Abstract
The objective of this study was to determine if experimental infection of neonatal calves with Mycobacterium avium subsp. paratuberculosis would invoke changes in the percentages of total B cells in the peripheral blood mononuclear cell population and of subpopulations of B cells as determined by CD5, CD25, and CD45RO markers during a 12-month period. Experimental infection groups included control (noninfected), oral (infected with M. avium subsp. paratuberculosis strain K-10), oral/DXM (pretreatment with dexamethasone before oral inoculation), i.p. (intraperitoneal inoculation), and oral/M (oral inoculation with mucosal scrapings from a cow with clinical disease) groups. Over the course of the study, the percentages of total B cells in nonstimulated and antigen-stimulated cell cultures increased for oral and i.p. group calves, with the highest percentages noted at 3 and 6 months. Oral/M group calves had increased percentages of activated B cells, as determined by CD5dim and CD5bright markers, at 9 and 12 months. Experimental infection by all methods resulted in increased expression of CD25+ and CD45RO+ B cells early in the study, but the most significant results were observed at 12 months for oral/DXM and oral/M group calves. Immunoblot analyses with a whole-cell sonicate of M. avium subsp. paratuberculosis demonstrated the most reactivity with sera from i.p. group calves and the least reactivity with sera from oral group calves. Further evidence of M. avium subsp. paratuberculosis-specific antibody responses in the i.p. group calves was demonstrated using the ethanol vortex enzyme-linked immunosorbent assay (EvELISA) method. In summary, an induction of B cell responses was noted after experimental infection with M. avium subsp. paratuberculosis, with differences in responses noted according to the method of experimental inoculation.
INTRODUCTION
Similar to the majority of mycobacterial diseases, paratuberculosis (Johne's disease) is controlled primarily by T cell responses following infection with Mycobacterium avium subsp. paratuberculosis. Little is known about the role that B cells may play in the immune responses to M. avium subsp. paratuberculosis infection, yet it is widely accepted that antibodies secreted by B cells provide little benefit to the host in controlling or clearing the infection (17). However, B cells also present cognate antigen to CD4+ T cells and secrete cytokines and, as such, play a significant role in maintaining the full complement of immunity necessary to control intracellular infections (14). A loss of cell-mediated immunity dominated by CD4+ T cells has been observed in the later stages of M. avium subsp. paratuberculosis infection and is concomitant with increasing numbers of B cells and an increase in humoral immune responses (16, 20). Yet little work has been done to define B cell responses or to elucidate B cell subpopulations during M. avium subsp. paratuberculosis infection.
There are several markers for B cells that are indicative of activation, such as CD5, CD25, and CD45. CD5 is a membrane glycoprotein that is expressed on T cells as well as B-1a lymphocytes (4). CD5 is recognized as a mediator of T cell-B cell interactions, and increased expression of this cell surface marker has been noted on B cells in autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease (13, 15, 21). Likewise, CD25 expression increases upon activation of B cells, and this activation has been associated with increased antigen presentation by cells (6, 9). The CD45 marker is associated with the memory phenotype when it is expressed on CD4+ T cells (CD4+ CD45RO+), but it has also been demonstrated for B cell populations, suggesting that a memory B cell phenotype is present (14). In human patients with inflammatory bowel diseases such as Crohn's disease and ulcerative colitis, the CD45 marker on B cells can be used as an indicator of the stage of disease (23). Expression of CD45RO on CD19+ B cells in the lamina propria and the peripheral blood was indicative of patients transitioning to more advanced stages of disease (23).
Previous observations have demonstrated that the stage of paratuberculosis has a significant impact on the number and phenotype of circulating B cells in cattle that are naturally infected with M. avium subsp. paratuberculosis (18). We proposed to further evaluate the expression of B cell markers in concordance with the appearance of M. avium subsp. paratuberculosis-specific antibody in calves experimentally infected with live M. avium subsp. paratuberculosis. In a previous publication, we demonstrated that the route of experimental inoculation with M. avium subsp. paratuberculosis (oral versus intraperitoneal [i.p.]) and the strain of M. avium subsp. paratuberculosis used for inoculation (laboratory strain versus clinical isolate) impacted the degree of tissue colonization in a 12-month study (19). Calves inoculated orally with a clinical isolate of M. avium subsp. paratuberculosis had the largest number of positive tissue sites (15/22 sites), whereas calves in the i.p. group had the smallest number (8.5/22 sites). Here we present further observations on the impact of oral or i.p. inoculation and bacterial strain on the temporal changes in activated B cell subpopulations.
MATERIALS AND METHODS
Animals and experimental infection.
As previously described (19), neonatal Holstein dairy calves obtained from status level 4 herds enrolled in the Voluntary Bovine Johne's Control Program were purchased at 1 to 2 days of age and housed in biosafety level 2 containment barns for the duration of the study. Control calves were housed in a barn separate from the experimentally infected animals. Calves were allowed to acclimate to their environment for 1 week prior to being assigned to the following treatment groups: (i) control noninfected group (n = 4), (ii) oral group (n = 4), (iii) oral/dexamethasone (oral/DXM) group (n = 4), (iv) i.p. group (n = 4), and (v) oral/mucosal (oral/M) group (n = 3). Descriptions of the inoculum preparation and the route of inoculation are provided in a previously published manuscript (19). Briefly, on day 0 of the study, the oral and oral/DXM groups were fed milk replacer containing, on average, 1 × 1011 live M. avium subsp. paratuberculosis organisms of the low-passage strain K-10 2 times per day for 14 consecutive days. However, the oral/DXM group was administered 0.25 mg DXM/kg of body weight (Azium; 23 Schering Corp., Kenilworth, NJ) intravenously (i.v.) for 3 days prior to the first bacterial challenge and then administered the same dosage of DXM on days 28 and 56 of the study. Intraperitoneal inoculation of calves was performed on days 0, 7, 14, and 21 of the study by inoculation of 1 ml of M. avium subsp. paratuberculosis (1011 bacteria) through a 3-mm incision in the right flank (19). The oral/M group calves were inoculated on days 0, 7, and 14 of the study by being fed milk replacer containing 2.6 × 1012 live M. avium subsp. paratuberculosis organisms obtained by scraping the ileal mucosa from a clinically infected cow. The numbers of viable M. avium subsp. paratuberculosis organisms in the inoculum preparations were verified by performing serial 10-fold dilutions of the stocks in phosphate-buffered saline (PBS), followed by plating onto Herrold's egg yolk medium (HEYM; Becton Dickinson, Sparks, MD) in duplicate and culturing for 12 weeks at 39°C. Based upon the average recovery of M. avium subsp. paratuberculosis by culture, approximate dosages of viable M. avium subsp. paratuberculosis organisms were 3.6 × 1012 for oral and oral/DXM group calves, 4 × 1011 for i.p. group calves, and 8 × 1012 for oral/M group calves during the study. All procedures performed on the animals were approved by the Institutional Animal Care and Use Committee of the National Animal Disease Center (NADC), Ames, IA. All calves, regardless of inoculation route, were confirmed to be infected by culture of intestinal tissues and associated lymph nodes, as well as the spleen and liver, taken at necropsy (19).
Blood collection and culture conditions.
Blood was collected from the jugular vein in 2× acid-citrate-dextrose (ACD; 1:10). For each animal, blood was collected on 2 consecutive days prior to initiation of the study, followed by sampling on days 7, 14, and 28 and monthly thereafter for 12 months. Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat fractions of blood. PBMCs were resuspended in complete medium (RPMI 1640 [Gibco, Grand Island, NY] with 10% fetal calf serum [Atlanta Biologics, Atlanta, GA], 100 U of penicillin G sodium [Gibco] per ml, 100 μg of streptomycin sulfate [Gibco] per ml, 0.25 μg of amphotericin B [Gibco] per ml, and 2 mM l-glutamine [Gibco]). Cells were cultured at 2.0 × 106/ml in replicate 48-well flat-bottomed plates (Corning Incorporated, Corning, NY) at 39°C in 5% CO2 in a humidified atmosphere. Duplicate wells were set up for each animal for each in vitro treatment. In vitro treatments consisted of no stimulation (NS; medium only), concanavalin A (ConA) (10 μg/ml; Sigma Chemical Co., St. Louis, MO), and M. avium subsp. paratuberculosis whole-cell sonicate (MPS; 10 μg/ml). Plates were incubated for 6 days, and cells were harvested for flow cytometric analyses.
Flow cytometric analysis.
Briefly, culture plates were centrifuged at 1,500 rpm for 5 min, and the supernatant was decanted. Cells were gently resuspended in 300 μl of PBS (0.15 M, pH 7.4). In 96-well round-bottom plates (Corning Incorporated), 50 μl of the cell suspension was added to wells containing 50 μl of primary monoclonal antibody to B cells, CD5, CD25, and CD45RO (Table 1). All wells received 10 μg/ml of DAPI (4′,6-diamidino-2-phenylindole; Sigma) to differentiate live from dead cells and to allow gating on viable cells. Cells were then incubated at 4°C for 30 min. After incubation, plates were centrifuged at 1,250 rpm for 2 min at 4°C, and the supernatant was decanted. One hundred microliters of secondary antibody cocktail, consisting of fluorescein-conjugated anti-mouse IgM (Southern Biotech, Birmingham, AL), R-phycoerythrin-conjugated goat F(ab)2 anti-mouse IgG2a (Southern Biotech), and peridinin chlorophyll protein complex-conjugated rat anti-mouse IgG1 (Becton Dickinson, San Jose, CA), diluted 1:312, 1:625, and 1:42, respectively, in PBS with 1% fetal calf serum and 0.04% sodium azide, was then added to designated wells, and plates were centrifuged again at 1,250 rpm for 2 min at 4°C. The cells were then suspended in 200 μl of BD FacsLyse (BD Biosciences, San Jose, CA) for immediate flow cytometric analysis. Samples were evaluated using 30,000 events per sample in a FACScan flow cytometer with Cell Quest software (Becton Dickinson). Analysis was conducted by gating on mononuclear cells, based on forward and side scatter characteristics (FlowJo; Tree Star, Inc., San Carlos, CA). The percentages of total B cells and CD25-, CD45RO-, and CD5-positive cells within the B cell population were also determined. During flow cytometric analysis, the CD5 subpopulation was further segregated into CD5bright and CD5dim populations as previously reported for bovine B cells (18).
Table 1.
Primary antibodies used in this studya
| Antigen | Antibody clone | Isotype | Working MAb concn (μg/ml)b | Specificity |
|---|---|---|---|---|
| B lymphocyte | BAQ155A | IgG1 | 7 | Total B cells |
| CD5 | B29a | IgG2a | 7 | Activation marker |
| CD25 | CACT116A | IgG1 | 15 | IL-2 receptor |
| CACT108A | IgG2a | 15 | IL-2 receptor | |
| LCTB2A | IgG3 | 15 | IL-2 receptor | |
| CD26 | CACT114A | IgG2b | 15 | Activation marker |
| CD45RO | GC42A1 | IgG1 | 10 | Memory/activation marker |
Obtained from VMRD Inc. (Pullman, WA).
Antibodies were diluted in PBS with 1% fetal calf serum and 0.04% sodium azide.
Immunoblotting of sera.
Electrophoresis and immunoblot assays were performed using previously reported procedures (3). Briefly, a whole-cell sonicate of M. avium subsp. paratuberculosis (K-10 strain) was prepared by sonication of M. avium subsp. paratuberculosis organisms (1 × 109/ml) in PBS at 25 W for 25 min on ice (Tekmar sonic disturber; Tekmar, Lorton, VA), and the protein concentration was determined. The MPS was diluted to a final concentration of 1 mg/ml and then stored at −20°C. The reactivities of serum and plasma samples from cattle against the whole-cell sonicate were assessed using a Mini-Protean II slot blot device (Bio-Rad, Richmond, CA). Antigen was electrophoresed through preparative 12% (wt/vol) polyacrylamide gels and transferred to nitrocellulose filters. These filters were placed in a blocking solution consisting of phosphate-buffered saline plus 0.1% Tween 20 and 2% (wt/vol) bovine serum albumin (PBST-BSA). After being blocked, the filters were placed into the slot blot device, and individual sera, diluted 1:200 in PBST-BSA, were added to independent slots. After a 2-h incubation with gentle rocking, blots were washed three times with PBST and incubated with horseradish peroxidase-conjugated anti-goat IgG heavy and light chains (Vector Laboratories, Burlingame, CA) diluted 1:20,000 in PBST-BSA for 1.5 h. Blots were again washed three times with PBST and were developed for chemiluminescence in SuperSignal detection reagent (Thermo Scientific, Rockford, IL).
EvELISA.
Surface antigens of M. avium subsp. paratuberculosis (strain Linda) were extracted using 80% ethanol as previously described (8). Strain Linda, an isolate from a human patient with Crohn's disease (ATCC, Manassas, VA), resulted in more sensitive detection of antibody in JD-positive sera than in strain K-10 extracts (unpublished results). Briefly, M. avium subsp. paratuberculosis was harvested at stationary phase and centrifuged at 4,500 rpm for 10 min, and the pellet was resuspended in 80% ethanol to make 80 mg wet weight of M. avium subsp. paratuberculosis/ml of ethanol solution. The suspension was then vortexed for 30 s at room temperature, followed by centrifugation at 9,000 rpm for 10 min. The supernatant was diluted by a factor of 80 with 80% ethanol and inoculated into each well of 96-well plates (50 μl/well) (PolySorp Nunc-Immuno 96-microwell plate; Nalge Nunc International, Rochester, NY). The plates were then incubated with the cover removed overnight in a fume hood at room temperature to allow the extracted antigens to adhere to the surfaces of the wells by evaporation. Each well of the plate was then incubated with 200 μl of buffer A at room temperature for 1 h, washed twice with 100 μl of PBST (10 mM phosphate-buffered saline, pH 7.0, containing 0.5% Tween 80), and inoculated with 50 μl of sera, including M. avium subsp. paratuberculosis-positive and M. avium subsp. paratuberculosis-negative control sera. Sera were diluted 1:100 with buffer A and then incubated at room temperature for 1 h. After washing of the wells four times with 100 μl of PBST, each well was inoculated with 50 μl of horseradish peroxidase-labeled goat anti-bovine IgG(H+L) polyclonal antibody (1:500 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) or with 50 μl of biotin-labeled goat anti-bovine IgG(H+L) polyclonal antibody (1:500 dilution; Jackson ImmunoResearch Laboratories) and then incubated at room temperature for 1 h. Plates were then incubated with horseradish peroxidase-labeled streptavidin (50 μl per well; 0.5 μg/ml) (Pierce Biotechnology, Rockford, IL) at room temperature for 1 h. After washing of the wells five times with 100 μl of PBST, ABTS (2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) tablets were used to develop color reactions according to the manufacturer's instructions, and optical densities (ODs) at 415 nm were determined by a microplate reader (model 680; Bio-Rad, Hercules, CA) at 2-min intervals for 16 min. Aliquots of the same serum samples were also tested using a commercial enzyme-linked immunosorbent assay (ELISA) (Herdchek; Idexx, Westbrook, ME) according to the manufacturer's instructions. Samples were determined as positive or negative based upon the sample-to-positive-control (S/P) ratio. Briefly, serum samples from known M. avium subsp. paratuberculosis-positive and -negative cows by prior screening were used as positive and negative controls in each ELISA. S/P ratios were then calculated as follows: S/P ratio = (sample serum OD − negative-control serum OD)/(positive-control serum OD − negative-control serum OD). S/P ratios for the ethanol vortex ELISA (EvELISA) varied with the known positive and negative samples used in each test but averaged 0.038 (mean + 2 standard deviations [SD]) for the noninfected calves in this study. The commercial test kit has an S/P ratio of 0.250, as the positive and negative controls are standardized.
For all cell analyses, nonstimulated (medium control) cultures were included to measure constitutive responses to infection and ConA-stimulated cultures were included as positive controls to determine general cell reactivity. However, our interest was primarily on antigen-specific recall responses after experimental infection, so the ConA responses are not shown. In general, few differences in ConA responses were noted between control noninfected animals and experimentally infected calves.
Statistical analysis.
The percentage of each cell population was analyzed by using the PROC MIXED analysis of SAS (SAS PC Windows, version 9.1.3, software). Values are reported as least-square means ± standard errors of the means (SEM) unless noted otherwise. When significant effects (P < 0.05) owing to treatment, day, or treatment-day interactions were detected, mean comparisons between the control group and infected treatment groups were conducted by the Tukey-Kramer post hoc test. For clarity in presentation, symbols are used in the figures to designate significant differences between control calves and experimentally infected calves within each time point. Differences due to day (time after infection) or treatment-day interactions are discussed within the text and are not represented by symbols in the figures.
RESULTS
Tissue colonization after infectious challenge.
The mode of experimental M. avium subsp. paratuberculosis infection was previously demonstrated to have an impact on the extent of tissue colonization and lesion formation (19). Briefly, calves that were orally inoculated with a clinical isolate of M. avium subsp. paratuberculosis (oral/M group) had the largest number of positive tissue sites (15/22 tissues), whereas i.p. group calves had the smallest number of positive tissue sites (8.5/22 tissues). Bacterial burdens within tissues were low across treatment groups, except for one calf within the oral/M group that had large numbers of M. avium subsp. paratuberculosis organisms. Lesion formation within tissues was fairly unremarkable and was greatest in oral/DXM group calves, followed by oral, oral/M, and, lastly, i.p. group calves. No consistent pattern between tissue infectivity or pathology and B cell responses could be discerned for calves in the present study.
B cell responses in infected calves.
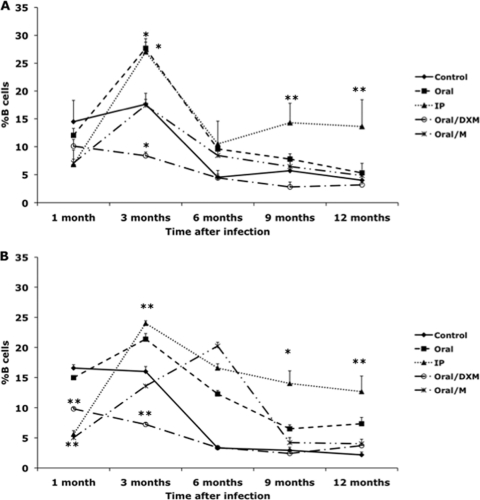
Culturing PBMCs with medium alone (NS) allowed us to discern differences in the percentage of total B cells due to infection status (Fig. 1 A). Differences due to the mode of infection were not demonstrated until 3 months of infection, with increases (P < 0.05) in B cells observed for oral and i.p. group calves. Thereafter, a decline in B cells was noted throughout the remainder of the study for all groups, yet i.p. group calves continued to demonstrate elevated numbers of B cells compared to the noninfected controls and other treatment groups. Interestingly, oral/DXM group calves were the only group with smaller numbers of B cells than the control calves, although this was significant (P < 0.05) only at 3 months. We also evaluated changes in B cell populations in fresh, noncultured PBMCs to determine if the mode of infection would affect B cell numbers, but treatment differences mirrored results for the NS cultured PBMCs, so those data are not shown. Upon stimulation of PBMCs with antigen (MPS), patterns of B cell numbers were similar to that observed for NS cultures, although at some time points the results were more definitive (Fig. 1B). The percentage of B cells in MPS-stimulated cultures increased (P < 0.05) for oral, oral/M, and i.p. group calves between 1 and 3 months, paralleling what was observed for nonstimulated cultures (Fig. 1A and B). Further increases (P < 0.05) in B cells were noted for oral/M group calves at 6 months, while in other treatment groups, B cell numbers had begun to decline by 6 months, with further reductions noted at 9 and 12 months. The percentages of antigen-specific B cells at 9 and 12 months were higher (P < 0.05) for i.p. group calves than for control noninfected calves (Fig. 1B), an effect that had been noted for nonstimulated PBMCs (Fig. 1A). Interestingly, for both the control noninfected calves and oral/DXM group calves, percentages of B cells were similar to those for other treatment groups at 1 month but had declined by 6 months and remained lower than those for the other treatment groups for the rest of the study, indicating that dexamethasone pretreatment disrupted the ability of calves to develop B cell responses to M. avium subsp. paratuberculosis infection at levels comparable to those for calves in other infection groups.
Fig. 1.
Percentages of B cells from PBMCs isolated from control noninfected calves (⧫) and calves infected with Mycobacterium avium subsp. paratuberculosis by the following methods: oral (▪), intraperitoneal (▴), oral with dexamethasone administration (○), and oral with mucosal scrapings from a cow with clinical disease (*). Data are for a 12-month study with nonstimulated PBMCs (A) and PBMCs stimulated with a whole-cell sonicate of Mycobacterium avium subsp. paratuberculosis (MPS) (B). Data are expressed as means ± SEM. Significant differences between control and infection groups for given time points are represented by asterisks (*, P < 0.01; **, P < 0.05).
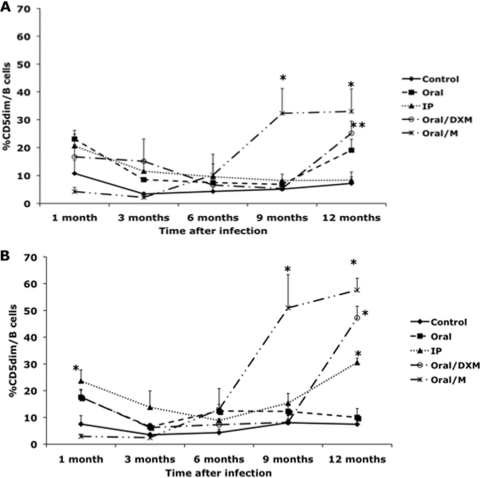
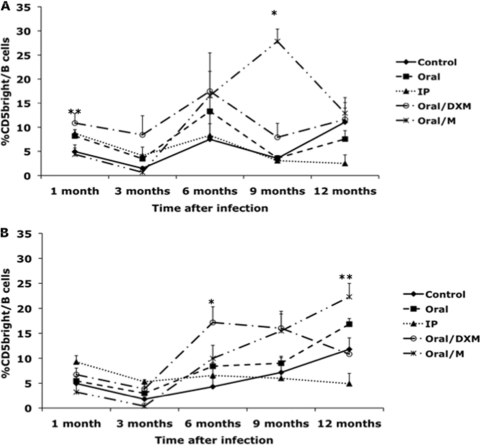
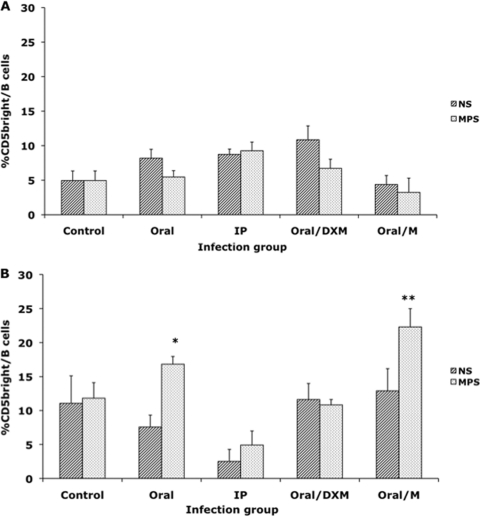
Inoculating the calves with a clinical isolate of M. avium subsp. paratuberculosis (oral/M group) resulted in an increase (P < 0.01) in the CD5dim B cell subpopulation at 9 and 12 months in both nonstimulated and MPS-stimulated cultures (Fig. 2 A and B). At 12 months postinfection, the percentage of CD5dim B cells in nonstimulated PBMCs was significantly (P < 0.05) higher for oral/M and oral/DXM group calves than for the control noninfected calves (Fig. 2A). A significant increase (P < 0.01) was also noted for MPS-stimulated PBMCs isolated from oral/M, oral/DXM, and i.p. group calves at 12 months. The percentage of CD5dim B cells remained fairly consistent throughout the study for both nonstimulated and MPS-stimulated PBMCs isolated from control calves. Oral/DXM group calves had larger numbers of CD5bright B cells in nonstimulated PBMC cultures than did control calves in the early months of the study (significant [P < 0.05] at 1 month) and tended to have higher levels than the other infection groups for most time points (Fig. 3 A). The exception was a marked (P < 0.05) increase in the CD5bright B cell subpopulation noted for oral/M group calves between 3 and 9 months of the study, resulting in higher (P < 0.05) expression levels than those for the other treatment groups at 9 months. The addition of MPS to unfractionated PBMCs resulted in similar trends shortly after experimental infection, with increases (P < 0.05) in CD5bright B cells noted for oral/DXM group calves at 6, 9, and 12 months and for oral/M calves at 9 and 12 months compared to time zero. Increases (P < 0.05) in CD5bright B cells were also noted for oral group and control noninfected calves at 12 months postinfection compared to the 3-month time point (Fig. 3B). Comparison of the ex vivo stimulation of unfractionated PBMCs with medium alone (nonstimulated) and MPS at 1 and 12 months suggested that additional stimulation with M. avium subsp. paratuberculosis antigen resulted in a shift in the phenotype of the CD5-positive cells for some treatment groups at 12 months (Fig. 4 A and B). At 1 month postinfection, there was little discernible effect due to stimulation of cells with MPS (Fig. 4A), but after 12 months of infection, a significant shift (P < 0.05) to increased numbers of CD5bright B cells was observed upon the addition of MPS to PBMC cultures for oral and oral/M group calves (Fig. 4B). Overall time effects between 1 and 12 months of infection demonstrated significant increases (P < 0.05) in CD5bright expression on B cells in MPS-stimulated cultures for control, oral, and oral/M group calves.
Fig. 2.
Percentages of CD5dim B cells from PBMCs isolated from control noninfected calves (⧫) and calves infected with Mycobacterium avium subsp. paratuberculosis by the following methods: oral (▪), intraperitoneal (▴), oral with dexamethasone administration (○), and oral with mucosal scrapings from a cow with clinical disease (*). Data are for a 12-month study with nonstimulated PBMCs (A) and PBMCs stimulated with MPS (B). Data are expressed as means ± SEM. Significant differences between control and infection groups for given time points are represented by asterisks (*, P < 0.01; **, P < 0.05).
Fig. 3.
Percentages of CD5bright B cells from PBMCs isolated from control noninfected calves (⧫) and calves infected with Mycobacterium avium subsp. paratuberculosis by the following methods: oral (▪), intraperitoneal (▴), oral with dexamethasone administration (○), and oral with mucosal scrapings from a cow with clinical disease (*). Data are for a 12-month study with nonstimulated PBMCs (A) and PBMCs stimulated with MPS (B). Data are expressed as means ± SEM. Significant differences between control and infection groups for given time points are represented by asterisks (*, P < 0.01; **, P < 0.05).
Fig. 4.
Comparison of in vitro stimulation with medium control (NS) and a whole-cell sonicate of Mycobacterium avium subsp. paratuberculosis (MPS) on the percentage of CD5bright B cells from PBMCs isolated from calves at 1 month of infection (A) and 12 months of infection (B). Data are expressed as means ± SEM. Significant differences between NS and MPS-stimulated PBMCs within a treatment group are represented by asterisks (*, P < 0.01; **, P < 0.05).
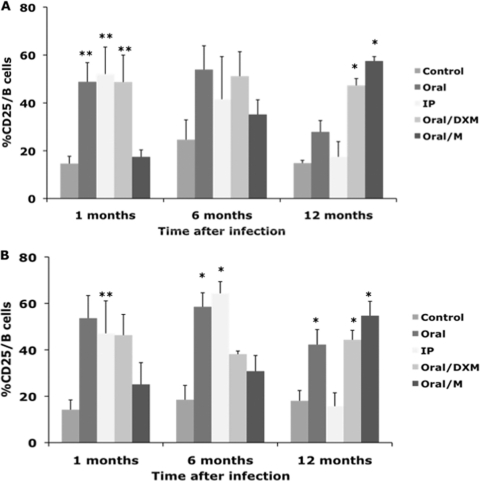
Experimental infection of calves resulted in significant (P < 0.05) increases in the percentage of CD25+ B cells in nonstimulated PBMCs from oral, i.p., and oral/DXM group calves, but not oral/M group calves, compared to control calves at 1 month postinfection (Fig. 5 A). By 6 months, these trends continued but were not significant. However, by 12 months postinfection, CD25 B cells had increased for oral/M group calves, resulting in larger (P < 0.05) numbers for oral/DXM and oral/M group calves than for control calves at the termination of the study. Although CD25 B cells decreased for oral and i.p. group calves at this time point, their levels still tended to be higher (P < 0.10) for oral group calves than for the controls. Similar patterns of CD25 expression were noted on B cells among MPS-stimulated PBMCs at 1, 6, and 12 months of the study (Fig. 5B), but increases in expression for oral and i.p. group calves were more significant at 6 months. Between 1 and 12 months, significant increases (P < 0.05) in the percentage of CD25 B cells were observed for oral/M group calves, regardless of ex vivo stimulation of PBMCs.
Fig. 5.
Percentages of CD25+ B cells from PBMCs isolated from control noninfected calves and calves infected with Mycobacterium avium subsp. paratuberculosis by the following methods: oral, intraperitoneal, oral with dexamethasone administration, and oral with mucosal scrapings from a cow with clinical disease. Data are for a 12-month study with nonstimulated PBMCs (A) and PBMCs stimulated with MPS (B). Data are expressed as means ± SEM. Significant differences between the control and infection groups for given time points are represented by asterisks (*, P < 0.01; **, P < 0.05).
The expression of CD45RO on B cells in MPS-stimulated PBMCs was upregulated in oral/DXM group calves (P < 0.01) and tended to be higher in oral group calves (P < 0.10) at 3 months postinfection than in control calves (Fig. 6). However, by 6 months postinfection, significant (P < 0.01) upregulation of CD45RO+ B cells was observed for all experimentally infected calves, except for oral/M group calves (due to high variability). At 12 months postinfection, this subpopulation of B cells was significantly greater for oral/DXM and oral/M group calves, averaging 57.6 and 55.2%, respectively, compared to 29.1% of the total B cell population for control calves at 12 months (Fig. 6). Similar trends in CD45RO expression on B cells were observed for nonstimulated PBMCs (Fig. 6A).
Fig. 6.
Percentages of CD45RO+ B cells from PBMCs isolated from control noninfected calves and calves infected with Mycobacterium avium subsp. paratuberculosis by the following methods: oral, intraperitoneal, oral with dexamethasone administration, and oral with mucosal scrapings from a cow with clinical disease. The data are for a 12-month study with nonstimulated PBMCs (A) and PBMCs stimulated with MPS (B). Data are expressed as means ± SEM. Significant differences between the control and infection groups for given time points are represented by asterisks (*, P < 0.01; **, P < 0.05).
Serum antibody responses in infected calves.
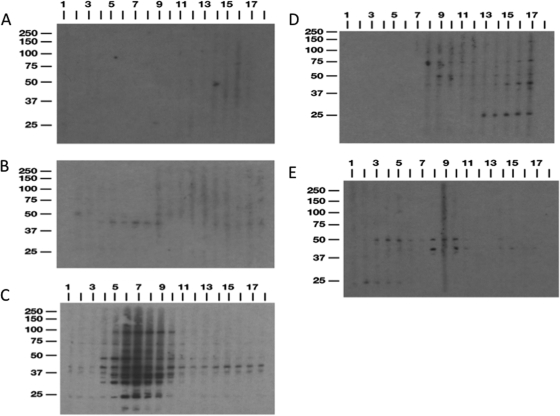
Immunoblot analysis was performed with sera from calves across all time points of the study (days −3, −2, 7, 14, and 21 and months 1 to 12). A whole-cell sonicate preparation of M. avium subsp. paratuberculosis strain K-10 was used as the antigen to assess the presence of serum antibodies. Sample blots for a representative calf from each treatment group are shown in Fig. 7. Banding patterns varied with treatment group, but major bands were apparent at 25, 42, and 50 kDa among the infection groups. Sera from control noninfected calves were consistently nonreactive on blots (Fig. 7A). Little immunoreactivity was observed for calves in the oral treatment group, with a major band apparent at approximately 42 kDa (Fig. 7B). Calves that were infected i.p. had the most robust responses, with numerous bands observed, including the 42-kDa band noted for oral group calves. Immunoreactivity was particularly robust between 1 and 6 months of infection, but strong reactivity was still discernible, with banding at 37, 42, 50, and 75 kDa, through the termination of the study (Fig. 7C). Another band of interest appeared at 25 kDa and was apparent for the majority of time points. Treatment of calves with dexamethasone prior to oral inoculation resulted in a delayed appearance of antibody, with bands appearing within 3 months and continuing through the 12-month study (Fig. 7D). This effect was observed for all 4 calves in the oral/DXM treatment group (data not shown), with 42-, 50-, and 75-kDa bands demonstrating the most consistency, although strong banding at 25 kDa was also noted in the latter part of the study. Surprisingly, the immunoblots for oral/M group calves demonstrated an early antibody response, with banding apparent at 25 and 50 kDa within 7 days of infection. Banding was markedly weaker after 5 months of infection but was still detectable at 42 and 50 kDa (Fig. 7E).
Fig. 7.
Immunoblotting of serum from a representative calf for each treatment group. (A) Control; (B) oral; (C) intraperitoneal; (D) oral with dexamethasone; (E) oral with mucosal scrapings from a cow with clinical disease. Sera were blotted against a whole-cell sonicate preparation of Mycobacterium avium subsp. paratuberculosis strain K-10. Lanes 1 to 17, days −3, −2, 7, 14, and 21 and months 1 through 12, respectively.
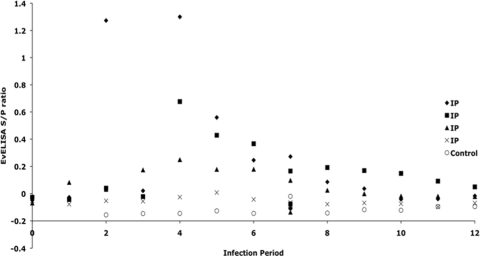
Further testing for M. avium subsp. paratuberculosis-specific antibody by the EvELISA method demonstrated the presence of antibody (determined by S/P ratio) only in the sera from i.p. inoculated calves (Fig. 8). Values for a control calf were plotted to demonstrate differences obtained between i.p. group calves and those from the other treatment groups. No antibody was detected by this method in any of the other treatment groups. As noted on the scattergram plot in Fig. 8, antibody was detected for 3 of 4 i.p. group calves (⧫, ▪, and ▴), ranging from 2 to 7 months postinoculation. Little antibody was detected by this method in the other i.p. group calf (×). Use of a commercial kit for the detection of M. avium subsp. paratuberculosis antibodies in the sera of experimentally infected calves was less sensitive, with positive S/P values of 0.417, 0.349, and 0.347 observed for only 1 i.p. group calf, at 7, 8, and 9 months postinfection, respectively (data not shown). Reactivity was observed in 2 additional i.p. group calves, but the level remained below the threshold of positive responses (S/P ratio of 0.250) outlined in the manufacturer's instructions. Interestingly, even though positive reactivity was attained to some degree for the same 3 i.p. group calves in both assays, the EvELISA detected antibody in the sera several months earlier than the commercial kit did.
Fig. 8.
Scattergram of EvELISA S/P ratios demonstrating antibody reactivity of sera from 4 calves in the i.p. treatment group compared to baseline values for one control noninfected calf. Values over the time course of the study are shown, and each symbol represents a single calf across time.
DISCUSSION
The progression of paratuberculosis from an asymptomatic subclinical state to one of clinical disease, as defined by signs of severe diarrhea and emaciation, coincides with a shift from Th1- to Th2-mediated immunity (16). The production of the Th2 regulatory cytokines interleukin-4 (IL-4), IL-5, and IL-10 supports a humoral immune response characterized by the proliferation of B cells, immunoglobulin secretion, and attenuation of Th1-mediated responses. The role that B cells may play in mycobacterial infections is not well understood, nor has it been addressed adequately. B cells are an essential component of adaptive immunity and are most noted for their ability to secrete antibodies that bind to pathogens and facilitate their uptake by phagocytic cells. B cells can also develop into memory cells, promoting an anamnestic response to antigens upon secondary exposure. However, B cells are antigen-presenting cells as well and play a role in the activation of CD4+ Th2 cells. Although it has not been explored fully, the decrease in CD4+ Th1-mediated function noted in the later stages of paratuberculosis may be associated closely with an increase in B cell activity (10).
An observation made previously by our group suggested that a shift in the percentage of B cells in the peripheral blood occurred with M. avium subsp. paratuberculosis infection, with increasing numbers in naturally infected cows demonstrating clinical signs of paratuberculosis compared to those in subclinically infected cows or healthy controls (19). Interestingly, the B cells isolated from cows with clinical disease were unresponsive to an M. avium subsp. paratuberculosis antigen preparation, whereas a normal proliferative response to the mitogen concanavalin A was observed. These observations were aligned with typical antemortem signs of clinical disease such as diarrhea and weight loss, concomitant with heavy shedding of M. avium subsp. paratuberculosis in the feces and a high serum antibody titer observed in the laboratory. Other studies have also suggested a link between B cell function and disease state. B cell knockout mice demonstrated delayed dissemination of M. tuberculosis from the lungs to the spleen and liver and delayed development of pulmonary lesions after challenge (5). A more recent study suggested that B cell involvement may not be so clear-cut, as infection of RAG−/− mice deficient in both B and T cells with M. tuberculosis did not affect dissemination into the spleen or the lungs or granuloma formation in the lungs (7). These observations suggest that B cell-T cell interactions are an integral component of the host response to mycobacterial infections in general and that B cells may play a significant role in regulating the pathogenesis of infection.
The present study was designed to evaluate host responses to different routes of experimental infection, including the evolution of B cell responses over a specific period of infection. Although calves in this study remained asymptomatic and subclinically infected during the 12-month term, as determined by recovery of viable M. avium subsp. paratuberculosis organisms from their tissues, an induction of total B cells and B cell subpopulations was observed, and this varied with the mode of experimental infection.
Although all experimentally inoculated calves in the present study would be considered subclinically infected, the oral/M group calves demonstrated the most significant degree of infection, as ascertained by recovery of M. avium subsp. paratuberculosis from tissues by culture or by PCR (19). Interestingly, the oral/M group calves also had increasing percentages of CD5+, CD25+, and CD45RO+ subpopulations at several time points throughout the study, suggesting that an increase in colonization of tissues may signal a concomitant shift to activated B cell subpopulations. Calves in the oral/DXM treatment group also showed significant shifts in the proportions of these B cell populations by 12 months of infection, correlating with the larger number of lesions noted in these calves (19). It is unknown if the upregulation of CD45RO on B cells in the oral/M and oral/DXM group calves is suggestive of a relationship between localized invasion and inflammation of the target tissues, but an upregulation of CD45RO expression on peripheral blood and lamina propria B cells has been observed in humans diagnosed with inflammatory bowel diseases such as Crohn's disease and ulcerative colitis (22, 23).
Similarly, increased expression of CD25 (IL-2 receptor) on B cells has been noted in rheumatic and inflammatory bowel diseases in humans and has also been associated with pathogen exposure in cattle (2, 9, 12, 22). CD25+ B cells constitute a more mature phenotype and are more efficient at antigen presentation but may secrete less immunoglobulin (1). Interestingly, neonatal calves vaccinated with either ovalbumin or M. bovis bacillus Calmette-Guérin (BCG) demonstrated increased CD25+ B cells in the superficial cervical lymph node cell population upon restimulation of cells with antigen (9). This effect was not associated with antibody secretion. Furthermore, 4.5 years after exposure of neonatal calves to bovine leukemia virus, cultured peripheral blood mononuclear cells demonstrated an increased expression of CD25 on B cells that was coincident with increased CD25 expression on CD4 and CD8 T cells (12). It is not clear whether CD25 expression on B cells is a positive indicator of host immunity, as it has been surmised that it may enhance interactions between B and T cell populations, whereas for human beings it has been suggested that CD25+ B cells are a unique subset of memory B cells that may play a role in the pathogenesis of autoimmune disorders (2). The implication that the CD25+ B cell phenotype does not play a major role in immunoglobulin secretion appears to corroborate results within the present study. Immunoblot and ELISA analyses demonstrated a higher degree of M. avium subsp. paratuberculosis-specific antibody in i.p. group calves, yet CD25 expression on B cells from these calves was not sustained throughout the study, resulting in a marked decline after 6 months of infection. This contrasted sharply with the increased expression of CD25 observed for calves inoculated via the oral route, regardless of which M. avium subsp. paratuberculosis isolate was given to calves or if the calves were administered DXM prior to inoculation. This suggests that the direct inoculation of M. avium subsp. paratuberculosis into the peritoneal cavity may have activated a different subset of B cells from those observed in the orally inoculated calves.
B cell populations may be segregated into subclasses by using activation markers such as CD5 to separate them into B-1 (CD5+) and B-2 (CD5−) classes. B-2 cells represent the normal circulating B cell population (13). The B-1 population can be subdivided further into B-1a and B-1b subclasses. Although both subclasses have the CD5+ phenotype, B-1b cells lack the surface receptor for CD5 (24). Recent work described the expansion of a CD5bright subpopulation of B cells in the peripheral blood of cattle subclinically infected with M. avium subsp. paratuberculosis that likely represents the B-1a population (18). This is significant in that it suggests that a shift in B cell subpopulations takes place during infection and may directly or indirectly affect pathogenesis induced by inflammatory T cells. Haas and Estes (11) demonstrated that bovine CD5− B cells could be activated to express CD5 by cross-linking the B cell receptor but not upon ligation with CD40. It would be interesting to explore this application in M. avium subsp. paratuberculosis-infected cattle to determine if CD5 expression on B cells relates to a T cell-independent mechanism.
The appearance of M. avium subsp. paratuberculosis-specific antibody was not expected in the present study due to the limited period of infection (12 months). M. avium subsp. paratuberculosis-specific antibody is most frequently associated with the later stages of infection and clinical disease, although antibody is detectable in asymptomatic infection as well (19a). Immunoblot analysis demonstrated that the reactivity of calf sera was highest and most consistent in i.p. group calves, although transient antibody was apparent in oral treatment groups as well. The EvELISA results were consistent with the immunoblot analyses, with positive responses noted only for i.p. group calves. The EvELISA protocol utilizes a novel method of antigen preparation and has previously demonstrated an improved sensitivity of detection compared to more traditional ELISAs for paratuberculosis (8). A mechanism to explain why i.p. group calves produced more M. avium subsp. paratuberculosis-specific antibody is not readily available, but the appearance and maintenance of antibody responses for i.p. group calves aligned with the higher percentage of total B cells during the 12-month study. It is possible that inoculation of M. avium subsp. paratuberculosis directly into the peritoneum allowed for a more rapid uptake by antigen-presenting cells in the draining lymph nodes, thereby invoking a more robust and protracted antibody response. No other B cell activation markers (CD5, CD25, or CD45RO) demonstrated any alignment with antibody responses.
In conclusion, it is apparent that M. avium subsp. paratuberculosis infection of neonatal calves, regardless of the route of experimental inoculation, invokes a strong activation of B cells. The temporal expression of CD5, CD25, and CD45RO markers on B cells suggests that the route of infection and the M. avium subsp. paratuberculosis isolate used to infect the calves have significant effects on the period of upregulation of these B cell markers. Presumably, this correlates with the period of sustainability of activated B cells in the peripheral circulation and could be a critical indicator of how neonatal calves respond to an enteric pathogen such as M. avium subsp. paratuberculosis in a natural infection. Further work needs to be conducted to elucidate the role that B cells play in host responses to M. avium subsp. paratuberculosis infection, particularly in regard to their interaction with T cells and how this either leads to or averts pathogenesis.
ACKNOWLEDGMENTS
We thank Bruce Pesch, Trudy Tatum, Tonia McNunn, and Megan Parlett for their excellent technical assistance, as well as Paul Amundson and Jerri Grove for their excellent care of the animals in this study.
This study was supported by a USDA-NRICAP (Johne's Disease Integrated Program) grant.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Amu S., Tarkowski A., Dörner T., Bokarewa M., Brisslert M. 2006. The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Scand. J. Immunol. 66:77–86 [DOI] [PubMed] [Google Scholar]
- 2. Amu S., Stromberg K., Bokarewa M., Tarkowski A., Brisslert M. 2007. CD25-expressing B-lymphocytes in rheumatics diseases. Scand. J. Immunol. 64:182–191 [DOI] [PubMed] [Google Scholar]
- 3. Bannantine J. P., Stabel J. R. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76:343–358 [DOI] [PubMed] [Google Scholar]
- 4. Bondada S., Bikah G., Robertson D. A., Sen G. 2000. Role of CD5 in growth regulation of B-1 cells. Curr. Top. Microbiol. Immunol. 252:141–149 [DOI] [PubMed] [Google Scholar]
- 5. Bosio C. M., Gardner D., Elkins K. L. 2000. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J. Immunol. 164:6417–6425 [DOI] [PubMed] [Google Scholar]
- 6. Brisslert M., et al. 2005. Phenotypic and functional characterization of human CD25+ B cells. Immunology 117:548–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chackerian A. A., Alt J. M., Perera T. V., Dascher C. C., Behar S. M. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eda S., et al. 2006. A highly sensitive and subspecies-specific surface antigen enzyme-linked immunosorbent assay for diagnosis of Johne's disease. Clin. Vaccine Immunol. 13:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foote M. R., Nonnecke B. J., Beitz D. C., Waters W. R. 2007. Antigen-specific B-cell responses by neonatal calves after early vaccination. J. Dairy Sci. 90:5208–5217 [DOI] [PubMed] [Google Scholar]
- 10. Gillian S., O'Brien R., Hughes A. D., Griffin J. F. T. 2010. Identification of immune parameters to differentiate disease states among sheep infected with Mycobacterium avium subsp. paratuberculosis. Clin. Vaccine Immunol. 17:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas K. M., Estes D. M. 2000. Activation of bovine B cells via surface immunoglobulin M cross-linking or CD40 ligation results in different B-cell phenotypes. Immunology 99:272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isaacson J. A., Flaming K. P., Roth J. A. 1998. Increased MHC class II and CD25 expression on lymphocytes in the absence of persistent lymphocytosis in cattle experimentally infected with bovine leukemia virus. Vet. Immunol. Immunopathol. 64:235–248 [DOI] [PubMed] [Google Scholar]
- 13. Le Pottier L., Devauchelle V., Pers J. O., Jamin C., Youinou P. 2007. The mosaic of B-cell subsets (with special emphasis on primary Sjögren's syndrome). Autoimmun. Rev. 6:149–154 [DOI] [PubMed] [Google Scholar]
- 14. McHeyzer-Williams L. J., McHeyzer-Williams M. G. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487–513 [DOI] [PubMed] [Google Scholar]
- 15. Polese L., et al. 2007. B1a lymphocytes in ulcerative colitis. Int. J. Colorectal Dis. 22:1005–1011 [DOI] [PubMed] [Google Scholar]
- 16. Stabel J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465–473 [DOI] [PubMed] [Google Scholar]
- 17. Stabel J. R. 2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim. Health Res. Rev. 7:61–70 [DOI] [PubMed] [Google Scholar]
- 18. Stabel J. R., Khalifeh M. S. 2008. Differential expression of CD5 on B lymphocytes in cattle infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 126:211–219 [DOI] [PubMed] [Google Scholar]
- 19. Stabel J. R., et al. 2009. Pathogenesis of Mycobacterium avium subsp. paratuberculosis in neonatal calves after oral or intraperitoneal experimental infection. Vet. Microbiol. 136:306–313 [DOI] [PubMed] [Google Scholar]
- 19a. Sweeney R. W., Whitlock R. H., Buckley C. L., Spencer P. A. 1995. Evaluation of a commercial enzyme-linked immunosorbent assay for the diagnosis of paratuberculosis in dairy cattle. J. Vet. Diagn. Investig. 7:488–493 [DOI] [PubMed] [Google Scholar]
- 20. Waters W. R., et al. 1999. Antigen-specific B-cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect. Immun. 67:1593–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Werner-Favre C., Vischer T. L., Wohlwend D., Zubler R. H. 1989. Cell surface antigen CD5 is a marker for activated human B cells. Eur. J. Immunol. 19:1209–1213 [DOI] [PubMed] [Google Scholar]
- 22. Yacyshyn B. R. 1993. Activated CD19+ B cell lamina propria lymphocytes in ulcerative colitis. Immunol. Cell Biol. 71:265–274 [DOI] [PubMed] [Google Scholar]
- 23. Yacyshyn B. R., Pilarski L. M. 1993. Expression of CD45RO on circulating CD19+ B-cells in Crohn's disease. Gut 34:1698–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Youinou P., Jamin C., Lydyard P. M. 1999. CD5 expression in human B-cell populations. Immunol. Today 20:312–316 [DOI] [PubMed] [Google Scholar]