Abstract
Some infectious diseases have been shown to halt the onset of autoimmune disease in animal models and have been suggested to also influence autoimmune pathology in humans. The isolation and study of small molecules and proteins from the infectious agents responsible for the protective effect will enable a mechanistic understanding of how these components may prevent or delay the onset of autoimmunity. In this study we confirm that the quorum-sensing signal molecule OdDHL from Pseudomonas aeruginosa can delay the onset of type 1 diabetes in the NOD mouse model. Furthermore, using an antigen-presenting cell-free system, we find not only that OdDHL inhibits the proliferation of naïve T cells but also that it directly inhibits the differentiation of T cell subsets. OdDHL was shown to have no effect on the inhibition of primed and committed differentiated T cell responses, suggesting that that immune mechanism mediated by this molecule may be more restricted to initial stages of infection.
INTRODUCTION
The ability of infectious agents to inhibit the onset of autoimmune disease or moderate inflammatory pathology has been demonstrated in a number of models of autoimmunity as well as in some human diseases (9, 15). Studies using animal models have demonstrated protection against autoimmune encephalomyelitis (EAE) and type 1 diabetes following some bacterial, viral, or helminth infections (3, 6, 7, 14, 25, 27, 30). It has also been demonstrated that a live infection may not be necessary for protection against type 1 diabetes, and parasite-derived products such as the soluble egg antigen (SEA) and soluble worm antigen (SWA) fractions of Schistosoma mansoni (5, 29) as well as some bacterial components (1, 17) can also be effective.
The nonobese diabetic (NOD) mouse provides a good model of the human autoimmune disease type 1 diabetes (12). Type 1 diabetes is a Th1-mediated autoimmune disorder (2, 10, 11), and early studies of the mechanism of diabetes prevention conferred by antigens of S. mansoni suggested that protection was attributed to the induction of high levels of the Th2-biased cytokines interleukin-4 (IL-4), IL-5, and IL-10, in addition to the reduced expression of gamma interferon (IFN-γ) (6, 29). Alongside a change in T helper cell bias, more recent studies with SEA have shown that this helminth extract, through its action on dendritic cells (DCs), is able to induce Foxp3 expression in naïve NOD T cells (28). As regulatory T (Treg) cells have been implicated in the protection against type 1 diabetes in NOD mice (4), this provides an additional mechanism by which infections can inhibit diabetes onset in NOD mice (5, 16, 18).
The natural synthetic version of a quorum-sensing signal molecule from Pseudomonas aeruginosa, N-3-(oxododecanoyl)-l-homoserine lactone (OdDHL), has been shown to delay the onset and reduce the final incidence of diabetes in female NOD mice (17). Initial in vitro studies suggested that OdDHL had a pronounced inhibitory effect on the production of cytokines associated with a proinflammatory Th1 response while permitting a Th2 response (24). Therefore, one possible mechanism by which OdDHL might inhibit diabetes development might be as a result of skewing the immune response toward a Th2 response. Subsequently, however, in vitro and in vivo studies examining responses in BALB/c and C57BL/6 mice have demonstrated that OdDHL can modulate either Th1 or Th2 cell responses, depending on the underlying cell bias of the different strains (21). This suggests that the mechanism by which OdDHL might protect against diabetes may not be simply as a result of selective suppression of Th1 activity. With regard to other potential mechanisms by which OdDHL might mediate immunomodulation, it has been shown that at low doses (<25 μM) OdDHL suppresses T cell proliferation and inhibits the production of proinflammatory cytokines IL-12 and tumor necrosis factor alpha (TNF-α) by lipopolysaccharide (LPS)-stimulated macrophages (23).
In this study, we have examined the effect of OdDHL on the differentiation and polarization of naïve NOD T cell populations in addition to determining whether such effects are also seen on already polarized T cell populations.
MATERIALS AND METHODS
Mice.
NOD mice were maintained under barrier conditions in the Biological Services Unit of the Pathology Department, University of Cambridge. All animal experiments were conducted under United Kingdom Home Office project license regulations after approval by the Ethical Review Committee of the University of Cambridge.
OdDHL.
N-(3-Oxododecanoyl)-l-homoserine lactone (OdDHL) was provided by David Pritchard, School of Pharmacy, University of Nottingham (19). OdDHL was dissolved in 1% dimethyl sulfoxide (DMSO) at a 100 μM concentration, and serial dilutions were made using Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco). The highest final concentration of DMSO used for in vitro studies was 0.05%.
In vivo analysis of OdDHL.
Four-week-old mice (10 per group) were injected intraperitoneally (i.p.) with OdDHL (1 mg/kg of body weight) in phosphate-buffered saline (PBS) containing 1% DMSO three times a week for 4 weeks. A control group received 1% DMSO in PBS using the same regimen. Mice were routinely monitored for glucosuria using Diastix (Bayer), and animals were deemed diabetic if urinary glucose exceeded 5 g/liter.
Cell culture and reagents.
Cells were cultured in IMDM supplemented with 10% heat-inactivated FCS, 2 × 10−3 M l-glutamine, 100 U/ml penicillin and streptomycin, and 50 μM 2-mercaptoethanol (all from Sigma-Aldrich). Anti-CD3 (145.2C11) and anti-CD28 (37.51) were obtained from BD Pharmingen and were used in solution at 0.5 and 5 μg/ml, respectively. Plates were precoated with 100 μl of antibodies diluted in PBS for 4 h at 37°C preceding cell culture. Cytokines used for the polarization of naïve T cells included IL-12 (Peprotech), IL-4 (Peprotech), IL-6 (Peprotech), IL-1β (Invitrogen), transforming growth factor β (TGF-β; R&D Systems), IFN-γ (R&D Systems), and human recombinant IL-2. Anti-IFN-γ (XMG1.2) and anti-IL-4 (1B11) and anti-IL-6 (15A7) were all grown in-house. Cell proliferation was measured either by incorporation of [3H]thymidine (added to cultures at 48 h; corrected counts per minute [ccpm] were measured after 12 h) or by carboxyfluorescein succinimidyl ester (CFSE) dye dilution at 48 h.
In vitro T cell differentiation.
CD4+ T cells were selected from splenic cell suspensions using an AutoMACS Pro instrument (Miltenyi). Further sorting of naïve CD4 cells (CD4+ CD25− CD44lo, where CD44lo indicates cells expressing CD44 at low levels) were carried out using a MoFlo (Beckman Coulter) to result in >98% purity.
For the study of the effect of OdDHL on the polarization of naïve T cells, these cells were seeded at a cell density of 5 × 105 cells/ml on anti-CD3- and anti-CD28-precoated 24-well plates for 4 days in the presence or absence of OdDHL (1, 5, or 10 μM) under the following polarizing conditions: 10 ng/ml IL-12 and 100 U/ml human recombinant IL-2 for polarization to Th1 cells; 2 ng/ml IL-4, 100 U/ml IL-2, and 50 μg/ml anti-IFN-γ for Th2 polarization; 25 ng/ml IL-6, 10 ng/ml IL-1β (Invitrogen), 2 ng/ml human TGF-β, 50 μg/ml anti-IFN-γ, and 10 μg/ml anti-IL-4 blocking antibodies for Th17 polarization; and 12 ng/ml TGF-β, 50 U/ml IL-2, and anti-IL-6 (10 μg/ml) for Treg cell polarization. These are conditions that have been used by ourselves and others to generate different T cell subsets from naïve T cells (2, 26).
For further study of polarized T cell subsets, cells grown in the absence of OdDHL were then transferred to fresh plates and rested for 2 to 3 days without stimulus. Cells were then harvested and plated at 5 × 105 cells/ml on anti-CD3- and anti-CD28-coated plates in the presence or absence of OdDHL (10 μM).
Flow cytometry.
Cells were stained using the following antibodies and fluorophores: CD4 (clone RM4-5)-peridinin chlorophyll protein (PerCP)-Cy5.5, CD25 (PC61)-phycoerythrin (PE), CD44 (IM7)-fluorescein isothiocyanate (FITC), major histocompatibility complex (MHC) class II (OX6)-FITC, anti-IFN-γ (XMG1.2)-FITC, anti-IL-17A (TC11-18H10)-PE, anti-IL-4 (11B11)-PE, and PE- or Cy7-conjugated controls. All were obtained from BD Bioscience. Foxp3 (FJK-16S)-FITC was obtained from eBioscience. Intracellular staining was carried out as described previously (5). Nonspecific binding was blocked using 2.4G2 anti-Fcγ receptor (FcγR) supernatant (prepared in-house). By staining CD4+ T cells intracellularly for specific cytokines and transcription factors, it was possible to identify CD4+ T cell subsets. The proportional representation of a single cytokine or transcription factor expressing CD4+ T cells was therefore indicative of Th1 (IFN-γ), Th2 (IL-4), Th17 (IL-17), and Treg (Foxp3) cells. Data were acquired on a FACSCalibur (BD Pharmingen) and analyzed using FlowJo software (TreeStar).
Statistics.
Statistical analysis was performed with GraphPad Prism software. Diabetes progression was analyzed by plotting Kaplan-Meier survival curves and performing a log rank test between the groups. For analysis of nonparametric data sets, a Mann-Whitney U test was performed. Results were deemed significant if P values were less than 0.05.
RESULTS
OdHDL delays diabetes onset in NOD mice.
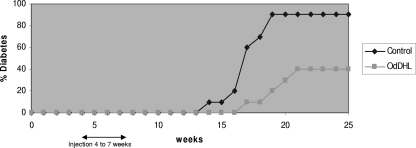
Previous studies have shown that OdHDL can alleviate diabetes in NOD mice (17). We have confirmed these findings, and we show in Fig. 1 that OdHDL delayed the onset of diabetes and reduced the final incidence of disease in OdHDL-treated mice compared to the control group given solvent alone. The OdHDL-treated group achieved a 40% incidence by 25 weeks of age in contrast to the 90% incidence seen in the DMSO-treated control group (P < 0.005). Having confirmed this effect, it was of interest to look at the possible mechanism by which diabetes onset is delayed.
Fig. 1.
OdDHL reduces the onset of spontaneous type 1 diabetes in NOD mice. Four-week-old mice were injected with OdDHL (1 mg/kg) i.p. three times a week for 4 weeks starting at 4 weeks of age. Control mice were injected with 1% DMSO in PBS for 4 weeks. The arrow indicates the start of injections. Each group contained 10 mice.
OdDHL influences dendritic cell function in vitro.
In vitro studies of the effect of OdDHL have demonstrated that this quorum-sensing signal molecule influences T cell responses, possibly through an initial effect on DC maturation (19, 22). Such an effect on the Th1 response would be beneficial for the successful establishment of infection by P. aeruginosa. An effect of OdDHL on Th1 responses has also been proposed as an explanation for the observed delay in the onset of the Th1-mediated autoimmune disease, type 1 diabetes (17).
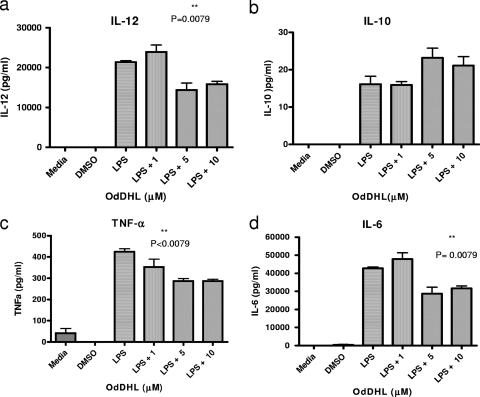
As strain differences in bone marrow-derived DC (BMDC) responses to exogenous signals have been observed (5), we examined the effects of OdDHL on NOD-derived BMDCs to determine whether OdDHL could influence NOD-derived DCs in a comparable fashion to that previously observed with C57BL/6 cells. In Fig. 2 it can be seen that OdDHL, at 5 and 10 μΜ, significantly inhibited LPS-induced production by NOD BMDCs of the proinflammatory cytokines IL-12, TNF-α, and IL-6 but not IL-10.
Fig. 2.
OdDHL reduces LPS-mediated secretion of IL-12, IL-6, and TNF-α from NOD BMDCs. NOD BMDCs were stimulated in vitro with LPS (100 ng/ml) in the presence or absence of OdDHL (1, 5, or 10 μM). DMSO-PBS at 0.1% or medium controls were also included. Supernatants were collected after 72 h and tested for IL-12 p40, IL-10, TNF-α, and IL-6, as indicated. Results are expressed as means of triplicates ± standard errors of the means. Differences in cytokine production between the OdDHL and control groups are statistically significant at the 1% level. Statistical analysis was carried out using a Mann-Whitney U test in GraphPad Prism.
The ability of OdDHL to inhibit IL-12 but not IL-10 is consistent with the observation using BMDCs from C57BL/6 mice that OdDHL might inhibit a Th1 response through an inhibition of proinflammatory cytokine production (22). However, in contrast to these previous studies, we did not observe any effect of OdDHL on the expression of CD80, CD40, or MHC class II in NOD BMDCs (data not shown).
The effect of OdDHL on T cell responses.
As previous studies had suggested that OdDHL might affect other T cell subsets apart from Th1 and be able to mediate its effect directly on T cells (19, 20), we decided to study its direct effect on all of the four major subsets of CD4+ T cells, Th1, Th2, Th17, and T regulatory (Treg) cells. Furthermore, since it was suggested that OdDHL inhibits both Th1 and Th2 cell subsets at an early stage in T cell activation, we decided to study its activity on the proliferation and polarization of naïve T cells into these four subsets, as outlined below.
(i) T cell proliferation.
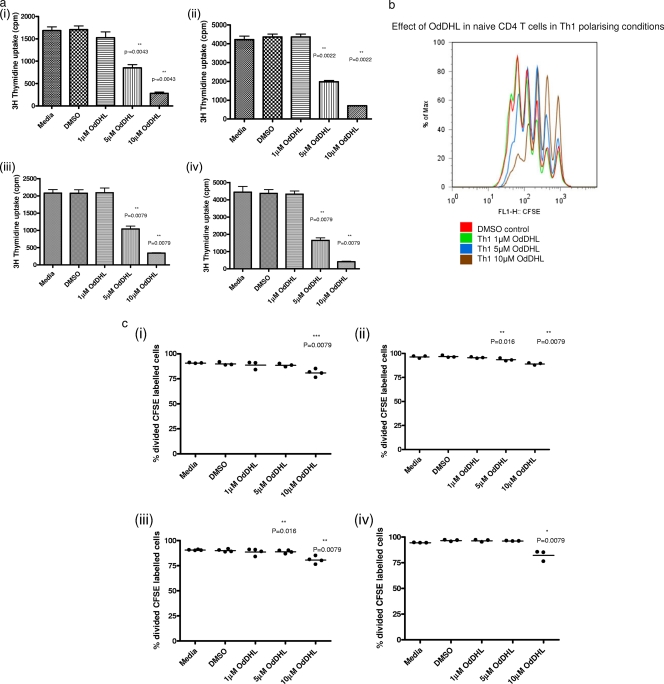
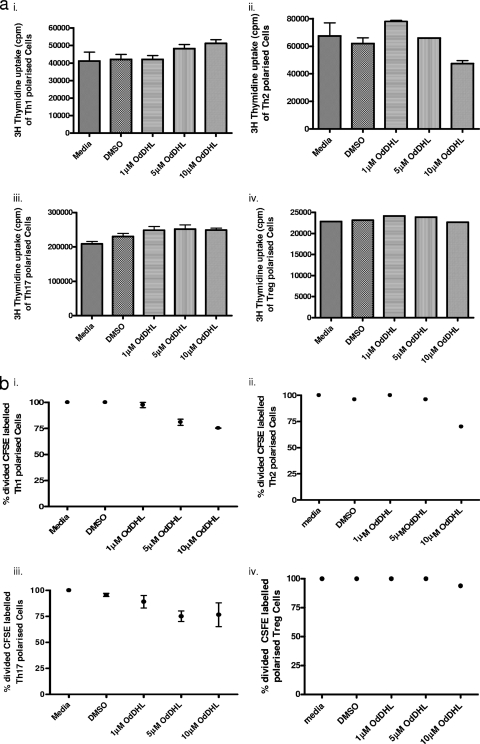
To determine whether OdDHL could directly affect naïve T cell proliferation and differentiation, naïve (CD4+ CD25− CD44lo) T cells were cultured under T cell-polarizing conditions in the presence of a range of concentrations of OdDHL (from 0 to 10 μM) with the appropriate DMSO control as described in Materials and Methods. As can be seen from Fig. 3, OdHDL significantly inhibited cell division of naïve cells in a dose-dependent manner whether T cell proliferation was assessed by uptake of [3H]thymidine (Fig. 3a) or CSFE dilution (Fig. 3b and c). This effect on T cell proliferation was seen under all T cell-polarizing conditions. Microscopic observations and apoptotic analysis demonstrated that cells remained dormant, appearing not to divide at 10 μM.
Fig. 3.
OdDHL inhibits proliferation of naïve CD4+ T cells. CD44− CD25lo cells (5 × 105) were grown on precoated anti-CD3/anti-CD28 plates under conditions conducive for Th1 (i), Th2 (ii), Th17 (iii), and Treg (iv) cell polarization. Cells were grown in the presence or absence of 10 μM OdDHL for 48 h, and proliferation was assessed using [3H]thymidine incorporation (a) or CFSE dilution (c). Two control conditions were also included, medium alone and PBS containing 0.01% DMSO. A representative FACS plot showing a dose-dependent inhibitory effect of OdDHL up to 10 μM (P = 0.006) on Th1 polarization is shown (b). Data are representative of three independent experiments. Statistical analysis was carried out by using the Mann-Whitney U test in GraphPad Prism.
(ii) T cell polarization.
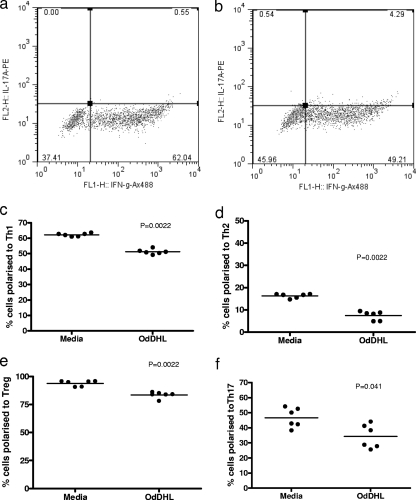
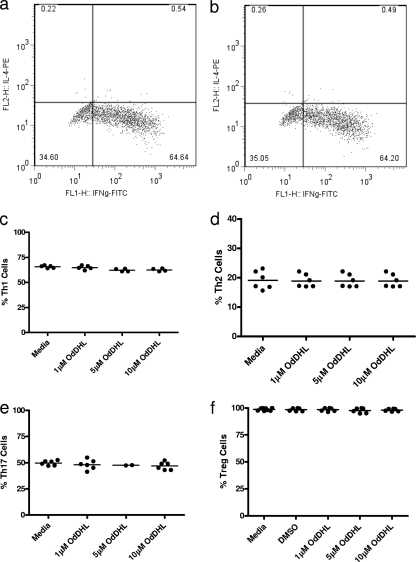
The ability of OdDHL to influence the differentiation of naïve T cells was assessed by examining its ability to inhibit the production of signature cytokines by T cells cultured under different polarizing conditions in the absence of antigen-presenting cells. OdDHL influenced the differentiation of naïve T cells into all T cell subsets (Fig. 4); there was no evidence of any differential effect. Figure 4a and b show representative fluorescence-activated cell sorting (FACS) plots for the effect on polarization of naïve CD4+ T cells to Th1 cells. Figure 4c to f summarize the effects of OdDHL on the proportional representation of individual T cell subsets. It is seen that, irrespective of the polarizing conditions employed, statistically significant inhibitory effects of OdDHL are seen on all subsets studied.
Fig. 4.
OdDHL inhibits CD4+ naïve T cell polarization in vitro. Naïve splenic T cells (5 × 105) were cultured for 4 days on anti-CD3/anti-CD28-precoated plates. Cells were grown under polarizing conditions specific to the T cell subset required. Representative FACS plots are shown of control cells (a) and of cells grown in the presence of 10 μM OdDHL (b) under Th1-polarizing conditions. A summary of between three and five independent experiments is shown where control and OdDHL-treated cells were grown under polarizing conditions for Th1 (c), Th2 (d), Th17 (e), and Treg (f) cells. Statistical analysis was carried out by using a Mann-Whitney U test in GraphPad Prism. Mean values are indicated by a horizontal bar. Ax488, Alexa Fluor 488.
To determine whether OdDHL had differential effects on already polarized T cell subsets, naïve T cells were first polarized in vitro and then exposed to OdDHL. Under our polarizing conditions, the percentage of polarized cells in the absence of OdDHL ranged from 60% for Th1, 20% for Th2, and 55 to 60% for Th17 to 90% for Treg cell populations. Polarized T cells were harvested, rested for 24 h, and then restimulated with anti-CD3 and anti-CD28 in the presence or absence of OdDHL. The ability of OdDHL to inhibit cell division was assessed by both [3H]thymidine and CFSE uptake. The results shown in Fig. 5a and b suggest that polarized T cells are less sensitive to the inhibitory effects of OdDHL than naïve T cells.
Fig. 5.
(a) OdDHL shows minimal inhibitory effect on the proliferation of polarized CD4+ T cells. Rested polarized T cells (5 × 105) were restimulated by plating in anti-CD3/anti-CD28-precoated plates under conditions essential for the maintenance of each T cell subset: Th1 (i), Th2 (ii), Th17 (iii), and Treg (iv). Proliferation was assessed using [3H]thymidine incorporation and CSFE dilution. Two control conditions were also included, medium alone and PBS containing 0.01% DMSO. A summary of five independent experiments is shown for [3H]thymidine (a) and CFSE dilution (b). Statistical analysis was carried out by using a Mann-Whitney U test in GraphPad Prism.
We next examined the effect of OdDHL on cytokine production by polarized T cells by carrying out intracellular cytokine analyses of viable cells following culture in the presence or absence of OdDHL. Figure 6a shows a representative FACS analysis of intracellular staining for IFN-γ and IL-17 of the polarized Th1 cell subset in the presence or absence of 10 μM OdDHL. From this plot it can be seen that that the percentage of viable polarized Th1 cells remains unaffected following culture with OdDHL. Summary data for all the studies on polarized T cell subsets are shown in Fig. 6b, from which it can be seen that OdDHL had little effect on cytokine production by already established T cell subsets
Fig. 6.
OdDHL shows minimal inhibitory effect on polarized CD4+ T cells in vitro. Rested polarized T cells (5 × 105) were cultured for 4 days on anti-CD3/anti-CD28-precoated plates under conditions essential for the maintenance of each T cell subset. Representative FACS plots are shown of Th1 cells grown in medium (a) or 10 μM OdDHL (b). A summary of between three and five independent experiments showing the effect of OdDHL on polarized cells subsets is also shown for Th1 (c), Th2 (d), Th17 (d), and Treg (f) cells. Statistical analysis was carried out by using a Mann-Whitney U test in GraphPad Prism.
Taking all our data together, it would seem that OdDHL does not have a selective effect on different T cell subsets and that any inhibitory effects on T cell differentiation and function occur at an early stage in the naïve T cell response.
DISCUSSION
It has been shown that OdDHL ameliorates diabetes onset in NOD mice. As type 1 diabetes is a Th1-mediated autoimmune disease (2, 10, 11), a possible explanation for its effect in delaying type 1 diabetes onset could be through a selective inhibitory action of OdDHL on Th1 responses and/or possibly through an increase in Treg cell activity. Recently Skindersoe and colleagues presented data suggesting that OdDHL inhibits maturation of BMDCs derived from C57BL/6 mice, thereby inhibiting naïve T cell stimulation (22). This therefore prompted us to examine whether we might find comparable effects using NOD BMDCs and T cells. It is possible that this might provide an explanation for our in vivo findings of the effect of this quorum-sensing molecule on the onset of type 1 diabetes. Examination of the effects of OdDHL on NOD BMDCs showed that OdDHL inhibited LPS-induced proinflammatory cytokine production with little effect on IL-10 production. This would be consistent with an effect of OdDHL on the proinflammatory response. However, in contrast to the observations of Skindersoe and colleagues using C57BL/6 cells, we could find no effect of OdDHL on the expression of CD80 and CD86 in NOD BMDCs. It has been previously shown that OdDHL was able to inhibit naïve T cell responses (19), and our own observations support this observation.
In studying the direct effect of this quorum-sensing molecule on both naïve NOD T cell subset development and on polarized T cells, we found not only that OdDHL inhibits proliferation of naïve T cells but also that it directly inhibits the differentiation of all T cell subsets in an antigen-presenting cell-free system. This lends support to previous observations that OdDHL acts on both antigen-presenting cells and directly on the T cells. This would also be consistent with the ability of this molecule to enter cells in an unregulated manner (20) and to have an effect on NF-κB (13).
As there was no evidence of a selective sparing of Treg cell responses, this suggests that OdDHL might simply inhibit diabetes onset at the stage of initial T cell priming and differentiation. It is thought that initial T cell priming may occur in the pancreatic lymph nodes and that primed and activated T cells then migrate into the pancreas. Mice were injected with OdDHL when they were 4 weeks of age, a time when pancreatic infiltration is only just commencing in NOD mice. The combined effect of OdDHL on both antigen-presenting cells and on T cell priming and proliferation suggests that this might be a critical time point for this quorum-sensing molecule to have efficacy in diabetes prevention. We were unable to find any significant effect of OdDHL on already polarized T cells, which might suggest that memory T cells would be less susceptible to in vivo inhibition by OdDHL. In terms of type 1 diabetes, we propose that this molecule would be unable to halt already primed T cells from mediating disease. This mode of action in diabetes prevention is similar to that found with helminth modulation of autoimmune disease, where effects are seen only at early time points. However, in the case of helminth infection, there is additional evidence of induction of Treg and invariant NK T (iNKT) cells to maintain an inhibition of diabetes prevention (5, 15). It should also be noted that OdDHL does not mediate the profound diabetes prevention observed with helminth-derived products, which would be consistent with a lack of induction of robust immunoregulatory circuits by OdDHL. As we did not find any evidence of an effect of this quorum-sensing molecule on expression of MHC class II or CD80 and CD86, this suggests that the effect of OdDHL on BMDCs is restricted to effects on cytokine production. This differs from the findings of Skindersoe and colleagues and may reflect differences in the mouse strains used to source the dendritic cells. There is evidence that NOD-derived BMDCs behave differently from those from other mouse strains with regard to cytokine responses (8).
Our data would support the view that elaboration of quorum-sensing signal molecules such as OdDHL by organisms such as P. aeruginosa might enable these infectious agents to evade initial immune responses and gain a foothold in the host. The inability of OdDHL to inhibit primed and committed differentiated T cell responses might suggest that immune evasion mediated by this molecule may be more restricted to initial stages of infection. This ability to inhibit T cell priming may additionally have the potential to prevent the onset of autoimmune pathologies.
ACKNOWLEDGMENTS
We are grateful to the Wellcome Trust for providing support for this research.
We also thank P. Zaccone, J. Phillips, and David Bending for their help and advice with this study. We are particularly grateful to S. Newland for all the technical help that he has provided.
Footnotes
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Alyanakian M. A., et al. 2006. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55:179–185 [PubMed] [Google Scholar]
- 2. Bending D., et al. 2009. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bras A., Aguas A. P. 1996. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology 89:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brode S., Raine T., Zaccone P., Cooke A. 2006. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+ CD25+ Foxp3+ regulatory T cells. J. Immunol. 177:6603–6612 [DOI] [PubMed] [Google Scholar]
- 5. Burton O. T., et al. 2010. Importance of TLR2 in the direct response of T lymphocytes to Schistosoma mansoni antigens. Eur. J. Immunol. 40:2221–2229 [DOI] [PubMed] [Google Scholar]
- 6. Cooke A., et al. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21:169–176 [DOI] [PubMed] [Google Scholar]
- 7. Drescher K. M., Kono K., Bopegamage S., Carson S. D., Tracy S. 2004. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329:381–394 [DOI] [PubMed] [Google Scholar]
- 8. Feili-Hariri M., Morel P. A. 2001. Phenotypic and functional characteristics of BM-derived DC from NOD and non-diabetes-prone strains. Clin. Immunol. 98:133–142 [DOI] [PubMed] [Google Scholar]
- 9. Gaisford W., Cooke A. 2009. Can infections protect against autoimmunity? Curr. Opin. Rheumatol. 21:391–396 [DOI] [PubMed] [Google Scholar]
- 10. Healey D., et al. 1995. In vivo activity and in vitro specificity of CD4+ Th1 and Th2 cells derived from the spleens of diabetic NOD mice. J. Clin. Invest. 95:2979–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz J. D., Benoist C., Mathis D. 1995. T helper cell subsets in insulin-dependent diabetes. Science 268:1185–1188 [DOI] [PubMed] [Google Scholar]
- 12. Kikutani H., Makino S. 1992. The murine autoimmune diabetes model: NOD and related strains. Adv. Immunol. 51:285–322 [DOI] [PubMed] [Google Scholar]
- 13. Kravchenko V. V., et al. 2008. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science 321:259–263 [DOI] [PubMed] [Google Scholar]
- 14. La Flamme A. C., Ruddenklau K., Backstrom B. T. 2003. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect. Immun. 71:4996–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehuen A., Diana J., Zaccone P., Cooke A. 2010. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10:501–513 [DOI] [PubMed] [Google Scholar]
- 16. Martins T. C., Aguas A. P. 1999. A role for CD45RBlow CD38+ T cells and costimulatory pathways of T-cell activation in protection of non-obese diabetic (NOD) mice from diabetes. Immunology 96:600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pritchard D. I., et al. 2005. Alleviation of insulitis and moderation of diabetes in NOD mice following treatment with a synthetic Pseudomonas aeruginosa signal molecule, N-(3-oxododecanoyl)-l-homoserine lactone. Acta Diabetol. 42:119–122 [DOI] [PubMed] [Google Scholar]
- 18. Richer M. J., Straka N., Fang D., Shanina I., Horwitz M. S. 2008. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-beta. Diabetes 57:1302–1311 [DOI] [PubMed] [Google Scholar]
- 19. Ritchie A. J., et al. 2005. The Pseudomonas aeruginosa quorum-sensing molecule N-3-(oxododecanoyl)-l-homoserine lactone inhibits T-cell differentiation and cytokine production by a mechanism involving an early step in T-cell activation. Infect. Immun. 73:1648–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ritchie A. J., et al. 2007. The immunomodulatory Pseudomonas aeruginosa signalling molecule N-(3-oxododecanoyl)-l-homoserine lactone enters mammalian cells in an unregulated fashion. Immunol. Cell Biol. 85:596–602 [DOI] [PubMed] [Google Scholar]
- 21. Ritchie A. J., Yam A. O., Tanabe K. M., Rice S. A., Cooley M. A. 2003. Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Infect. Immun. 71:4421–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skindersoe M. E., et al. 2009. Pseudomonas aeruginosa quorum-sensing signal molecules interfere with dendritic cell-induced T-cell proliferation. FEMS Immunol. Med. Microbiol. 55:335–345 [DOI] [PubMed] [Google Scholar]
- 23. Tateda K., et al. 2003. The Pseudomonas aeruginosa autoinducer N-3- oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Telford G., et al. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tracy S., Drescher K. M. 2007. Coxsackievirus infections and NOD mice: relevant models of protection from, and induction of, type 1 diabetes. Ann. N. Y. Acad. Sci. 1103:143–151 [DOI] [PubMed] [Google Scholar]
- 26. Veldhoen M., et al. 2008. Transforming growth factor-beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9:1341–1346 [DOI] [PubMed] [Google Scholar]
- 27. Walsh K. P., Brady M. T., Finlay C. M., Boon L., Mills K. H. 2009. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J. Immunol. 183:1577–1586 [DOI] [PubMed] [Google Scholar]
- 28. Zaccone P., et al. 2010. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J. Biomed. Biotechnol. 2010:795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaccone P., et al. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33:1439–1449 [DOI] [PubMed] [Google Scholar]
- 30. Zaccone P., et al. 2004. Salmonella typhimurium infection halts development of type 1 diabetes in NOD mice. Eur. J. Immunol. 34:3246–3256 [DOI] [PubMed] [Google Scholar]