Abstract
The performance of a rapid and simple immunochromatographic test (ICT) with recombinant Em18 (rEm18) antigen for serological follow-up of Echinococcus multilocularis infection was evaluated by comparison with that of an enzyme-linked immunosorbent assay (ELISA) with rEm18, using serum samples from patients who underwent surgery and/or received antiparasitic chemotherapy. The degree of Em18-band intensity on the ICT correlated highly with the absorbance value obtained by the ELISA. The kinetics of antibody levels obtained by the ICT paralleled those of the ELISA. These data suggest that the ICT has high potential as an easy-to-handle, fast, and reliable follow-up tool to monitor the status of alveolar echinococcosis in different stages.
INTRODUCTION
Alveolar echinococcosis (AE), caused by the larval stage (metacestode) of Echinococcus multilocularis, is a serious parasitic disease of humans in countries in higher latitudes of the Northern Hemisphere. In the previous decade, a lot of new data have been published on the prevalence of E. multilocularis in final and intermediate hosts in areas where it had previously not been recorded (5). The metacestodes propagate asexually, like a tumor, leading to organ dysfunction, mainly in the liver. Because of their slow growth, clinical symptoms usually do not become evident until 10 or more years after initial infection (13). Complete removal of alveolar lesions by surgery has been strongly recommended as a primary treatment (3). In addition, therapy with benzimidazole derivatives is also important in patients with AE. Although such imaging techniques as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) with [18F]fluorodeoxyglucose are used to monitor the efficacies of treatments, atypical imaging results lead to difficulties in terms of interpreting disease status (progression or regression) (2). In contrast, serologic analysis is considered to be useful for monitoring disease activity (4, 8, 10). Studies by an enzyme-linked immunosorbent assay (ELISA) with recombinant E. multilocularis 18-kDa antigen (rEm18) (14) and serum samples from patients in different clinical stages of AE according to the World Health Organization (WHO)-PNM (P, parasitic mass in the liver; N, involvement of neighboring organs; M, metastasis) system (11) have revealed that specific immunoglobulin G (IgG) antibody levels in a patient's serum shows a close relationship between the clinical status and the individual treatment (7, 9, 17, 18). However, the ELISA is time-consuming and requires special materials and equipment, which renders this test unsuitable for direct clinical applications. To overcome this problem, we recently developed an immunochromatographic test (ICT) using rEm18 and demonstrated its reliability (15). Another group also has developed an immune filtration assay with multiple native antigens for rapid serodiagnosis of human cystic echinococcosis and AE (6). The ICT has been known as a simple and rapid method for detection of specific antigens of infectious agents or specific antibodies to them semiquantitatively. In this study, we evaluated the ICT with rEm18 as a follow-up tool for monitoring AE patients after treatment in different stages.
MATERIALS AND METHODS
Patients.
All patients described in this study were seen at the University Hospital and Medical Center Ulm, Ulm, Germany. A total of 12 patients (72 sera) with a history of hepatic AE and a follow-up period of 2.5 to 6.5 years were included in the study. The patients were assigned to different clinical WHO-PNM stages of the disease (11). All patients had acquired AE in Germany and received benzimidazole therapy. Three patients had curatively resected lesions, 3 had recurrences after surgery, 5 had unresectable lesions but stable disease, and 1 had apparently dead, fully calcified lesions (Table 1). All serum samples were tested at the Department of Parasitology, Asahikawa Medical University, Japan, in a blind test. The classification of curative resection, stable disease, progressive disease, or presence of an apparently dead, fully calcified lesion was established by magnetic resonance imaging based on lesion size and morphology at the respective follow-up intervals. Ethical approval was obtained from the University of Ulm.
Table 1.
Characteristics of patients with alveolar echinococcosis included in this study
| Patient no. | Stage | PNM code | Statusa | Age (yr)b | Sex | Follow-up duration (yr) |
|---|---|---|---|---|---|---|
| 1 | I | P1N0M0 | Apparently dead, fully calcified lesion | 58 | M | 4 |
| 2 | I | P1N0M0 | Unresectable, stable disease | 61 | M | 3.5 |
| 3 | II | P2N0M0 | Curative resection | 38 | F | 3 |
| 4 | II | P2N0M0 | Unresectable, stable disease | 59 | F | 6.5 |
| 5 | II | P2N0M0 | Recurrence after resection | 74 | F | 2.5 |
| 6 | IIIa | P3N0M0 | Recurrence after resection | 17 | F | 3.5 |
| 7 | IIIa | P3N0M0 | Recurrence after resection | 19 | F | 3 |
| 8 | IIIb | P4N0M0 | Unresectable, stable disease | 57 | M | 3 |
| 9 | IIIb | P4N0M0 | Unresectable, stable disease | 86 | F | 5.5 |
| 10 | IV | P4N1M0 | Curative resection | 30 | M | 3 |
| 11 | IV | P4N1M0 | Curative resection | 52 | M | 2.5 |
| 12 | IV | P4N1M0 | Unresectable, stable disease | 54 | M | 4 |
Assessed by imaging (apparently dead lesion, progressive disease, and stable disease) or imaging and histologic analysis (curative resection).
Age when first blood sample was obtained.
Enzyme-linked immunosorbent assay and immunochromatography test.
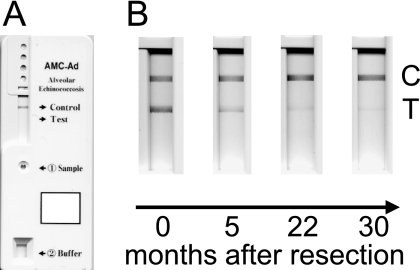
For the ELISA, recombinant Em18 antigen (14) was used to coat microtiter plates at a concentration of 10 ng/well. The wells were blocked with 250 μl of blocking buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% casein) at 37°C for 1 to 2 h. After the wells were rinsed twice with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBST), 100-μl serum samples diluted 1:100 in blocking buffer were added, and the mixtures were incubated at 37°C for 1 h. The wells were rinsed five times with PBST, incubated with 100 μl of recombinant protein G conjugated with peroxidase (Zymed) at 37°C for 1 h, and rinsed five times with PBST. After incubation with 100 μl of substrate (0.4 mM 2,2′-azino-di-[3-ethyl-benzthiazoline sulfonate] in 0.2 M citric acid buffer, pH 4.7) for 30 min at room temperature, the absorbance at 405 nm with a reference wavelength of 630 nm was measured. For the ICT, the immunochromatographic strip and cassette were prepared as described previously (15). For the assay, 10 μl of the respective serum sample was mixed with 20 μl of a serum dilution buffer containing 0.1 mg/ml alkaline phosphatase-conjugated goat anti-human IgG antibody (Dako, Japan) in a tube, and the mixed serum sample was applied to the sample window of the plastic device. Soon after applying the serum sample (within 30 s), 200 μl of the substrate solution was loaded onto the substrate reservoir pad and the result was judged after 20 min. 5-Bromo-4-chloro-3-indolylphosphate was used for color development. A sample was considered positive if two color lines (control line, anti-goat IgG; test line, rEm18) appeared in the result window, and a sample was considered negative if only a control line appeared in the result window (see Fig. 1). In the case of no appearance of a control line, the assay was invalid even if a colored test line appeared. The color intensity of the stained blue line was confirmed by observation with the naked eye and quantified by an immunochromatography reader, C10066 (Hamamatsu, Japan) according to the manufacturer's instructions. The results are expressed as milli-absorbance (mABS) units per capture zone, and relative intensity was calculated by dividing mABS of the test line by mABS of the control line.
Fig. 1.
Examples of ICT tests with negative and positive sera. (A) Result with a negative serum sample. The inscriptions “Sample” and “Buffer” represent the positions for loading of sample and of substrate solution, respectively. (B) Results with positive sera from patient 11. Only result windows are shown. C, control line; T, test line.
In both methods, serum samples giving values greater than the mean value plus four standard deviations for negative-control samples collected from healthy Japanese people not showing any clinical features of AE were considered seropositive.
Statistical analyses.
The binomial test was applied to compare the sensitivity of the ICT and the ELISA, and Spearman's rank test was used for the correlation analysis of the ICT and the ELISA.
RESULTS AND DISCUSSION
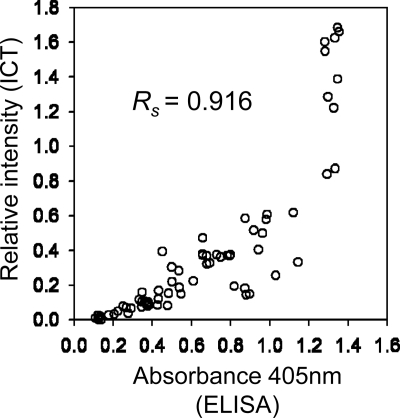
A total of 72 serum samples from 12 AE patients were examined by the ICT and the ELISA. For the ICT, the intensity of the band which appeared at the test line (Fig. 1) was quantified to obtain objective judgment after observation by the naked eye. No discrepancies between the results based on relative intensity values and visual inspections were observed. Five ELISA-negative samples, one from patient 1 and four from patient 5, with absorbance values close to the cutoff absorbance value, showed very weak ICT-positive results. There was no significant difference in detection of specific antibody to rEm18 between the ICT and the ELISA (Table 2, binomial test; P = 0.0625). As shown in Fig. 2, the values obtained by the ICT and the ELISA for individual sera correlated very well (Spearman's rank test; Rs = 0.916; P = 0.0000002), which indicated that the ICT had ability to assay antigen-specific IgG semiquantitatively and the band intensity was in proportion to antibody levels.
Table 2.
Comparison of ICT with ELISA
| ICT result | No. (%) of samples with ELISA result |
Total (%) | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 64 (88.9) | 5 (6.9) | 69 (95.8) |
| Negative | 0 (0) | 3 (4.2) | 3 (4.2) |
| Total (%) | 64 (88.9) | 8 (11.1) | 72 (100) |
Fig. 2.
Scatter graph showing the correlation between relative intensity in the ICT and absorbance values in the ELISA using 72 serum samples from AE patients. Spearman's rank correlation coefficient (Rs) was 0.916 (P = 0.0000002).
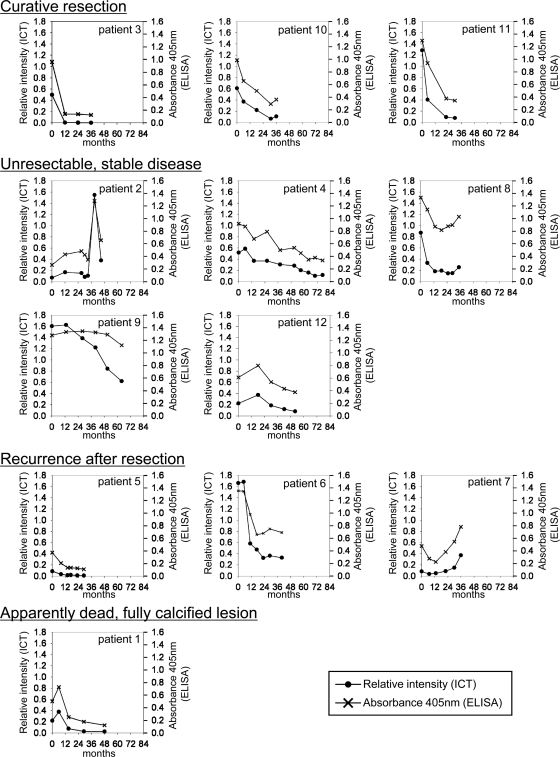
Comparisons of relative intensity values with absorbance values in individual patients revealed that the kinetics of levels of antibody to rEm18 obtained by the ICT were similar to those obtained by the ELISA (Fig. 3). In cases with curative resections, continuous and dramatic decreases in antibody levels after resection of an alveolar hydatid cyst were observed. These results are consistent with those of previous studies using Em18 and other native E. multilocularis metacestode antigens (1, 7, 9, 12, 16, 17, 18). In cases with unresectable and stable diseases, the kinetics of antibody levels varied in individual patients. However, antibody levels had an inclination to decrease slowly during the observation period. A drastic increase in the antibody level was observed in patient 2 after 39 months. In patient 8, antibody levels began to increase slowly after 29 months. In patient 9, ELISA absorbance values did not change until 51 months, although relative intensities of the ICT began to decrease from 13 months. Because high antibody levels in serum samples until 51 months possibly made ELISA absorbance values reach a plateau level, a change in ELISA absorbance values might not have been observed. Further experiments with a higher serum dilution for ELISA are needed to make clear the reason for the difference in kinetics of antibody levels observed between two methods. In cases with recurrences, the initial decreases in antibody levels were observed for all three patients. For patient 5, after 14 months, antibody levels dropped below the cutoff as determined by the ELISA and remained undetectable throughout the observation period, in contrast to all samples being positive by the ICT. In two other patients, increases in antibody levels were observed after 29 months (patient 6) and 13 months (patient 7). In a case with a calcified lesion (patient 1), a slow decrease in antibody levels was observed, and the last serum sample became negative by the ELISA but was ICT positive. Because antibody levels in patients are very closely related to the progression of AE (1, 7, 9, 12, 16, 17, 18), the efficacy of chemotherapy using benzimidazole against parasites might influence the variability of antibody responses.
Fig. 3.
The kinetics of anti-rEm18 antibody levels obtained by using the ICT and the ELISA in patients after surgical operation and/or chemotherapy. The cutoff values for the ICT and the ELISA were 0.009 and 0.162, respectively. Time of resection and start of chemotherapy are at month 0.
In conclusion, the results of this study strongly indicated that the band intensity observed in ICT-positive tests was reflected in the height of antibody levels against rEm18. In other words, the ICT has high potential as a follow-up tool for monitoring the progression of AE, since antibody levels against rEm18 are closely related to the parasite's activity in the human host. In addition, the ICT shows several advantages: (i) expertise, experience, and special equipments which are required for conventional laboratory-based ELISA are not needed; (ii) time consumption is only 20 min, which is much lower than the 4 h taken by the ELISA. Therefore, as a rapid and easy-to-handle (bedside) test, the ICT is able to monitor levels of antibody to Em18, which is a marker for the presence of active or inactive/abortive lesions and the lesion status after radical resection, which can facilitate and accelerate proper clinical management. However, further analysis of reproducibility among different lots of ICT devices and a large-scale evaluation might be necessary, and in the case of positive ICT results without prior imaging, imaging should still be included as a diagnostic tool.
ACKNOWLEDGMENTS
This study was financially supported in part by the Hokkaido Translational Research Project from the Ministry of Education, Japan (MEXT) (2007 to 2011), by the Special Coordination Funds for Promoting Science and Technology from MEXT (2010 to 2012), and by the International Research Fund from the Japan Society for the Promotion of Science (21256003) to A.I.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Ammann R. W., et al. 2004. Immunosurveillance of alveolar echinococcosis by specific humoral and cellular immune tests: long-term analysis of the Swiss chemotherapy trial (1976–2001). J. Hepatol. 41:551–559 [DOI] [PubMed] [Google Scholar]
- 2. Bresson-Hadni S., et al. 2006. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol. Int. 55(Suppl.):S267–S272 [DOI] [PubMed] [Google Scholar]
- 3. Brunetti E., Kern P., Vuitton D. A. 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 114:1–16 [DOI] [PubMed] [Google Scholar]
- 4. Carmena D., Benito A., Eraso E. 2007. The immunodiagnosis of Echinococcus multilocularis infection. Clin. Microbiol. Infect. 13:460–475 [DOI] [PubMed] [Google Scholar]
- 5. Eckert J., Conraths F. J., Tackmann K. 2000. Echinococcosis: an emerging or re-emerging zoonosis? Int. J. Parasitol. 30:1283–1294 [DOI] [PubMed] [Google Scholar]
- 6. Feng X., et al. 2010. Dot immunogold filtration assay (DIGFA) with multiple native antigens for rapid serodiagnosis of human cystic and alveolar echinococcosis. Acta Trop. 113:114–120 [DOI] [PubMed] [Google Scholar]
- 7. Fujimoto Y., et al. 2005. Usefulness of recombinant Em18-ELISA to evaluate efficacy of treatment in patients with alveolar echinococcosis. J. Gastroenterol. 40:426–431 [DOI] [PubMed] [Google Scholar]
- 8. Gottstein B. 1992. Molecular and immunological diagnosis of echinococcosis. Clin. Microbiol. Rev. 5:248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikawa Y., et al. 2009. Serological monitoring of progression of alveolar echinococcosis with multiorgan involvement by use of recombinant Em18. J. Clin. Microbiol. 47:3191–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito A., Nakao M., Sako Y. 2007. Echinococcosis: serological detection of patients and molecular identification of parasites. Future Microbiol. 2:439–449 [DOI] [PubMed] [Google Scholar]
- 11. Kern P., et al. 2006. WHO classification of alveolar echinococcosis: principles and application. Parasitol. Int. 55(Suppl.):S283—S287 [DOI] [PubMed] [Google Scholar]
- 12. Ma L., et al. 1997. Alveolar echinococcosis: Em2plus-ELISA and Em18-Western blots for follow-up after treatment with albendazole. Trans. R. Soc. Trop. Med. Hyg. 91:476–478 [DOI] [PubMed] [Google Scholar]
- 13. McManus D. P., Zhang W., Li J., Bartley P. B. 2003. Echinococcosis. Lancet 362:1295–1304 [DOI] [PubMed] [Google Scholar]
- 14. Sako Y., et al. 2002. Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J. Clin. Microbiol. 40:2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sako Y., Fukuda K., Kobayashi Y., Ito A. 2009. Development of an immunochromatographic test to detect antibodies against recombinant Em18 for diagnosis of alveolar echinococcosis. J. Clin. Microbiol. 47:252–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schantz P. M., Wilson J. F., Wahlquist S. P., Boss L. P., Rausch R. L. 1983. Serologic tests for diagnosis and post-treatment evaluation of patients with alveolar hydatid disease (Echinococcus multilocularis). Am. J. Trop. Med. Hyg. 32:1381–1386 [DOI] [PubMed] [Google Scholar]
- 17. Tappe D., et al. 2009. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am. J. Trop. Med. Hyg. 80:792–797 [PubMed] [Google Scholar]
- 18. Tappe D., et al. 2010. Immunoglobulin G subclass responses to recombinant Em18 in the follow-up of patients with alveolar echinococcosis in different clinical stages. Clin. Vaccine Immunol. 17:944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]