingly demonstrate the suitability of the PSQ technique for quantitative molecular typing of VZV. By comparing the three vaccines through quantification of the PSQ signals, we detected on average 54% wt alleles in Varivax VZV which exceeded the wt allele fractions in Varilrix and Priorix-Tetra VZV (both 35% wt alleles) by about 20% (Fig. 5).
Fig. 5.
Percentage of wild-type markers determined by PSQ for 32 SNPs of vaccine VZV genomes from three varicella vaccines. The vertical bars indicate the individual detection limits. They were defined as described in the text. Black bars, Varivax; white bars, Varilrix; hatched bars, Priorix-Tetra.
PSQ revealed 100% vaccine VZV markers at positions 560, 105705, 106262, 107252, and 108111 in all three vaccines. All other loci revealed mixed markers for Varilrix and Priorix-Tetra VZV (5 to 90% wt alleles) and Varivax VZV (11 to 90% wt alleles) except four loci in Varivax VZV, where only wt markers were detected (nt 12779, 31732, 89734, and 107599) (Fig. 5).
SNP positions containing 100% vaccine VZV markers in vaccines as well as in vaccine-type VZV from vaccinees are suitable for differentiation between vaccine-type VZV and wt VZV in routine diagnostics. Pure (100%) vaccine VZV markers have been detected previously by PSQ at positions 560, 105705, 106262, 107252, and 108111 in the Varivax vaccine VZV as well as in patient isolates (except SNP 560) (10). In accordance, vaccine-type VZV alleles were also detected in our PSQ analysis, albeit with weak wt signals at positions 560 (5 to 9%) and 107252 (5 to 7%), just below the detection limits of 10% and 8%, respectively (Fig. 5). To assay these SNPs in more depth, we analyzed all five positions by cloning the respective five PCR fragments from Varivax in E. coli K-12 and screened 73 to 125 E. coli clones per SNP position for inserts with wt alleles. One of 100 clones (1%) revealed the wt allele at position 560, 0/96 at position 105705, 7/125 (6%) at position 106262, 0/99 at position 107252, and 4/73 (5%) at position 108111. This indicated very low wt allele minorities at positions 560, 106262, and 108111 but not at positions 105705 and 107252. Likewise, the minor, mostly doubtful wt signals in Varilrix VZV at positions 105310, 105544, and 107136 were evaluated by cloning. Inserts with wt alleles were identified in 3%, 0%, and 4% of the clones, respectively. This confirmed very low wt allele minorities at positions 105310 and 107136 in Varilrix VZV (Fig. 1).
SNP analysis of (i) vaccine-type VZV from vaccinees and (ii) wild-type VZV from nonvaccinated individuals and vaccinees with PSQ.
In the nine specimens from vaccinees with vaccine-type VZV, five SNPs in orf62 homogeneously displayed the vaccine-type allele (nt 560, 105705, 106262, 107252, and 108111), being in accordance (except nt 560) with the findings of previous analyses of vaccinees with rashes (6, 9, 10, 11). All specimens also displayed mixed alleles and therefore contained mixtures of variants (see below) (Fig. 1). The high variability of the mixed allele percentage (6.2 to 40.6%) may be in part due to the fact that the specimens contained highly different total VZV copy numbers (100 to 107) and that the firm determination of a minority allele is limited to specimens with a sufficient copy number. In addition, the samples were most likely derived from different numbers of lesions, which may have influenced the numbers of variants detected.
At four positions (nt 12779, 31732, 107599, and 107797), all specimens revealed 100% wt alleles. This is in accordance with reports of previous studies that observed 90 to 100% wt alleles at these positions (9, 10). Furthermore, it largely matched the Varivax VZV profile (100% wt allele at positions 12779, 31732, and 107599; 52% wt allele at nt 107797) rather than the profiles of the GSK vaccines, which uniformly revealed mixed markers (Fig. 1).
The remaining 23 SNPs of the specimens displayed vaccine, wt, or mixed markers, apparently in a random fashion. There was no specimen that revealed wt alleles in all 23 variable positions (maximum, 18/23 for specimen no. 8) (Fig. 1). The percentage of wt markers in the different specimens varied by between 34% and 69%, and that of mixed markers varied by between 6% and 41% (Fig. 2). This indicated the presence of more than one vaccine variant in each specimen. Likewise, Loparev and coauthors reported on the presence of more than one vaccine variant in specimens from vaccinees. In accordance with our results (on average, 54% vaccine, 34% wt, and 12% mixed markers), these authors observed 59% vaccine, 33% wt, and 8% mixed markers in vaccine-related rashes (9).
Finally, PSQ of wt VZV from nonvaccinated (n = 4) and vaccinated (n = 5) individuals uniformly revealed alleles at all 32 SNP positions that had been defined as wt on the basis of the sequences of wt strains Oka-P and Dumas (Fig. 1). This showed that there is most likely no variation among wt strains in the 32 SNPs analyzed in this study (Fig. 1).
Comparison of orf62 sequences from vaccines with those from vaccinees suffering from rashes.
To determine whether vaccine-type VZV strains in patient specimens are genetically identical to vaccine-type VZV strains present as minorities in vaccines, we examined orf62, which encodes a transactivator protein thought to contribute to the attenuation of the vaccine (4, 5). orf62 encompasses more than one-third of the SNPs analyzed here and therefore is a highly representative locus in the genome. To analyze SNP profiles of single molecules, we cloned the entire orf62 in E. coli. Clones from the three vaccines were completely sequenced by CTSQ, and 11 SNP positions were analyzed. It appeared that Varivax contains a larger diversity of VZV variant strains than Varilrix and Priorix-Tetra. Thirty different SNP profiles were observed for Varivax and only 10 were observed for Varilrix and Priorix-Tetra (Fig. 4A and B, respectively). Furthermore, the Varivax variant strains on average contained more wt SNPs (37%; Fig. 4A) than the variant strains of Varilrix and Priorix-Tetra (17%; Fig. 4B). In addition, the SNP profiles of the cloned vaccine variant orf62 sequences were compared with the profiles of the vaccine-type VZV in the nine patient specimens with vaccine-type VZV. Importantly, one or more vaccine clone profiles were identical to one of the orf62 profiles originating from the specimens and were almost exclusively detected in Varivax (Fig. 3). It has previously been speculated (but not formally demonstrated) that the vaccine VZV variants detected in patient specimens preexist in the administered vaccine preparations (9, 10). Our data are the first to indicate that this is most likely the case (Fig. 3). However, our analysis was restricted to orf62, which covers only one-third of the SNPs differentiating vaccine strains from the parent wt strain Oka-P. Further analysis of complete genomes is required to settle this issue.
SNPs for diagnostic differentiation of wild-type versus vaccine-type VZV.
Different commercial preparations of varicella vaccine (Varilrix and Priorix-Tetra, both from GSK; Varivax from SPMSD) were compared by PSQ for the first time. Due to the higher sensitivity of PSQ than CTSQ, unprecedentedly high percentages of mixed SNPs were found in VZV strains in all three vaccine brands. However, even SNPs that were found by PSQ to be pure vaccine type and that had previously been reported to be pure (7, 9, 10) revealed very low levels of wt SNPs when larger numbers of E. coli clones were analyzed (Fig. 1). Four SNPs (nt 105705, 106262, 107252, and 108111) have formerly been reported to be pure vaccine type in vaccines and individuals with vaccine-related rashes (7, 9, 10). One of them, nt 106262, has been used in real-time PCR to firmly discriminate wt strains from vaccine strains (8). In this study, we detected 5 to 6% wt SNPs at two of the four positions (nt 106262 and 108111) by PSQ of a large number of E. coli clones of the vaccine Varivax VZV (Fig. 1). In addition, a complete orf62 of Varivax VZV was cloned in E. coli and revealed the wt allele at position 108111 (Fig. 4A). Of note, wt alleles were previously detected by CTSQ at positions 560, 105705, 107252, and 108111 in VZV strains from some older lots of Varilrix. In contrast, Varivax lots revealed VZV strains with pure vaccine types at these positions (13, 14). These data and those presented here show that the mixtures of variant strains in the vaccines as well as the relative strain proportions change with time in different vaccine batches. It is therefore reasonable to assume that the SNP profiles of vaccine-type VZV strains causing rash-like illnesses in vaccinees may change over time as well. On the basis of this assumption, the firm discrimination of vaccine-type VZV from wt VZV strains should include more than one SNP. Rather, the four-position core set (nt 105705, 106262, 107252, and 108111) should be used for diagnostic differentiation of wild-type and vaccine-type VZV.
Conclusions.
In this study of VZV variants, mixed alleles with small wt minorities were detected with a high sensitivity by PSQ. While CTSQ usually does not detect allele or mutant minorities present at levels below 20% in mixed alleles (2, 15), most PSQ assays detect them with a detection limit well below 10% (15; this study). Furthermore, alleles can be quantified by PSQ, in contrast to CTSQ. An additional advantage is that PSQ is much faster than CTSQ, enabling the analysis of 96 PCR products in 10 min. By PSQ, we determined an estimated 3-fold higher diversity of VZV variants with 20% more wt SNPs in Varivax than Varilrix and Priorix-Tetra. Furthermore, by analyzing the complete orf62, we observed VZV variants that represented minor components of the Varivax vaccine in vaccinated individuals suffering from mild rash-associated syndromes. Therefore, Varivax resembles a vaccine that appears to have more “wild-type-like” VZV. This might be the reason why Varivax, on the one hand, seems to be related to comparatively higher frequencies of rash-associated syndromes, i.e., mild forms of varicella or zoster, and, on the other hand, protects against varicella more efficiently. In the future, the ongoing German varicella sentinel will provide further insight into this issue. Finally, from our PSQ data on vaccine virus cloned in E. coli, it is concluded that the analysis of a core set of four SNPs is required as a minimum for a firm diagnostic differentiation of vaccine-type VZV from wt VZV.
Supplementary Material
ACKNOWLEDGMENTS
The technical assistance of Cornelia Walter and Sonja Liebmann is kindly acknowledged.
This work was funded by the Robert Koch Institute.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 11 May 2011.
REFERENCES
- 1. Breuer J., Schmid D. S. 2008. Vaccine Oka variants and sequence variability in vaccine-related skin lesions. J. Infect. Dis. 197(Suppl. 2):S54–S57 [DOI] [PubMed] [Google Scholar]
- 2. Castor J., Cook L., Corey L., Jerome K. R. 2007. Rapid detection directly from patient serum samples of human cytomegalovirus UL97 mutations conferring ganciclovir resistance. J. Clin. Microbiol. 45:2681–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davison A. J., Scott J. E. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67(Pt 9):1759–1816 [DOI] [PubMed] [Google Scholar]
- 4. Gomi Y., Imagawa T., Takahashi M., Yamanishi K. 2001. Comparison of DNA sequence and transactivation activity of open reading frame 62 of Oka varicella vaccine and its parental viruses. Arch. Virol. Suppl. 2001:49–56 [DOI] [PubMed] [Google Scholar]
- 5. Gomi Y., Imagawa T., Takahashi M., Yamanishi K. 2000. Oka varicella vaccine is distinguishable from its parental virus in DNA sequence of open reading frame 62 and its transactivation activity. J. Med. Virol. 61:497–503 [DOI] [PubMed] [Google Scholar]
- 6. Gomi Y., et al. 2008. DNA sequence analysis of varicella-zoster virus gene 62 from subclinical infections in healthy children immunized with the Oka varicella vaccine. Vaccine 26:5627–5632 [DOI] [PubMed] [Google Scholar]
- 7. Gomi Y., et al. 2002. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 76:11447–11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harbecke R., et al. 2009. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J. Med. Virol. 81:1310–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loparev V. N., Rubtcova E., Seward J. F., Levin M. J., Schmid D. S. 2007. DNA sequence variability in isolates recovered from patients with postvaccination rash or herpes zoster caused by Oka varicella vaccine. J. Infect. Dis. 195:502–510 [DOI] [PubMed] [Google Scholar]
- 10. Quinlivan M. L., et al. 2007. Natural selection for rash-forming genotypes of the varicella-zoster vaccine virus detected within immunized human hosts. Proc. Natl. Acad. Sci. U. S. A. 104:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quinlivan M. L., Gershon A. A., Steinberg S. P., Breuer J. 2004. Rashes occurring after immunization with a mixture of viruses in the Oka vaccine are derived from single clones of virus. J. Infect. Dis. 190:793–796 [DOI] [PubMed] [Google Scholar]
- 12. Ronaghi M. 2001. Pyrosequencing sheds light on DNA sequencing. Genome Res. 11:3–11 [DOI] [PubMed] [Google Scholar]
- 13. Sauerbrei A., Rubtcova E., Wutzler P., Schmid D. S., Loparev V. N. 2004. Genetic profile of an Oka varicella vaccine virus variant isolated from an infant with zoster. J. Clin. Microbiol. 42:5604–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sauerbrei A., Zell R., Harder M., Wutzler P. 2006. Genotyping of different varicella vaccine strains. J. Clin. Virol. 37:109–117 [DOI] [PubMed] [Google Scholar]
- 15. Schindele B., et al. 2010. Improved detection of mutated human cytomegalovirus UL97 by pyrosequencing. Antimicrob. Agents Chemother. 54:5234–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siedler A., Arndt U. 2010. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill. 15(13):pii=19530. [PubMed] [Google Scholar]
- 17. Spackova M., et al. 2010. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine 28:686–691 [DOI] [PubMed] [Google Scholar]
- 18. Tillieux S. L., et al. 2008. Complete DNA sequences of two Oka strain varicella-zoster virus genomes. J. Virol. 82:11023–11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.