Abstract
Previous studies suggest that altered virus-specific T-cell responses observed during chronic ethanol exposure may be due to abnormal functioning of dendritic cells (DCs). Here we explored the effects of ethanol on exogenous antigen presentation by DCs. BALB/c, C57BL/6, and CBA/caj mice were fed ethanol or an isocaloric control diet for 8 weeks. The splenic DC population was expanded using an Flt3L expression plasmid via tail vein injection. DCs were purified and assessed for antigen presentation and processing and for peptide-major histocompatibility complex class I and II (MHCI and MHCII) formation on the cell surface. Interleukin-2 (IL-2) was measured as an indicator of antigen-specific T-cell activation by DCs in coculture. Antigen processing and peptide-MHCII complexes were evaluated by flow cytometry. We observed that ethanol not only suppressed allogeneic peptide presentation to T cells by DCs but also altered presentation of exogenous ovalbumin (OVA) peptide 323-339 to an OVA-specific DO11 T-cell line as well as to OVA-sensitized primary T cells. Smaller amounts of peptide-MHCII complexes were found on the DCs isolated from the spleens of ethanol-fed mice. In contrast to MHCII presentation, cross-presentation of exogenous OVA peptide via MHCI by DCs remained intact. More importantly, ethanol-exposed DCs had reduced B7-DC and enhanced ICOS-L (inhibitory) costimulatory molecule expression. Ethanol inhibits exogenous and allogeneic antigen presentation and affects the formation of peptide-MHCII complexes, as well as altering costimulatory molecule expression on the cell surface. Therefore, DC presentation of peptides in a favorable costimulatory protein environment is required to subsequently activate T cells and appears to be a critical target for the immunosuppressive effects of ethanol.
INTRODUCTION
Long-term excessive ethanol consumption has been associated with increased susceptibility to bacterial and viral infections in alcoholics (3, 32, 34). Ethanol has been reported to inhibit the functioning of multiple components of the immune system; both innate immune cells, such as neutrophils, monocytes, macrophages, and dendritic cells (DCs), and B and effector T cells involved in adaptive immunity are adversely affected in both in vitro and in vivo ethanol exposure models. Several signaling pathways found in innate immune cells, involving cytokines, Toll-like receptors (TLRs) 2, 3, 4, and 9, and their downstream targets, such as NF-κB as well as signal transducers and activators of transcription (STAT), have been reported to be affected negatively by acute and chronic ethanol exposure (11, 12, 22–24, 27). In addition, secretion of the proinflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 has been found to be altered as well (1).
In this regard, we have provided evidence by use of a murine model of chronic ethanol feeding that CD4+ T-cell proliferation and cytotoxic T-lymphocyte (CTL) responses generated by genetic immunization against hepatitis C virus (HCV) core and nonstructural 5 (NS5) proteins were substantially reduced compared to those in isocaloric pair control mice (6, 9, 10). Further investigation revealed that CTL activity could be restored partly with additions of IL-2 and fully by coimmunization with granulocyte-macrophage colony-stimulating factor (GM-CSF) expression plasmids, suggesting that antigen-presenting cells (APCs) may be a critical target of ethanol's action to promote impaired CD4+ and CD8+ T-cell priming (6, 9, 10, 33). Indeed, subsequent studies revealed that adoptive transfer of splenic DCs derived from control but not ethanol-fed mice restored the generation of virus-specific CTL activity in the chronically ethanol-fed animals (1). This finding implied that depressed effector T-cell functions in the setting of chronic ethanol feeding may be due in part to intrinsic defects in antigen presentation capacity by DCs. This hypothesis was further supported by the finding that the alloreactivity of APCs isolated from ethanol-fed mice was impaired compared with that obtained from isocaloric pair control mice. In contrast, ethanol feeding had no effect on alloreactivity when healthy APCs derived from isocaloric pair-fed mice were cocultured with T cells isolated from ethanol-fed mice, as measured by T-cell proliferation (1). Such intrinsic defects in DCs were subsequently shown to produce abnormal T-cell activation due to impaired CD40, CD80, and CD86 costimulatory molecule expression and abnormal cytokine secretion (1). However, it was also possible that DC antigen processing and presentation pathways may have been altered by chronic ethanol consumption.
DCs are a heterogeneous population of professional APCs of importance not only to the activation of naïve cells (2, 17) but also to the recall phase (36) of the adaptive immune response against viral and bacterial pathogens. There is a repertoire of distinct DC subsets (14) that are specialized to take up, process, and present exogenous and endogenous antigens to CD4+ T cells via major histocompatibility complex class II (MHCII) molecules and to CD8+ T cells by MHCI molecules, respectively (29). Recently, ethanol was shown to inhibit MHCI presentation of peptides by interfering with the proteasomal degradation of antigens in hepatocellular carcinoma cells in a CYP2E1-dependent manner (25, 26). However, the influence of ethanol on antigen processing and presentation by DCs, along with the formation of the peptide-MHCII complex on the cell surface as it relates to impaired T-cell activation, has not been investigated previously.
Therefore, we explored the effects of chronic ethanol exposure in vivo on antigen processing and presentation following endocytosis by DCs in a murine model. It was observed that ethanol impairs not only allogeneic peptide presentation but also presentation of exogenous antigens by DCs to CD4+ T cells. The processing of exogenous antigens within the endocytic pathway appeared unaffected, yet smaller amounts of peptide-MHCII complex molecules were found on the DC surface when cells were derived from ethanol-fed animals than when they were isolated from isocaloric pair control animals. Impaired T-cell activation by DCs derived from ethanol-fed animals was independent of previously observed increases in IL-10 production as well as reduced TNF-α, IL-6, IL-12p70, and gamma interferon (IFN-γ) cytokine secretion, since the neutralization of the former and restoration by addition of the latter cytokines to T-cell-DC cocultures did not restore abnormal T-cell activation produced by chronic ethanol administration. Our results also suggest that chronic ethanol feeding under these experimental conditions has no impact on cross-presentation of exogenous antigens to CD8+ T cells. These investigations emphasize how ethanol may suppress cellular immune responses by interfering with peptide presentation by conventional spleen-derived CD11c+ DCs.
MATERIALS AND METHODS
Antibodies and reagents.
Fluorescein isothiocyanate (FITC)-CD11c, phycoerythrin (PE)-CD11c, PE-T-cell receptor beta (PE-TCR-β), peridinin chlorophyll protein (PerCP)-CD8a, anti-mouse-FITC, anti-rabbit-FITC, PE-anti-41BB-L, PE-anti-B7DC, PE-anti-B7-H3, PE-anti-B7-H4, PE-anti-OX40L, PE-anti-ICOSL, and FITC-anti-CD86 were purchased from eBioscience (San Diego, CA). Ovalbumin (OVA), hen egg lysozyme (HEL), lipopolysaccharide (LPS), and rabbit anti-Ova (C 6534) were obtained from Sigma-Aldrich. OVA peptides 323-339 and 257-264 were purchased from GenScript (Piscataway, NJ). Medium for DCs and DO11 cell culture, known as clone food (CF), contained 25 mM HEPES-buffered RPMI supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids, 5 mg/ml gentamicin, 100 U/ml penicillin G, 2 mmol/liter glutamine, 5 × 10−5 2-mercaptoethanol, 1 mmol/liter sodium pyruvate, and 100 mg/ml streptomycin, which were purchased from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD).
Animals.
Six-week-old female BALB/c (H2d), CBA/caj (H2k), CBA/j (H2k), and C57BL/6 (H2b) mice were obtained from Jackson or Harlan Laboratories. Rag1KO OT-I mice were purchased from Taconic. All animal procedures were approved by the Life Span Animal Care and Use Committee.
Ethanol feeding regimen.
Six-week-old female BALB/c, CBA, and C57BL/6 mice were placed on a chronic ethanol diet (Bioserv liquid diet; Bioserv, Frenchtown, NJ) that was prepared according to the manufacturer's instructions as previously described (1). Mice were fed either liquid ethanol or an isocaloric control diet for 8 weeks. The ethanol content in the diet was gradually increased from 1.25% (wt/vol) to 2.5% by the second week and to 3.75% by the third week. Mice were injected with Flt3L plasmid to expand the DCs in vivo 12 days before sacrifice.
Generation of DCs.
To boost the number of DCs obtained from the spleen, mice were given hydrodynamic injections with 10 μg of Flt3L-encoding plasmid in 2 ml saline via the tail vein (twice), as previously described (13). The injections were performed 12 and 7 days prior to the end of the 8-week feeding period. The animals were sacrificed at the 8th week. DCs were isolated from spleens by use of CD11c microbeads (Miltenyi Biotec) and a magnetic separator. The purity of the Flt3L-induced CD11c+ population was >95% after each isolation process, as measured by flow cytometry.
Alloreactivity assays.
Allostimulation assays were performed with negatively selected T cells isolated from C57BL/6 spleens by use of pan-T-cell purification microbeads (Miltenyi Biotec). Stimulator DCs were isolated from the BALB/c or CBA/caj strain after 8 weeks of ethanol or isocaloric pair feeding following Flt3L expansion in the spleen. After isolation, 107 DCs were incubated with or without 200 ng/ml LPS overnight. DCs were washed three times with serum-free medium and cocultured with various numbers of T cells for 3 days (for enzyme-linked immunosorbent assay [ELISAs]) or 6 days (for proliferation assays) at 37°C. For ELISAs and thymidine incorporation assays, 105 T cells were plated in 96-well round-bottom plates. For carboxyfluorescein succinimidyl ester (CFSE) assays, 106 T cells were plated in 24-well plates.
Antigen degradation assays.
OVA and green fluorescent protein (GFP) (Millipore, Temecula, CA) degradation was measured by two different methods. OVA degradation was assessed as previously described (28). Briefly, OVA protein was conjugated covalently to 3-μm latex beads (Polysciences) according to the manufacturer's instructions. Conjugation was confirmed by flow cytometry. Next, 1 × 107 CD11c+ DCs derived from ethanol or control groups were resuspended in 200 μl of CF medium. Protease inhibitors (Roche minitablet) were added to the medium as a separate control for assessment of protein degradation. Another control included DCs pulsed with unconjugated beads. All tubes were incubated at 37°C for 5 min prior to being pulsed with beads (18 μl). OVA-bead or bead-only suspension was added and thoroughly mixed with the cells. After 15 min of incubation at 37°C, 1 ml of cold medium was added to stop phagocytosis, and the free beads were washed away by overlaying the cell suspension on a 2-ml FBS flotation cushion. After 5 min of centrifugation at 150 × g and 4°C, the pellet was resuspended in 1 ml medium and the overlaying step was repeated twice. Next, the cells were resuspended in 2 ml CF medium and incubated at 37°C. At various times, 300 μl was taken from tubes, spun, and lysed overnight, followed by staining with rabbit anti-OVA and FITC-anti-rabbit antibodies. The samples were then analyzed by FACSCalibur flow cytometry.
The GFP degradation assay was performed by conjugating GFP to 3-μm immunomagnetic beads (Calbiochem) according to the manufacturer's instructions. Flt3-expanded splenocytes derived from ethanol and control spleens were incubated with the conjugated or unconjugated beads for 5 h. The phagocytic cells (including DCs and others) were selected using magnets, and the free beads were separated by Lympholyte (Cedarlane, Hornby, Canada) density gradient centrifugation. Cells containing beads were incubated at 37°C, stained at different times with PE-CD11c, and examined on a FACSCalibur flow cytometer for GFP loss. As controls, GFP-bead-pulsed and fixed phagocytic cells or unconjugated bead-pulsed cells were included in the analysis.
HEL48-61 peptide presentation.
CD11c+ splenic DCs were cultured in CF containing 1 mg/ml HEL protein for 25 h. Cells (1 × 106) were removed at different times, washed with Hanks' balanced salt solution (HBSS), and stained with PE-CD11c and mouse Aw.3.18 antibody (4), followed by staining with FITC-anti-mouse antibody. CD11c+ cells were gated and analyzed for FITC signals via FACSCalibur analyses. The mean fluorescence intensity (MFI) at each time point was subtracted from that at time point 0 and divided by that at time point 0 [relative MFI = (tx − t0)/t0].
Peptide presentation assays.
Stimulator DCs, isolated from ethanol or control diet-fed mice, were pulsed with soluble OVA323-339 peptide at the indicated concentrations (10 and 1 μg/ml), using 107 cells/ml in CF medium, for 20 (long pulse) or 2 (short pulse) hours. Cells were then washed extensively and used for coculture with responder T cells. Responder T cells were either OVA-specific DO11 CD4+ T-cell hybridomas or sensitized primary T cells. When DO11.10 cells were employed for ELISAs, IL-2 levels were measured at 18 h of coculture.
To obtain antigen-sensitized T cells, BALB/c mice were immunized subcutaneously (s.c.) on the back with 100 μg of OVA-complete Freund's adjuvant (OVA-CFA) and (incomplete) emulsion on days 0 and 7, respectively. The mice were sacrificed on days 12 to 15, and T cells were isolated from the spleen by use of pan-T-cell or CD4+ T-cell isolation microbead kits (Miltenyi Biotec). For ELISA measurements, 1 × 105 T cells were cocultured with 1 × 102 to 1 × 105 DCs in 96-well plates for 1 to 3 days.
Cell proliferation assay.
CFSE (Molecular Probes) labeling of T cells was performed with 5 μM carboxyfluorescein. Cells were labeled in a 50-ml Falcon tube with 1 × 107 cells/ml in HBSS supplemented with 5% FBS. Cells were washed three times with 10 volumes of HBSS containing 5% FBS and subsequently used for coculture experiments. On day 6, the cells were harvested and stained with PE-TCR-β and PerCP-CD8a. TCR-β+ cells were gated, and CD8+ and CD8− cells were analyzed for CFSE dilution.
As a second approach to measure T-cell proliferation, [3H]thymidine was added to 96-well plates at a concentration of 1 μCi/well 18 h before measurement. After being labeled, cells were transferred to 96-well V-bottom plates and washed extensively (three times), and their radioactivity was determined by use of a TopCount scintillation counter.
ELISAs.
IL-2 (eBioscience) measurements were performed according to the manufacturer's instructions.
Costimulatory molecule expression.
Freshly isolated or mature DCs [cultured overnight with 200 ng/ml LPS or poly(I:C) for 24 h] were stained for costimulatory molecule expression as well as for CD11c. The CD11c+ cells were gated and analyzed for the expression of the indicated costimulatory molecules.
RESULTS
Ethanol suppresses allogeneic antigen presentation.
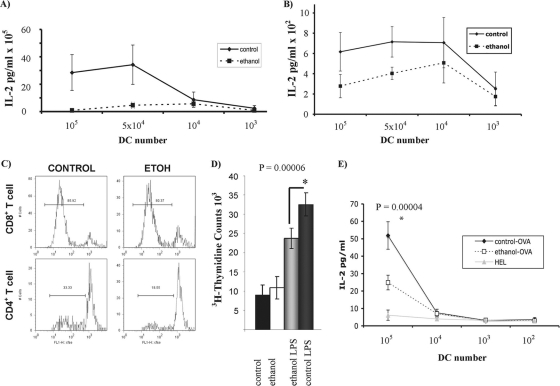
The inhibitory effect of ethanol on allogeneic peptide presentation by CD11c+ DCs to T cells (1, 20, 21) was assessed further in this study. In this regard, CD11c+ DCs isolated from spleens of both BALB/c and CBA/caj mice were cocultured with enriched T cells derived from C57BL/6 mouse spleens. T-cell activation was determined via IL-2 secretion measured by ELISA in cocultures, while proliferation of CD4+ and CD8+ T cells was detected by both CFSE labeling and [3H]thymidine incorporation. Ethanol suppressed proliferation and IL-2 secretion by T cells in DC-T-cell cocultures (Fig. 1 A to D).
Fig. 1.
Chronic ethanol exposure alters endogenous peptide presentation by murine BALB/c and CBA/caj DCs to allogeneic C57BL/6 T cells. (A) LPS-matured splenic CD11c+ DCs were purified from ethanol or isocaloric diet-fed CBA/caj mice and were cultured for 3 days with negatively selected T cells obtained from C57BL/6 mice. IL-2 levels in cocultures were measured via ELISA as an indicator of T-cell activation. Control DCs were significantly more potent at activating T cells (P < 0.01). (B) DCs were purified from ethanol or control diet-fed BALB/c mice and then cultured with C57BL/6 T cells. BALB/c DCs derived from control diet-fed mice were also significantly more potent activators of T cells (P < 0.01). (C) T-cell proliferation responses were assessed by CFSE dilution via flow cytometry. Ethanol or control diet-fed CBA/caj mouse-derived DCs were cocultured with C57BL/6 mouse-derived T cells. Labeled T cells were cultured for 5 days with DCs, and TCR-β+ CD8+ and TCR+ CD8− cells were gated for analyses. Proliferation was reduced in ethanol group-derived DC cocultures (results are representative of three experiments). (D) Ethanol or control diet-fed BALB/c mouse-derived DCs were cocultured with C57BL/6 mouse-derived T cells. [3H]thymidine incorporation was used to monitor cell proliferation. T-cell proliferation induced by mature BALB/c DCs derived from chronically ethanol-fed mice was significantly reduced. (E) DCs obtained from ethanol or control diet-fed BALB/c mice were pulsed with full-length OVA for 20 h and cocultured with the DO11 T-cell line overnight. Subsequently, IL-2 levels were measured in the supernatant via ELISA as an indicator of antigen-specific activation of T cells. DCs derived from control-fed mice presented OVA more efficiently than those derived from the ethanol-fed animals.
Ethanol does not alter the kinetics of exogenous antigen degradation following endocytosis.
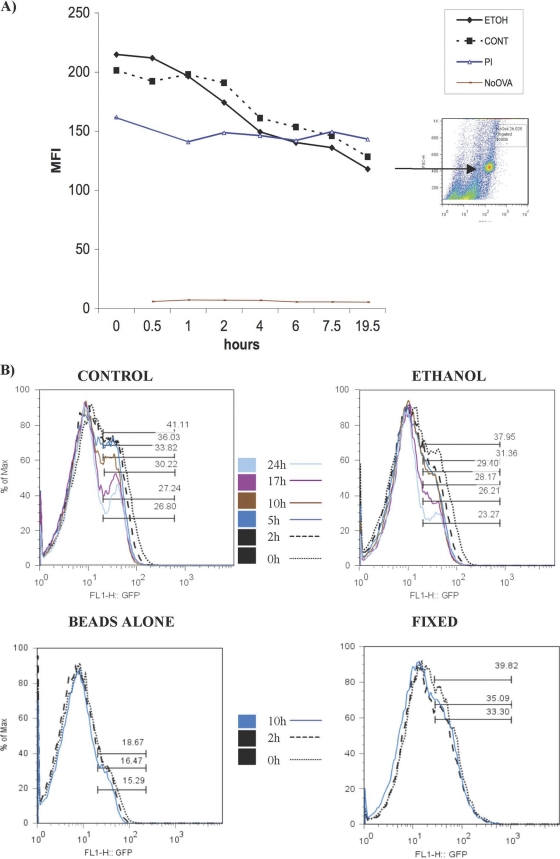
Previous studies suggested that ethanol does not alter phagocytosis or pinocytosis of exogenous antigens by DCs (1). However, Osna et al. reported that ethanol inhibited proteasomal degradation of peptides and thus reduced their presentation by MHCI molecules in hepatocytes (26). To resolve these discordant observations and to further explore the possible inhibitory influence of ethanol on the processing of exogenous antigens taken up through an endocytic process as a potential mechanism for depressed T-cell activation, we compared the kinetics of endocytosed OVA protein degradation by DCs isolated from control and ethanol-fed BALB/c mice. In this experiment, latex beads were conjugated with OVA, and CD11c+ splenic DCs were briefly pulsed. Cells were washed to remove free beads and then incubated at 37°C (28). At various times, DCs were harvested, lysed, stained with OVA-specific and FITC-coupled secondary antibodies, and analyzed by flow cytometry (Fig. 2 A). Protease inhibitors were added as a control for antigen degradation by DCs; uncoated beads were included as an additional control for specificity. There was comparable and equal degradation of OVA by DCs derived from both ethanol-fed and control mice (Fig. 2A). This finding was also recapitulated by the use of another protein (GFP), which confirmed the results observed with the OVA molecule (Fig. 2B). Therefore, there appears to be no defect in exogenous antigen uptake and degradation by DCs derived from chronically ethanol-fed mice under these experimental conditions.
Fig. 2.
Chronic ethanol consumption in mice does not alter processing of exogenous endocytosed antigens by DCs. (A) DCs derived from control and ethanol-fed mice degraded OVA with similar kinetics. As a control, DCs were pulsed with uncoated beads (no OVA) or with OVA-coated beads in the presence of a protease inhibitor cocktail (PI). The former (negative control) showed no signal; the latter (positive control) showed uniform signals across different time points. The inset shows the uncoated bead population measured by flow cytometry. (B) A second antigen degradation assay was performed with GFP-coated beads. Flt3L-expanded splenocytes were pulsed with GFP-coated magnetic beads or uncoated beads (beads only). Phagocytic cells were selected through a magnetic field, cultured at 37°C, and monitored over time for degradation of GFP. As a further control, DCs were pulsed with GFP-beads and fixed to ensure that the signal loss was specific for proteolytic degradation.
Pulsing of DCs with OVA323-339 peptide demonstrates impaired antigen-specific T-cell activation.
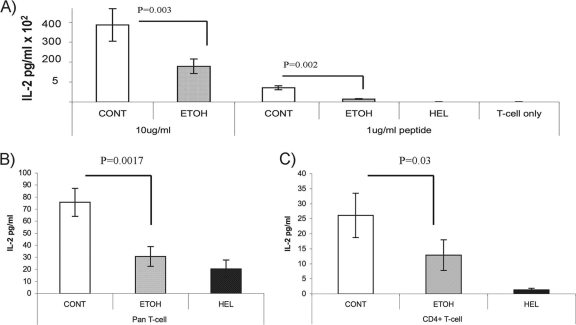
To further clarify the role of antigen processing, we pulsed ethanol-derived and control DCs with a short, “ready-to-present” 17-amino-acid OVA peptide (OVA323-339). These experiments revealed that when DCs were pulsed with small amounts of OVA323-339 peptide (1 μg/ml), presentation of the peptide by DCs derived from ethanol-fed animals to both DO11 T cells and OVA-sensitized primary T cells (CD4+ and pan-T cells) was impaired, as seen by IL-2 measurements (Fig. 3A to C). These results suggest that ethanol may alter the transport of peptide-MHCII complexes to the cell surface and/or influence peptide loading of MHCII molecules rather than affecting the degradation of exogenous antigens into immunogenic small peptides as shown in Fig. 2.
Fig. 3.
Pulsing of DCs with “ready-to-present” OVA323-339 peptide demonstrates impaired antigen-specific T-cell activation produced by chronic ethanol consumption. (A) DCs were pulsed with OVA323-339 peptide at different concentrations and cocultured with DO11 T cells for 18 h. IL-2 levels measured via ELISA were used to assess T-cell activation. HEL was used as a control for antigen specificity, and “T cells only” was a negative control that lacked DC additions. DCs derived from chronically ethanol-fed mice had impaired OVA peptide presentation. DCs were pulsed with OVA323-339 peptide and cocultured with pan-T cells (B) or CD4+ T cells (C) isolated from mice previously immunized with OVA protein. Ethanol diet-fed mouse-derived DCs pulsed with the OVA peptide were unable to restore T-cell activation to levels observed with control DCs.
Ethanol influences the abundance of peptide-MHCII complex formation on the DC surface.
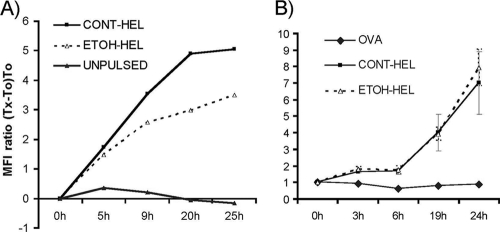
In order to assess the possible effects of ethanol on the presentation of specific peptide-MHCII complexes on the surfaces of DCs, we employed the monoclonal Aw3.18 antibody, which detects HEL48-61 peptide-MHCII complexes in the I-Ak genetic background (Fig. 4A). (4). The kinetics of pHEL48-61-MHCII accumulation on the surfaces of CD11c+ DCs isolated from ethanol or control diet-fed CBA/caj mouse spleens were compared at various times after pulsing of cells with HEL protein for approximately 24 h. We detected substantially larger amounts of peptide-MHCII complexes in DCs isolated from control diet-fed mice than in those from ethanol-fed mice (Fig. 4A). This difference between DCs derived from ethanol-fed and control mice was observed after 8 weeks of the diet regimen but not after 4 weeks of ethanol feeding (Fig. 4B). This finding confirms previous studies which observed equal amounts of MHC class II molecules expressed on the surfaces of DCs isolated from 4-week chronically ethanol-fed and isocaloric pair controls under the same experimental conditions (1).
Fig. 4.
Chronic ethanol consumption in mice results in reduced peptide-MHCII complex formation on the surfaces of DCs. (A) CD11c+ splenic DCs isolated from control or ethanol diet-fed mice (8 weeks) were pulsed with HEL protein. At different times, cells were stained with Aw3.18 antibody followed by FITC-anti-mouse IgG and were visualized via FACSCalibur flow cytometry. The signal for each time studied was calculated as follows: relative MFI = (tx − t0)/t0. Unpulsed DCs were used as a negative control. DCs derived from control diet-fed mice displayed strikingly higher peptide MHCII levels than those isolated from ethanol-fed animals. The graph is representative of three independent experiments. (B) DCs purified from 4-week ethanol-fed CBA/caj mice were pulsed with HEL, washed, and incubated at 37°C for analyses of peptide-MHCII complex formation on the DC surface. OVA protein pulsing was used as a specificity control/negative control. As observed previously, 4 weeks of ethanol feeding was not sufficient to alter the immune response associated with depressed DC function (1, 6, 8–10); rather, 8 weeks of chronic ethanol exposure was required.
Peptide-MHCII complex abundance is not sufficient to differentially activate T cells.
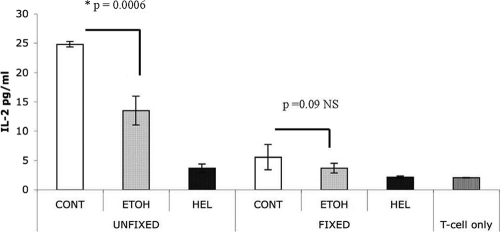
To test whether lower levels of peptide-MHCII complexes found on DCs isolated from ethanol-fed mice were responsible for reduced DO11 T-cell activation, we fixed DCs after a 20-h pulse with OVA. Because fixation of DCs following their pulse with antigen eliminates secretion of cytokines and the clustering of costimulatory molecules, an assay in which DCs are fixed prior to coculture with T cells was deemed informative for testing the role of reductions in the abundance of peptide-MHCII complexes in the suppressed T-cell activation by DCs following ethanol exposure (Fig. 4A). Such an assay would suggest the additional requirement for DC cytokine secretion to activate T cells. However, we did not observe a significant difference in T-cell activation between fixed DCs derived from chronically ethanol-fed and control animals (Fig. 5), although control DCs tended to be better stimulators of T-cell activation, consistent with higher levels of peptide-MHCII complex expression on their surfaces.
Fig. 5.
Effect of DC fixation on activation of T cells. OVA- or HEL-pulsed CD11c+ splenic DCs were fixed before their coculture with DO11 T cells. Subsequently, IL-2 levels were measured by ELISA after 18 h of culture. There was no statistical difference between fixed DCs derived from both ethanol and control diet-fed mice, since they presented endogenous OVA in the absence of DC cytokine secretion. However, IL-2 levels of fixed DCs were much lower than those observed in nonfixed DCs (P = 0.0006).
Ethanol does not impact cross-presentation of exogenous antigens.
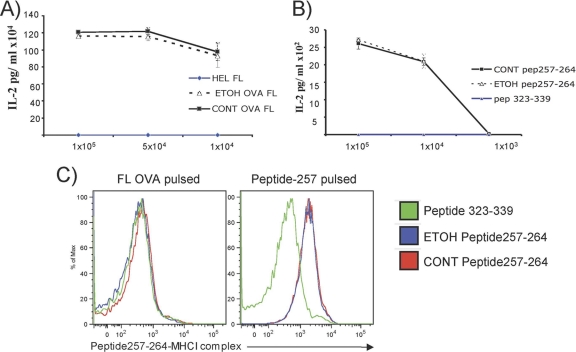
To determine the potential effects of ethanol on antigen cross-presentation by DCs, we cultured DCs purified from ethanol-fed and isocaloric pair control C57BL/6 mice, pulsed them with full-length OVA (Fig. 6A) or OVA peptide 257-264 (SIINFEKL) for 20 h (Fig. 6B), and cocultured them with OT-I CD8+ T cells derived from transgenic mice. We observed a comparable activation of T cells. Similarly, we detected comparable amounts of peptide 257-264-MHCI complexes on the surfaces of control and ethanol-derived DCs, as quantified by use of a monoclonal antibody specific to the SIINFEKL-MHCI complex for flow cytometry (Fig. 6C).
Fig. 6.
Chronic ethanol consumption does not adversely affect cross-presentation of exogenous antigens. DCs obtained from 8-week chronic ethanol or control diet-fed C57BL/6 mice were pulsed with full-length OVA (A) or OVA peptide 257-264 (B) for 20 h and then cocultured with OT-I T cells purified from transgenic mice overnight. Both full-length protein and short peptides of exogenous OVA were cross-presented via MHCI at similar efficiencies by ethanol-exposed and control DCs. (C) Peptide-MHCI complexes on the DC surface were quantified by use of a monoclonal antibody specific to SIINFEKL-MHCI after pulsing of cells with full-length OVA protein or with peptide 257-264. Eight-week ethanol exposure had no effect on peptide-MHCI presentation on the DC surface after 24 h of pulsing with peptide 257-264. Peptide 323-339 was used as an antigen specificity control for monoclonal antibody reactivity.
Analysis of costimulatory molecule expression.
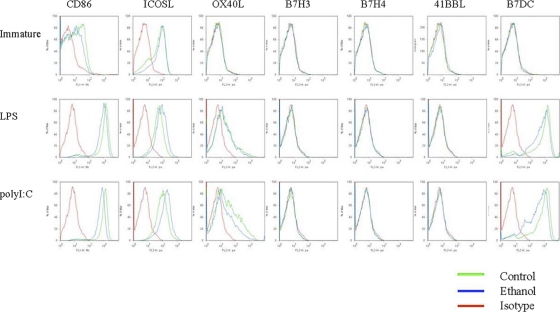
The expression of costimulatory molecules on the DC surface was assessed in response to chronic ethanol exposure. Previously, CD80, CD86, and CD40 levels were examined (1, 20). We extended these experiments by examining the expression of other costimulatory molecules which may have activating and/or inhibitory functions, since they may contribute to the reduced T-cell priming by ethanol-derived DCs. To do this, immature or LPS- or poly(I:C)-matured DCs were stained with anti-41BB-L, anti-B7-DC, anti-B7-H3, anti-B7-H4, anti-OX40L, and anti-ICOS-L antibodies, with anti-CD86 as a positive control (Fig. 7). Immature DCs expressed both CD86 and ICOS-L. However, DCs derived from ethanol-fed animals exhibited further reductions in CD86 expression, as previously described (1). After maturation with LPS or poly(I:C), DCs did not express the 41BBL, B7-H3, and B7-H4 cell surface molecules. However, CD86, ICOS-L, OX40L, and B7-DC (PD-L2) were highly expressed. More importantly, CD86 and B7-DC surface expression was reduced in matured DCs exposed to chronic ethanol. Interestingly, higher expression levels of the inhibitory ICOS-L marker (16, 35) were observed in DCs derived from ethanol-fed mice after maturation with both ligands. In contrast, OX40L levels were comparable between ethanol-fed and control mice. Thus, chronic ethanol consumption has a major effect on the expression of these DC costimulatory molecules, which may contribute to depressed T-cell activation.
Fig. 7.
Inhibitory and activating costimulatory molecules are expressed differentially in DCs after chronic ethanol exposure. DCs freshly isolated from ethanol or control diet-fed BALB/c mice or after maturation for 24 h with LPS or poly(I:C) were stained with CD11c antibodies and for the indicated costimulatory molecules. The CD11chigh cells were gated and analyzed for the expression of costimulatory molecules. ICOS-L was upregulated in ethanol-fed mouse-derived DCs, whereas B7-DC and CD86 were downregulated in DCs derived from ethanol-fed mice. OX40L was unchanged, while B7-H3, B7-H4, and 41BB-L were not expressed by mature DCs. The results represent three independent experiments.
DISCUSSION
Suppressive effects of ethanol exposure on the ability of DCs to present endogenous peptides to allogeneic T cells have been demonstrated by several in vitro and in vivo investigations (5, 8, 20, 21, 30). Moreover, the inhibitory effects of ethanol on DC presentation of exogenous antigens were recently explored (15, 21). In this regard, Mandrekar et al. found that in vitro exposure of monocyte-derived human myeloid DCs to ethanol inhibited presentation of tetanus toxoid antigen to memory T cells (21). Heinz and Waltenbaugh revealed that OVA presentation by CD8+ splenic DCs to transgenic primary DO11.10 CD4+ T cells was impaired. Furthermore, these cocultures were characterized by reduced secretion of IL-2, IL-12p70, IFN-γ, IL-17, and IL-6 (15). In addition, the generation of HCV NS5-specific CTL activity via genetic immunizations was also impaired in a chronic ethanol-fed murine model. Collectively, these studies suggest that priming of both CD4+ and CD8+ T cells by DCs is diminished as a consequence of chronic ethanol exposure (1). It appears, however, that the antigen-specific CD4+ and CD8+ T cells themselves are not abnormal; rather, it is the action of ethanol on DCs that produces such T-cell defects as those shown here and in a previous investigation (1). Despite accumulating evidence revealing impaired allogeneic and autologous antigen presentation by DCs derived from chronically ethanol-fed mice compared to that of DCs obtained from animals on an isocaloric control diet, the underlying cellular mechanisms responsible for reduced antigen presentation are poorly understood. Previously, it was suggested that chronic ethanol exposure does not impair antigen uptake pathways by DCs (1). Therefore, in this investigation, the fate of exogenous OVA protein following uptake by DCs was examined, with the aim of determining the possible effects of ethanol on antigen processing, as measured by degradation, to the final destination of peptide arrival on the cell surface in the context of MHCII presentation as well as cross-presentation by MHCI.
Through phagocytosis or pinocytosis, dendritic cells capture exogenous antigens and process them in the lysosome after they enter the endocytic pathway (18). In such a process, ethanol has the potential to disrupt the uptake and degradation of the antigen into its peptides, which would in turn affect the loading of the peptide on the MHCI/II complex. Our findings, however, reveal that the degradation of complex antigens is not affected by high ethanol intake, because the kinetics of the endocytosed full-length OVA protein or GFP were comparable in DCs derived from ethanol- and isocaloric control diet-fed mice (Fig. 2).
Furthermore, processing of the full-length intact protein in order to form the peptide-MHCII complex is necessary to trigger T-cell activation. If antigen processing was indeed altered by ethanol, then presenting DCs with a short, ready-to-present peptide could restore T-cell activation in affected ethanol-derived DCs. However, when the DCs were pulsed with low concentrations of the highly immunogenic OVA323-339 peptide—the 17-amino-acid epitope to which DO11 T cells are reactive—the DO11 T-cell line and both CD4+ T-cell and pan-T-cell activation by DCs prepared from ethanol-fed mice were still impaired, as seen by their lower IL-2 levels than those with DCs isolated from control mice (Fig. 3A to C). These observations collectively reveal that ethanol not only inhibits alloreactivity exhibited by T cells cocultured with DCs but also affects the presentation of exogenous small peptides by DCs to subsequently activate CD4+ T cells, and this presentation defect does not rely on an antigen degradation pathway.
Previously, MHC class II but not class I antigens were reported to be expressed differentially on DC surfaces derived from 8-week chronically ethanol-fed and isocaloric control diet-fed mice under these experimental conditions (1). In line with this observation, our results demonstrated that the individual peptide 48-61-MHCII complexes were strikingly lower in dendritic cells obtained from ethanol-fed mice than in those isolated from control mice after they were each pulsed with HEL protein (Fig. 4A). This finding suggests that the observed low abundance of MHCII molecules on the DC surface, as well as impaired peptide loading or interrupted transport of the peptide-MHCII complex to the DC surface, may together or separately be responsible for reduced peptide-MHCII complex presentation.
Cross-presentation, on the other hand, seems to be refractory to the effects of ethanol. In studying this class of molecules in C57BL/6 mice, LPS-matured DCs derived from ethanol-fed mice did not exhibit smaller amounts of peptide-MHCI complexes after being pulsed with the SIINFEKL peptide than those with control-derived DCs. These results confirm those found by Fan et al. (7), who argued that signal 1, the presentation of the processed peptide by the DC on the DC surface, is not affected by ethanol. It is noteworthy, however, that though this statement is true for MHCI molecules, as demonstrated with C57BL/6 mice, it does not apply to MHCII complexes; we demonstrated in this study a reduced peptide- MHCII complex formation in DCs from ethanol-fed mice compared to that in DCs from control mice when we examined the CBA/caj strain, which carries the H2 I-Ak MHCII molecule haplotype that is necessary for peptide-MHCII complex detection experiments (Fig. 6C).
Nevertheless, reduced T-cell activity may also be due to impaired direct T-cell interaction with molecules on the dendritic cell surface. Lower expression of CD80, CD86, and CD40 by mature (CpG) and immature splenic DCs derived from chronic ethanol-fed murine models has been established (1, 20). However, the evaluation of new costimulatory molecules revealed new inhibitory and stimulatory roles. Not only did we show that B7-H3, B7-H4, and 41BB-L are not expressed by immature DCs or by poly(I:C)- or LPS-matured DCs and that OX40L exhibits comparable expression in ethanol diet-fed mouse DCs and control mouse DCs following maturation, but we also demonstrated that B7-DC levels were consistently lower in matured DCs derived from ethanol-fed animals (Fig. 7). More importantly, we report here that ICOS-L was consistently found to be expressed highly in ethanol diet-fed mouse DCs matured with LPS and poly(I:C) compared to its expression in control DCs. Recently, high ICOS-L expression was reported to induce naïve CD4+ T cells to produce IL-10, which reduces TH1 responses. Thus, ICOS-L appears to be an inhibitory molecule for T-cell activation by DCs (16), although further studies will be required to determine ICOS-L's specific role in ethanol-induced DC functional abnormalities. These observations reveal that the reduced expression or overexpression of these costimulatory molecules can prevent their interaction with those present on the T-cell surface, which in turn decreases the dendritic cell's ability to generate an immunogenic response.
Additionally, Aloman et al. reported that chronic ethanol feeding of mice resulted in decreased IL-12, IL-6, TNF-α, and IFN-γ secretion and elevated IL-10 secretion by mature splenic CD11c+ DCs (1). If these soluble factors were mediating inhibition of T-cell activation, then adding back those cytokines which were found to be suppressed or neutralizing IL-10, which was observed to be upregulated, could restore some of the suppressive effects exhibited by ethanol on DC priming of T cells. Neither “add-back” experiments with IL-12, IL-6, TNF-α, or IFN-γ nor neutralizing IL-10 alone or in combination with cytokine additions restored the impaired DO11 T-cell activation, as measured by IL-2 production by T cells activated by DCs derived from ethanol-fed animals (data not shown). These results, taken in the context of OVA peptide presentation, are different from the findings of others regarding allogeneic stimulation, in which IL-12 additions restored the impaired T-cell proliferation driven by ethanol-exposed allogeneic DCs. However, we observed that defective OVA peptide presentation by DCs derived from ethanol-fed animals could not be restored by addition of recombinant IL-12 back to DC-T-cell cocultures, as measured by IL-2 secretion, and thus the assay conditions for T-cell function were quite different between the studies (31). In addition, there were several major differences in the experimental approaches between these two studies, including the antigen of interest, which may explain such disparate observations. Yet our findings allow us to conclude that disproportionate cytokine levels in ethanol-fed mice are not the sole explanation for their low T-cell activity but further indicate that ethanol's detrimental effects occur via more than one signal, i.e., via antigen presentation capability, costimulatory expression, and cytokine release.
Indeed, ethanol impairs DC-mediated T-cell activation; 8 weeks of ethanol feeding substantially reduced endogenous antigen presentation to T cells compared with that of DCs derived from isocaloric diet-fed controls, as assessed by IL-2 secretion and T-cell proliferation (Fig. 1A to D). These results support and extend previous findings of ethanol's effects on alloreactivity of bone marrow-derived splenic and hepatic murine DCs or monocyte-derived inflammatory DCs (5, 20, 21, 30). Moreover, they confirm recent findings by Fan et al. (36), who showed reduced T-cell functionality via T-cell proliferation studies when dendritic cells from ethanol-fed mice were cocultured with T cells. C57BL/6 mice, however, did not exhibit lower IL-2 levels after ethanol exposure; in fact, their IL-2 levels were comparable to those from control-fed mice, regardless of the pulsing of DCs with full-length OVA protein or the short OVA peptide 257-264 (Fig. 6A and B).
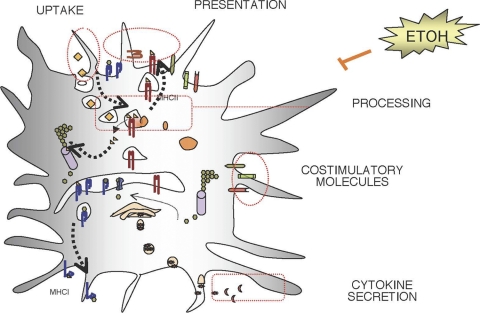
The results reported in this study help to elucidate the mechanism by which ethanol inhibits DC activation of T cells. As shown by the cartoon presented in Fig. 8, our investigations demonstrate that both endogenous antigen presentation by DCs to allogeneic T cells and exogenous OVA peptide presentation to DO11 and primary CD4+ T cells are impaired during chronic ethanol consumption in mice. The results suggest that there are no defects in uptake and degradation of exogenous complex antigens in the endocytic pathway. However, DCs derived from chronically ethanol-fed mice demonstrated reduced peptide-MHCII complex presentation compared to those from control mice, with higher expression of the inhibitory costimulatory molecule ICOS-L as well as deregulation of other costimulatory molecules, such as CD86 and B7-DC. Moreover, ethanol seems to impede cytokine release in order to repress host T-cell responses, which appears to be particularly important with respect to exogenous viral protein (1, 19). Therefore, our results suggest that the reduced T-cell activity in ethanol-fed mice is due to the combination of these three disrupted signals that work together to ensure proper T-cell activation when DCs detect an immunogenic antigen.
Fig. 8.
Summary of ethanol's effects on DC activation of T cells. The cartoon illustrates the global effects of chronic ethanol exposure on DC activation of T cells, as demonstrated by the above-mentioned results and by the previous studies of others (1, 5, 8, 15, 20, 21, 30, 34). DCs derived from chronically ethanol-fed mice exhibit normal antigen uptake (1) and degradation. However, the functional effect of ethanol on DC activation of antigen-specific T cells (1, 15, 21) is multifactorial and involves abnormalities of cytokine secretion (1, 5, 31), upregulation and downregulation of costimulatory molecule expression (1, 20), peptide-MHCII complex formation, and subsequent presentation of these complexes on the cell surface; all of these alterations may contribute to depressed cellular immune responses in mice with chronic ethanol consumption.
ACKNOWLEDGMENT
This study was supported in part by grant AA-08169 from the National Institutes of Health.
Footnotes
Published ahead of print on 11 May 2011.
REFERENCES
- 1. Aloman C., Gehring S., Wintermeyer P., Kuzushita N., Wands J. R. 2007. Chronic ethanol consumption impairs cellular immune responses against HCV NS5 protein due to dendritic cell dysfunction. Gastroenterology 132:698–708 [DOI] [PubMed] [Google Scholar]
- 2. Banchereau J., Steinman R. M. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 3. Cook R. T. 1998. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin. Exp. Res. 22:1927–1942 [PubMed] [Google Scholar]
- 4. Dadaglio G., Nelson C. A., Deck M. B., Petzold S. J., Unanue E. R. 1997. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity 6:727–738 [DOI] [PubMed] [Google Scholar]
- 5. Dolganiuc A., et al. 2003. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin. Exp. Res. 27:1023–1031 [DOI] [PubMed] [Google Scholar]
- 6. Encke J., zu Putlitz J., Geissler M., Wands J. R. 1998. Genetic immunization generates cellular and humoral immune responses against the nonstructural proteins of the hepatitis C virus in a murine model. J. Immunol. 161:4917–4923 [PubMed] [Google Scholar]
- 7. Fan J., et al. Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation. Alcohol Clin. Exp. Res. 35:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng D., Eken A., Ortiz V., Wands J. R.posting date. Chronic alcohol-induced liver disease inhibits dendritic cell function. Liver Int. Mar 21, 2011. doi. 10.1111/j.1478-3231.2011.07514.x/abstract. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 9. Geissler M., Gesien A., Tokushige K., Wands J. R. 1997. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J. Immunol. 158:1231–1237 [PubMed] [Google Scholar]
- 10. Geissler M., Gesien A., Wands J. R. 1997. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J. Immunol. 159:5107–5113 [PubMed] [Google Scholar]
- 11. Goral J., Choudhry M. A., Kovacs E. J. 2004. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J. Leukoc. Biol. 75:553–559 [DOI] [PubMed] [Google Scholar]
- 12. Goral J., Kovacs E. J. 2005. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 174:456–463 [DOI] [PubMed] [Google Scholar]
- 13. He Y., et al. 2000. Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum. Gene Ther. 11:547–554 [DOI] [PubMed] [Google Scholar]
- 14. Heath W. R., Carbone F. R. 2009. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10:1237–1244 [DOI] [PubMed] [Google Scholar]
- 15. Heinz R., Waltenbaugh C. 2007. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin. Exp. Res. 31:1759–1771 [DOI] [PubMed] [Google Scholar]
- 16. Ito T., et al. 2007. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung S., et al. 2002. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurts C., Robinson B. W., Knolle P. A. 2010. Cross-priming in health and disease. Nat. Rev. Immunol. 51:1430–1437 [DOI] [PubMed] [Google Scholar]
- 19. Kuzushita N., et al. 2006. Vaccination with protein-transduced dendritic cells elicits a sustained response to hepatitis C viral antigens. Gastroenterology 130:453–464 [DOI] [PubMed] [Google Scholar]
- 20. Lau A. H., Abe M., Thomson A. W. 2006. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J. Leukoc. Biol. 79:941–953 [DOI] [PubMed] [Google Scholar]
- 21. Mandrekar P., Catalano D., Dolganiuc A., Kodys K., Szabo G. 2004. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J. Immunol. 173:3398–3407 [DOI] [PubMed] [Google Scholar]
- 22. Mandrekar P., et al. 2002. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/Toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin. Exp. Res. 26:1609–1614 [DOI] [PubMed] [Google Scholar]
- 23. Norkina O., et al. 2008. Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes. Alcohol Clin. Exp. Res. 32:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norkina O., et al. 2007. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J. Leukoc. Biol. 82:752–762 [DOI] [PubMed] [Google Scholar]
- 25. Osna N. A. 2009. Hepatitis C virus and ethanol alter antigen presentation in liver cells. World J. Gastroenterol. 15:1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osna N. A., et al. 2008. Proteasome activation by hepatitis C core protein is reversed by ethanol-induced oxidative stress. Gastroenterology 134:2144–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pruett S. B., Schwab C., Zheng Q., Fan R. 2004. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J. Immunol. 173:2715–2724 [DOI] [PubMed] [Google Scholar]
- 28. Savina A., Vargas P., Guermonprez P., Lennon A. M., Amigorena S. 2010. Measuring pH, ROS production, maturation, and degradation in dendritic cell phagosomes using cytofluorometry-based assays. Methods Mol. Biol. 595:383–402 [DOI] [PubMed] [Google Scholar]
- 29. Shortman K., Naik S. H. 2007. Steady-state and inflammatory dendritic- cell development. Nat. Rev. Immunol. 7:19–30 [DOI] [PubMed] [Google Scholar]
- 30. Szabo G., Catalano D., White B., Mandrekar P. 2004. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin. Exp. Res. 28:824–828 [DOI] [PubMed] [Google Scholar]
- 31. Szabo G., Dolganiuc A., Mandrekar P., White B. 2004. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol 33:241–249 [DOI] [PubMed] [Google Scholar]
- 32. Szabo G., Mandrekar P. 2009. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin. Exp. Res. 33:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Szabo G., Mandrekar P., Dolganiuc A., Catalano D., Kodys K. 2001. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcohol Clin. Exp. Res. 25:1766–1772 [PubMed] [Google Scholar]
- 34. Szabo G., et al. 2010. Alcohol and hepatitis C virus—interactions in immune dysfunctions and liver damage. Alcohol Clin. Exp. Res. 34:1675–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tuettenberg A., et al. 2009. The role of ICOS in directing T cell responses: ICOS-dependent induction of T cell anergy by tolerogenic dendritic cells. J. Immunol. 182:3349–3356 [DOI] [PubMed] [Google Scholar]
- 36. Zammit D. J., Cauley L. S., Pham Q. M., Lefrancois L. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]