Abstract
Several studies have reported that intramuscular injection of DNA vaccines against infectious bronchitis virus (IBV) induces protective immune responses. In the present study, we developed oral and nasal DNA vaccines that carried the S1 gene and N gene of IBV delivered by attenuated Salmonella enterica serovar Typhimurium strains SL/pV-S1 and SL/pV-N, respectively. The safety and stability of recombinant Salmonella vaccine were evaluated. Following oral and nasal administration to chickens, the serum and mucosal samples were collected and antibodies against IBV were measured. Chickens were then challenged with IBV strain M41 by the nasal-ocular route 3 weeks after boosting. The results showed that oral and nasal immunization with coadministered SL/pV-S1 and SL/pV-N elicited significant IBV-specific humoral and mucosal immune responses and conferred protective efficacy against IBV challenge higher than that in chickens immunized only with SL/pV-S1. The current study shows that novel DNA vaccines delivered by attenuated S. Typhimurium may be promising candidates for the prevention of infectious bronchitis (IB).These vaccines are efficacious, easily produced economically, and able to be delivered orally and nasally rather than injected. Coadministration of SL/pV-S1 and SL/pV-N may represent an effective mucosal vaccination regimen.
INTRODUCTION
Infectious bronchitis (IB) is an acute and highly contagious respiratory infectious disease of chickens which has a worldwide distribution and continues to be a major health problem in the poultry industry (30, 32). Chickens of all ages may be infected, and virus replication can occur in multiple tissues. IB can cause mortality in young chickens and produces severe economic losses due to poor weight gain and a reduction in egg quality and quantity (4, 26).
IB is caused by infectious bronchitis virus (IBV), a member of the Coronaviridae family that has an unsegmented, single-stranded, and positive-sense RNA genome of >27 kb in length (3, 35). The genome encodes four major structural proteins: the surface spike glycoprotein (S), consisting of S1 and S2 subunits; the membrane (M) glycoprotein; the phosphorylated nucleocapsid (N) protein; and the envelope (E) protein. Of these, S1 glycoprotein and N protein can elicit protective immune responses in chickens. S1 glycoprotein is reported to attach to the host cell membrane and induce neutralizing and hemagglutination-inhibiting antibodies (2, 5, 14). N protein is the most abundant virus-derived protein produced throughout infection and is conserved across various IBV strains. This protein is also highly immunogenic and carries epitopes that induce cross-reactive antibodies (26). Furthermore, N protein may act as a relevant target for immune recognition in both mice and chickens (1, 27, 40, 41).
To date, vaccination is the most effective means of preventing and controlling IB in the poultry industry. However, traditional live and inactivated virus vaccines that have not provided effective long-term control of IB outbreaks are associated with poultry loss (12, 37). Increased numbers of new serotypic variants have also been observed and may be attributed to the widespread use of live attenuated vaccines that can potentially recombine with other IBV strains (14, 34). Such problems associated with traditional vaccines have spurred a major effort to develop new candidate vaccines that are safe and effective for the control of this disease.
Since the first avian DNA vaccine for avian influenza virus was shown to be efficacious in chickens in 1993, DNA vaccines have become potential candidates for protecting against a variety of different viral, bacterial, and parasitic infections (9, 15, 18, 33). Studies showed that vaccination with IBV DNA vaccines is able to induce protective immune responses in some cases (1, 10, 29, 41). Compared to traditional vaccines, DNA vaccines for avian species offer many advantages. DNA vaccines may be useful in immunizing avian chicks by overcoming the limitations of an immature immune system and produce minimal interference from passive maternal antibodies (24, 28). DNA vaccination requires the injection of naked DNA, which is not suitable for vaccinating large numbers of commercial chickens. To overcome this limitation, an attenuated Salmonella enterica serovar Typhimurium strain has been used as a DNA vaccine delivery system in multiple studies, whereby recombinant antigens of pathogens are delivered orally or nasally to induce strong humoral and cellular immune responses (23, 25). The aim of the present study was to develop DNA vaccines carrying the S1 and N genes of IBV to be delivered by the attenuated S. Typhimurium strains SL/pV-S1 and SL/pV-N. The immune responses and protective efficacy of oral and nasal immunization with SL/pV-S1 and the coadministration of SL/pV-S1 and SL/pV-N were also evaluated to provide information about DNA vaccines against IBV in chickens.
MATERIALS AND METHODS
Virus strain, vaccines, and attenuated Salmonella strain.
IBV strain M41 was purchased from the China Institute of Veterinary Drugs Control (IVDC; Beijing, China) and propagated in the allantoic cavities of 9- to 10-day-old specific-pathogen-free (SPF) embryonated chicken eggs before the allantoic fluid was harvested at 48 h postinoculation. The IB attenuated live vaccine (strain H120) was purchased from Qianyuanhao Biological Co. Ltd. (Beijing, China), and the attenuated S. Typhimurium aroA mutant strain SL7207 (S. Typhimurium 2337-65 derivative hisG46 Δ407 (aroA::Tn10 [tetracycline susceptible]) (11) was kindly provided by B. A. D. Stocker (Stanford University, CA).
Plasmid and cells.
The pVAX1 plasmid vector was purchased from Invitrogen and contained elements that complied with the Food and Drug Administration document Points to Consider on Plasmid DNA Vaccines for Preventative Infectious Disease Indications (22). COS-7 cells were purchased from the Shanghai Institute for Biological Sciences (CAS) and cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at pH 7.2 and 37°C with 5% carbon dioxide.
Construction and in vitro expression of DNA vaccine plasmids.
Specific primers were designed to amplify the S1 gene or the N gene of IBV strain M41. The BamHI and XhoI sites were added to the 5′ and 3′ ends of the primers, respectively. The S1 and N genes were first amplified from virus genomic RNA using reverse transcription-PCR (RT-PCR) and then TA cloned into the pMD-18T vector (TaKaRa). Both genes were isolated from respective recombinant plasmids by BamHI and XhoI digestion and subcloned into the same sites in the eukaryotic expression vector pVAX1 to form pVAX1-S1 and pVAX1-N. All constructs were verified by double-enzyme digestion and sequencing.
Six-well tissue culture plates were seeded with COS-7 cells. Monolayers of 70 to 80% confluent cells were transiently transfected with plasmids pVAX1-S, pVAX1-N, and pVAX1 (control), using a Lipofectamine 2000 kit (Invitrogen Life Technologies, CA) according to the manufacturer's instructions. At 48 h posttransfection, the expression of the recombinant plasmids in COS-7 cells was detected using an indirect immunofluorescent assay. The primary antibodies used were antiserum of chickens to IBV, and the secondary antibodies were fluorescein isothiocyanate-conjugated rabbit anti-chicken IgG (Sigma).
Transformation of S1 gene and N gene into attenuated S. Typhimurium.
To produce competent cells, attenuated S. Typhimurium strain SL7207 was inoculated into LB broth at 37°C with agitation. When they reached an optical density at 600 nm (OD600) of 0.5 to 0.6, the bacteria were incubated in an ice-cold water bath for 30 min. Bacteria were then centrifuged before the pellet was washed and resuspended in ice-cold, ultrapure H2O.
Purified recombinant plasmids pVAX1-S1 and pVAX1-N and control plasmid pVAX1 were subsequently transformed separately into attenuated S. Typhimurium competent cells by electroporation. Positive transformants were selected on LB agar plates containing 50 μg/ml kanamycin and further confirmed by PCR and restriction enzyme digestion analysis. The attenuated S. Typhimurium strains carrying plasmid pVAX1-S1, pVAX1-N, or pVAX1 were designated SL/pV-S1, SL/pV-N, and SL/pV, respectively.
In vitro stability of plasmid in S. Typhimurium.
The stability of the DNA vaccine plasmids carried by S. Typhimurium was assessed as previously described (19). Briefly, the recombinant attenuated S. Typhimurium strains were inoculated on LB agar with or without kanamycin for one generation. Ten colonies of bacteria were randomly picked every 5 generations for up to 20 generations for PCR and restriction enzyme digestion. The optimum culture conditions for production were further determined for shake-flask culture and static culture by inoculating the recombinant attenuated S. Typhimurium strains into LB broth with kanamycin and harvesting at 3, 5, 7, 9, and 11 h postinoculation. Stability was calculated as the percentage of bacteria that retained plasmid, i.e., the ratio of the kanamycin-resistant colony number on LB agar with kanamycin to the total colony number on LB agar without kanamycin.
Preparation and safety of recombinant S. Typhimurium vaccines.
Recombinant S. Typhimurium vaccine strains (SL/pV-S1, SL/pV-N, and SL/pV) were grown statically in LB with kanamycin at 37°C to an OD600 of 0.6 to 0.8. Bacterial cells were harvested by centrifugation, washed, and then suspended in phosphate-buffered saline (PBS; pH 7.2) to a concentration of 5 × 109 CFU/ml, as determined by plating serial dilutions on LB agar plates.
Four-day-old commercial broiler chickens were purchased from Wuxi Poultry Farm in Jiangsu Province, China. To verify the safety of DNA vaccines delivered by attenuated S. Typhimurium, chickens were divided into three groups (10 chickens per group) and simultaneously inoculated orally and nasally with an equal amount of recombinant S. Typhimurium at total doses of 1 × 109 CFU, 5 × 109 CFU, and 1 × 1010 CFU, respectively. All chickens were monitored daily for 2 weeks for clinical signs, including demeanor, volume of fluid and food consumed, and consistency of feces.
Immunization.
Chickens were divided into five groups (15 chickens per group) and fasted for 3 h prior to immunization. Each group of chickens was simultaneously inoculated orally and nasally (half the total dose per route) with one of the following: 5 × 109 CFU of SL/pV-S1, 5 × 109 CFU of SL/pV, 2.5 × 109 CFU each of SL/pV-S1 and SL/pV-N (coadministered), strain H120, or PBS as a control. All groups of immunized chickens were boosted with the same vaccine components at the same dose 2 weeks later.
Evaluation of antibody responses.
Serum samples were collected from the wing vein 2 weeks after priming and 3 weeks after boosting. Prevaccination sera were also collected for control chickens. The sera were centrifuged and stored at −20°C until analysis. Intestinal mucosal samples were collected at 3 weeks postboosting as described by Pan et al. (25). Briefly, the entire small intestine was collected and shaken in a PBS solution containing Tween 20 (0.05%), EDTA (0.05 mg/ml), and phenylmethylsulfonyl fluoride (0.35 mg/ml). Intestinal lavage solutions were then centrifuged to remove particulate matter, and the supernatants were stored at −20°C. Serum IgG antibody titers and intestinal mucosal IgA antibody titers specific for IBV were determined by an indirect enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with 50 μl IBV (10 μg/ml) in 0.05 M bicarbonate/carbonate coating buffer (pH 9.6) overnight at 4°C. The plates were then washed three times with PBS containing 0.05% Tween 20 and blocked overnight with 100 μl 10% fetal calf serum in PBS at 4°C. For specific IgG or IgA determination, chicken serum (diluted 1:10) or intestinal extract (diluted 1:2) was used as the primary antibody. Horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG or IgA (Sigma) was used as secondary antibody. The specific IgG or IgA titers were determined in triplicate by endpoint-dilution ELISA.
Challenge.
Three weeks after the boosting immunization, chickens in each group were weighed and challenged with 104 50% egg infective doses of IBV strain M41 via the nasal-ocular route. Chickens were killed at 5 and 10 days after the viral challenge. Tracheal tissues and kidneys were subsequently collected aseptically and used to inoculate embryos to isolate the challenge virus (13). If embryos were stunted or dead and had IBV-specific lesions, samples were considered positive for IBV. Negative embryos were inoculated for three additional blind passages. Allantoic fluid in all chicken embryos was collected and analyzed using hemagglutination (HA) (20).
Statistical analysis.
The Statistical Package for the Social Sciences (SPSS; version 15.0; SPSS, Inc., Chicago, IL) was used to analyze differences between groups. By using one-way analysis of variance (ANOVA), probability (P) values of less than 0.05 were considered significant and P values of less than 0.01 were considered highly significant.
RESULTS
Construction and expression of DNA vaccine plasmids.
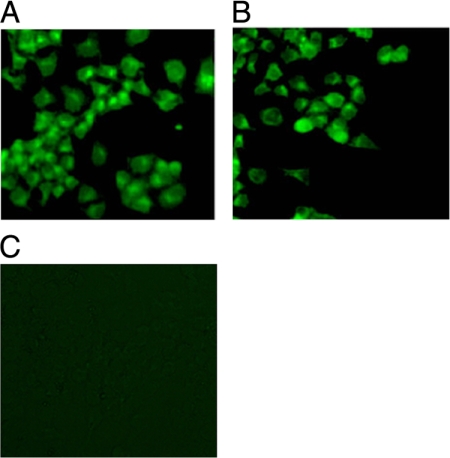
S1 and N genes were successfully amplified from RNA extracted from IBV using RT-PCR. DNA vaccine plasmids were successfully constructed by subcloning the S1 and N genes into the DNA vector pVAX1, as confirmed by restriction enzyme digestion analysis and PCR sequencing. The DNA vaccines were subsequently transfected into COS-7 cells to confirm expression using an indirect immunofluorescent assay. Cells transfected with DNA vaccines displayed strong cytoplasmic fluorescence and revealed that individual DNA vaccines encoding S1 or N protein can be expressed in a eukaryotic system (Fig. 1). Cells transfected with backbone plasmid did not show fluorescence.
Fig. 1.
S1 protein and N protein expression from the DNA vaccine in COS-7 cells. COS-7 cells were transfected with pVAX1-S1 (A), pVAX1-N (B), and backbone plasmid (C); 48 h later, protein expression was analyzed using an immunofluorescence assay.
Identification and in vitro stability of recombinant S. Typhimurium.
PCR and restriction enzyme digestion analyses showed that recombinant plasmids pVAX1-S1 and pVAX1-N were successfully transformed into attenuated S. Typhimurium SL7207 by electroporation. The plasmids were genetically stable in the recombinant attenuated S. Typhimurium population under conditions with or without kanamycin after 20 generations of growth. Furthermore, to further optimize culture conditions with kanamycin for production, we observed that plasmid stability was maintained in over 91% of the attenuated S. Typhimurium population in the static culture until 11 h postinoculation, while plasmid stability in the shake-flask culture was lower (data not shown). These results indicate that the static culture can confer higher plasmid stability in attenuated S. Typhimurium.
Safety of recombinant S. Typhimurium in chickens.
No abnormal clinical signs were observed in the test chickens receiving recombinant S. Typhimurium at doses of 1 × 109 CFU, 5 × 109 CFU, or 1 × 1010 CFU during the 2-week observation period. On day 35 after the first immunization, immunized chickens were weighed, and there was no significant difference in body weights between the recombinant S. Typhimurium-immunized groups and the other groups (P > 0.05) (Table 1). These results suggested that the recombinant S. Typhimurium had no negative effect on body weight gain.
Table 1.
Effect of oral and nasal DNA vaccines delivered by attenuated S. Typhimurium on chicken's body weighta
| Group | Inoculum dose | Mean body wt (kg) at 5 wk postinoculation |
|---|---|---|
| SL/pV-S1 | 5 × 109 CFU | 0.411 ± 0.038 |
| SL/pV-S1+SL/pV-N | 2.5 × 109 CFU each | 0.415 ± 0.062 |
| SL/pV | 5 × 109 CFU | 0.417 ± 0.061 |
| H120 | 0.2 ml | 0.407 ± 0.044 |
| Control | 0.2 ml PBS | 0.413 ± 0.060 |
The body weight in all groups was recorded at 35 days after the prime immunization. The body weight of each group is presented as the mean ± standard deviation. No significant difference in body weight was detected between groups (P > 0.05).
Antibody responses analysis.
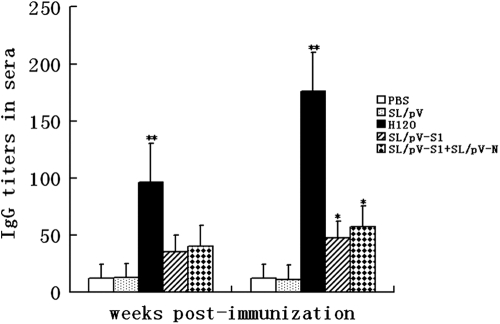
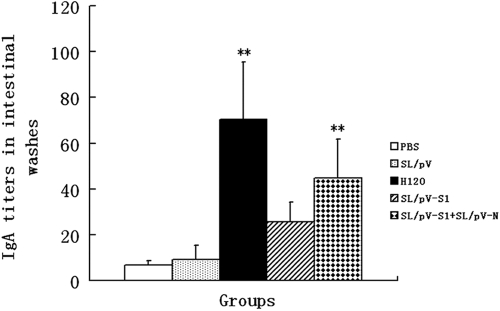
The presence of IBV antigen-specific antibodies in sera and intestinal samples of immunized chickens was determined by ELISA. As shown in Fig. 2, serological antibody responses were detected in groups that received recombinant S. Typhimurium at 2 weeks postpriming, and there was no significant difference in antibody levels in the recombinant S. Typhimurium-vaccinated group (P > 0.05). However, a substantial increase in serum antibody titers was observed in the group coadministered SL/pV-S1 and SL/pV-N and in the SL/pV-S1 group at 3 weeks postboosting. No significant differences were observed between the group coadministered SL/pV-S1 and SL/pV-N and the SL/pV-S1 group (P > 0.05), and titers in both groups were significantly higher than those in the control groups (P < 0.05) (Fig. 2). Furthermore, intestinal mucosal immune responses were evident in chickens immunized with recombinant S. Typhimurium or H120 vaccine, of which the group that received H120 had the highest mucosal antibody titers, while titers in the group coadministered SL/pV-S1 and SL/pV-N were higher than those in the SL/pV-S1 group (P < 0.01) (Fig. 3). No serum or intestinal antibodies were detected in the SL/pV and control groups.
Fig. 2.
Determination of serological antibody responses in different immunization groups. Groups of five chickens were inoculated orally and nasally with SL/pV-S1, SL/pV-S1 and SL/pV-N, SL/pV, H120 (IB attenuated live vaccine), or PBS. Serum samples were collected at 2 weeks after primary vaccination and 3 weeks after postboosting vaccination. Anti-IBV IgG antibody titers were determined by ELISA. Data represent the sample mean (n = 10) ± standard deviation. * and **, significant differences at P < 0.05 and P < 0.01, respectively.
Fig. 3.
Determination of intestinal mucosal antibody responses in different immunization groups. Groups of five chickens were inoculated orally and nasally with SL/pV-S1, SL/pV-S1 and SL/pV-N, SL/pV, H120 (IB attenuated live vaccine), or PBS. Intestinal samples were prepared at 3 weeks postboosting vaccination. Intestinal extracts (diluted 1:2) were used as primary antibodies, and HRP-conjugated rabbit anti-chicken IgA (Sigma) antibodies were used as secondary antibodies. Anti-IBV IgA antibody titers in intestinal washes were determined by ELISA. Data represent the sample mean (n = 5) ± standard deviation. **, significant difference at P < 0.01.
Immunoprotection efficacy assay.
To evaluate whether recombinant S. Typhimurium could induce immunoprotection against IBV, all groups were challenged with M41 IBV strain via the nasal-ocular route at 3 weeks postboosting. No mortality was observed in any of the groups. Protective response levels after challenge were analyzed by isolating the virus from tracheal and kidney tissue samples. The percentages of birds with virus isolated on day 5 were 80%, 20 to 40%, and 40 to 60% for the SL/pV-S1 group, the group coadministered SL/pV-S1 and SL/pV-N, and the H120 group, respectively (Table 2). These values were lower than those for the control and SL/pV groups (both 100%). On day 10, the challenge virus was nearly cleared from the group coadministered SL/pV-S1 and SL/pV-N and the H120 group and was partly cleared from the SL/pV-S1 group. These results indicated that chickens immunized with coadministered SL/pV-S1 and SL/pV-N had an increased level of resistance against IBV, similar to that observed in chickens immunized with H120.
Table 2.
Protective efficacy of oral and nasal DNA vaccines delivered by S. Typhimurium in commercial chickensa
| Group | No. positive/no. tested (% isolated) |
% protectionb |
|||
|---|---|---|---|---|---|
| Day 5 postchallenge |
Day 10 postchallenge |
||||
| Trachea | Kidney | Trachea | Kidney | ||
| SL/pV-S1 | 4/5 (80) | 4/5 (80) | 2/5 (40) | 1/5 (20) | 40 |
| SL/pV-S1+ SL/pV-N | 2/5 (40) | 1/5 (20) | 1/5 (20) | 0/5 (0) | 70c |
| SL/pV | 5/5 (100) | 5/5 (100) | 3/5 (60) | 3/5 (60) | 20 |
| H120 | 3/5 (60) | 2/5 (40) | 1/5 (20) | 0/5 (0) | 60 |
| Control | 5/5 (100) | 5/5 (100) | 3/5 (60) | 2/5 (40) | 20 |
Chickens were challenged with IBV strain M41 nasally and ocularly at 3 weeks postboosting. The challenge virus was isolated from the trachea and kidney, and the immunoprotection efficacy was assayed. Protective response levels after challenge were analyzed by isolating the virus from tracheal and kidney tissue samples. The percentage of isolated virus was determined as the number of positive chickens/the total number of chickens.
Percent protection was determined by the number of unaffected chickens/the total number of chickens.
Fisher's exact test, P <0.05.
DISCUSSION
Vaccination with DNA vaccines, also known as third-generation vaccines, is a promising approach for the effective control of many diseases in the fields of veterinary medicine and food animal production (7, 8). Recently, several studies have shown that DNA vaccines can elicit protective immune responses against IBV (1, 14, 18, 42), with the intramuscular route of injection being the most common route of DNA vaccination in avian species. However, the cost constraints involved with livestock production and the need for mass immunization have limited the application of DNA vaccines to the commercial poultry industry. Such limitations may be overcome by using attenuated Salmonella as a carrier of DNA vaccines. It has been reported that attenuated Salmonella has been used most extensively to deliver orally or nasally target antigens to the mucosal and systemic immune system (21). Salmonella-based vaccines are stable, convenient, and relatively inexpensive to produce. Currently, Salmonella-based DNA vaccines have been used to express foreign antigens in animals and to induce protective efficacy against a variety of pathogens, especially those invading via the respiratory or genital tract (16, 19, 23, 25). Therefore, we have constructed recombinant Salmonella vaccine strains against IBV and evaluated their immune efficacy.
In this study, evaluation of the humoral and mucosal immune responses elicited in vaccinated animals indicated that DNA vaccination using recombinant attenuated S. Typhimurium induced higher IBV-specific serum and intestinal mucosal antibody titers in the vaccinated group than in the negative-control group. Furthermore, coadministration of SL/pV-S1 and SL/pV-N induced serum and mucosal antibody titers higher than those induced by SL/pV-S1 alone. Antibody responses, especially mucosal immune responses, might play a critical role in the elimination of the virus during infection. However, because there have been only a few reports of IBV DNA vaccine delivered by attenuated Salmonella, the dose, duration, and times of vaccination need further evaluation to enhance their immune efficacy.
Several studies have shown that combining multiple DNA vaccines by intramuscular injection generates greater levels of protection than a single DNA vaccine (17, 31, 36). Our study found that oral immunization with SL/pV-S1 induced only partial protection against IBV in chickens (20%) at 5 days postchallenge, which was similar to that obtained using subcutaneous (s.c.) vaccination of a recombinant fowlpox virus expressing S1 (38). A lower percentage of challenge virus was isolated from the SL/pV-S1 group, the coadministered SL/pV-S1 and SL/pV-N group, and the H120 group than from the control and SL/pV groups. The eradication of challenge virus in the group coadministered SL/pV-S1 and SL/pV-N was earlier than that in the SL/pV-S1 group. By day 10, the challenge virus was almost fully cleared from the group coadministered SL/pV-S1 and SL/pV-N and the H120 group and was partly cleared from the SL/pV-S1 group. The protection levels obtained with an oral vaccine in our study were very similar to the values obtained with vaccines administrated by parenteral routes (38). These results suggest that oral immunization of coadministered DNA vaccines, which encode the IBV protective antigen and are delivered by attenuated S. Typhimurium, is a successful strategy to induce significant protection against IBV.
Attenuated Salmonella cells with low pathogenicity and high invasion capacity have been demonstrated to transfer DNA vaccine to phagocytic cells, such as macrophages and dendritic cells. A mechanism generally imagined for the processes includes the following: Salmonella cells first enter the host via M cells in the intestine and are taken up in the dome areas by phagocytes, which possibly migrate into the spleen and lymph nodes after activation (6, 39). Subsequently, the bacteria replicate and die due to their metabolic attenuation and liberate the DNA vaccine into the nucleus of host cells through some kind of secretion mechanism. Finally, the encoded antigen is transcribed and expressed (39). In order to optimize this vaccine system in the future, it is necessary to understand the mechanisms for Salmonella-mediated mucosal DNA vaccination, which may influence the strength and the type of immune responses induced.
In summary, our study demonstrates that coadministration of recombinant S. Typhimurium SL/pV-S1 and SL/pV-N can induce strong mucosal and humoral immune responses and conferred partial protective efficacy against IBV challenge in chickens. This study should contribute to the development of an orally and nasally delivered DNA vaccine, which may be more practical to control IBV infection. Future studies should assess the protective efficacy of coadministering recombinant S. Typhimurium SL/pV-S1 and SL/pV-N against heterologous virus strains and the effects of combining recombinant S. Typhimurium with a traditional vaccine against IBV.
ACKNOWLEDGMENTS
This work was supported by grants from the National Department Public Benefit Research Foundation (grant 200803020), the National Nature Science Foundation of China (grant 30871860), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Boots A. M. H., et al. 1992. Induction of anti-viral immune responses by immunization with recombinant-DNA encoded avian coronavirus nucleocapsid protein. Vaccine 10:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosch B. J., Zee V. D. R., De Haan C. A., Rottier P. J. 2003. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 77:8801–8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavanagh D. 2005. Coronaviruses in poultry and other birds. Avian Pathol. 34:439–448 [DOI] [PubMed] [Google Scholar]
- 4. Cavanagh D., Naqi S. 1997. Infectious bronchitis, p. 11–26 In Calnek B. W. (ed.), Diseases of poultry. Iowa State University, Ames, IA [Google Scholar]
- 5. Cavanagh D., et al. 1992. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 21:33–43 [DOI] [PubMed] [Google Scholar]
- 6. Cochlovius B., Stassar M. J. J. G., Schreurs M. W., Brenner A., Gosse J. A. 2002. Oral DNA vaccination: antigen uptake and presentation by dendritic cells elicits protective immunity. Immunology 80:89–96 [DOI] [PubMed] [Google Scholar]
- 7. Dhama K., Mahendran M., Gupta P. K., Rai A. 2008. DNA vaccines and their applications in veterinary practice: current perspectives. Vet. Res. Commun. 32:341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunham S. P. 2002. The application of nucleic acid vaccines in veterinary medicine. Res. Vet. Sci. 73:9–16 [DOI] [PubMed] [Google Scholar]
- 9. Fodor I., et al. 1999. Induction of protective immunity in chickens immunised with plasmid DNA encoding infectious bursal disease virus antigens. Acta Vet. 47:481–492 [DOI] [PubMed] [Google Scholar]
- 10. Guo Z. C., et al. 2010. Priming with a DNA vaccine and boosting with an inactivated vaccine enhance the immune response against infectious bronchitis virus. J. Virol. Methods 167:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoiseth S. K., Stocker B. A. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239 [DOI] [PubMed] [Google Scholar]
- 12. Jia W., Karaca K., Parrish C. R., Naqi S. A. 1995. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch. Virol. 140:259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson M. A., Pooley C., Ignjatovic J., Tyack S. G. 2003. A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against challenge with infectious bronchitis virus. Vaccine 21:2730–2736 [DOI] [PubMed] [Google Scholar]
- 14. Kapczynski D. R., Hilt D. A., Shapiro D., Sellers H. S., Jackwood M. W. 2003. Protection of chickens from infectious bronchitis by in ovo and intramuscular vaccination with a DNA vaccine expressing the S1 glycoprotein. Avian Dis. 47:272–285 [DOI] [PubMed] [Google Scholar]
- 15. Klotz C., Gehre F., Lucius R., Pogonka T. 2007. Identification of Eimeria tenella genes encoding for secretory proteins and evaluation of candidates by DNA immunisation studies in chickens. Vaccine 25:6625–6634 [DOI] [PubMed] [Google Scholar]
- 16. Kulkarni R. R., Parreira V. R., Sharif S., Prescott J. F. 2008. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine 26:4194–4203 [DOI] [PubMed] [Google Scholar]
- 17. Leachman S. A., et al. 2000. Granulocyte-macrophage colony-stimulating factor priming plus papillomavirus E6 DNA vaccination: effects on papilloma formation and regression in the cottontail rabbit papillomavirus-rabbit model. J. Virol. 74:8700–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J. R., et al. 2003. Plasmid DNA encoding antigens of infectious bursal disease viruses induce protective immune responses in chickens: factors influencing efficacy. Virus Res. 98:63–74 [DOI] [PubMed] [Google Scholar]
- 19. Li L., et al. 2006. Oral DNA vaccination with the polyprotein gene of infectious bursal disease virus (IBDV) delivered by attenuated Salmonella elicits protective immune responses in chickens. Vaccine 24:5919–5927 [DOI] [PubMed] [Google Scholar]
- 20. Mahmood M. S., Siddique M., Hussain I. 2004. Carrier state studies of infectious bronchitis virus in asymptomatic layer in Pakistan. Int. J. Poultry Sci. 3:547–549 [Google Scholar]
- 21. Mielcarek N., Alonso S., Locht C. 2001. Nasal vaccination using live bacterial vectors. Adv. Drug Deliv. Rev. 51:55–69 [DOI] [PubMed] [Google Scholar]
- 22. Morishita R., et al. 2004. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. American Heart Association. Hypertension 44:203–209 [DOI] [PubMed] [Google Scholar]
- 23. Ning J. F., Zhu W., Xu J. P., Zheng C. Y., Meng X. L. 2009. Oral delivery of DNA vaccine encoding VP28 against white spot syndrome virus in crayfish by attenuated Salmonella typhimurium. Vaccine 27:1127–1135 [DOI] [PubMed] [Google Scholar]
- 24. Oshop G. L., Elankumaran S., Heckert R. A. 2002. DNA vaccination in the avian. Vet. Immunol. Immunopathol. 89:1–12 [DOI] [PubMed] [Google Scholar]
- 25. Pan Z. M., et al. 2009. Priming with a DNA vaccine delivered by attenuated Salmonella typhimurium and boosting with a killed vaccine confers protection of chickens against infection with the H9 subtype of avian influenza virus. Vaccine 27:1018–1023 [DOI] [PubMed] [Google Scholar]
- 26. Seah J. N., Yu L., Kwang J. 2000. Localization of linear B-cell epitopes on infectious bronchitis virus nucleocapsid protein. Vet. Microbiol. 5:11–16 [DOI] [PubMed] [Google Scholar]
- 27. Seo S. H., Wang L., Smith R., Collisson E. W. 1997. The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 71:7889–7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegrist C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331–3346 [DOI] [PubMed] [Google Scholar]
- 29. Song C. S., et al. 1998. Induction of protective immunity in chickens vaccinated with infectious bronchitis virus S1 glycoprotein expressed by a recombinant baculovirus. J. Gen. Virol. 79:719–723 [DOI] [PubMed] [Google Scholar]
- 30. Sun L., et al. 2007. A Massachusetts prototype like coronavirus isolated from wild peafowls is pathogenic to chickens. Virus Res. 130:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tian L., et al. 2008. The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochem. Biophys. Res. Commun. 377:221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tseng C. C., Li N. Z., Yao C. H., Wang C. H. 1996. Isolation and adaptation of infectious bronchitis virus in Taiwan from 1993 to 1995. J. Chin. Soc. Vet. Sci. 22:113–120 [Google Scholar]
- 33. Vanrompay D., Cox E., Vandenbussche F., Volckaert G., Goddeeris B. 1999. Protection of turkeys against Chlamydia psittaci challenge by gene gun-based DNA immunizations. Vaccine 17:2628–2635 [DOI] [PubMed] [Google Scholar]
- 34. Wang L., Junker D., Collisson E. W. 1993. Evidence of natural recombination within the S1 gene of infectious bronchitis virus. Virology 192:710–716 [DOI] [PubMed] [Google Scholar]
- 35. Wang L., Xu Y., Collission E. W. 1997. Experimental confirmation of recombination upstream of S1 hypervariable region of infectious bronchitis virus. Virus Res. 49:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang S. X., et al. 2008. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 26:1098–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X., Khan M. I. 2000. Molecular characterization of an infectious bronchitis virus strain isolated from an outbreak in vaccinated layers. Avian Dis. 44:1000–1006 [PubMed] [Google Scholar]
- 38. Wang Y. F., et al. 2009. Protection of chickens against infectious bronchitis by a recombinant fowlpox virus co-expressing IBV-S1 and chicken IFN-γ. Vaccine 27:7046–7052 [DOI] [PubMed] [Google Scholar]
- 39. Weiss S. 2003. Transfer of eukaryotic expression plasmids to mammalian hosts by attenuated Salmonella spp. J. Med. Microbiol. 293:95–106 [DOI] [PubMed] [Google Scholar]
- 40. Williams A. K., Wang L., Sneed L. W., Collisson E. W. 1992. Comparative analyses of the nucleocapsid genes of several strains of infectious bronchitis virus and other coronaviruses. Virus Res. 25:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu L., Liu W., Schnitzlein W. M., Tripathy D. N., Kwang J. 2001. Study of protection by recombinant fowl poxvirus expressing C-terminal nucleocapsid protein of infectious bronchitis virus against challenge. Avian Dis. 45:340–348 [PubMed] [Google Scholar]
- 42. Zhang D. Y., Zhou J. Y., Chen W. Q., Chen J. G. 2009. Co-expression of IBV structural proteins and chicken interleukin-2 for DNA immunization. Vet. Med. 4:69–74 [Google Scholar]