Abstract
The aim of a malaria transmission-blocking vaccine is to block the development of malaria parasites in the mosquito and thus prevent subsequent infection of the human host. Previous studies have demonstrated that the gametocyte/gamete surface protein Pfs230 can induce transmission-blocking immunity and have evaluated Escherichia coli-produced Pfs230 as a transmission-blocking vaccine candidate. In this study, we used the wheat germ cell-free expression system to produce N-terminal fragments of Pfs230 and evaluated the transmission-blocking activity of antisera raised against the recombinant Pfs230 protein. The rabbit antisera reacted to the surface of cultured gametocytes and gametes of the Plasmodium falciparum NF54 line, recognized the 360-kDa form of parasite-produced Pfs230 by Western blot assay, and reduced the infectivity of NF54 parasites to Anopheles stefensi mosquitoes in the presence of complement in a standard membrane feeding assay. Thus, our data demonstrate that the N-terminal pro domain of Pfs230 is sufficient to induce complement-dependent transmission-blocking activity against P. falciparum.
INTRODUCTION
Malaria is a major infectious disease caused by protozoa of the genus Plasmodium and is transmitted by anopheline mosquitoes. There were an estimated 225 million clinical cases of malaria and 781,000 malaria-related deaths in 2009 (39). Among four species of human malaria parasites, Plasmodium falciparum causes the most severe form of malaria and is globally distributed. Elimination strategies are, however, limited and confounded by the emergence of multidrug-resistant parasites and insecticide-resistant mosquitoes (15). Therefore, the development of malaria vaccines is an essential component of malaria elimination and, further, eradication (13, 14). Malaria vaccines are generally divided into three groups based on the parasite life cycle target stage: preerythrocytic, asexual blood stage, and transmission-blocking vaccines (TBVs). Preerythrocytic vaccines act against sporozoites and liver stage parasites and are intended to prevent infection. Asexual blood stage vaccines are aimed at reducing parasite multiplication and growth to protect against clinical symptoms. TBVs block malaria transmission by interrupting the parasite life cycle in the mosquito. Thus, TBVs are considered to be essential components of combination vaccines that target multiple stages of the parasite's life cycle aimed at malaria eradication (1, 30).
Target antigens for TBV development are sexual and mosquito stage-specific surface molecules (5). Antigens expressed on the surface of zygotes and ookinetes in the mosquito midgut (e.g., P25 and P28 in P. falciparum and P. vivax), referred to as postfertilization antigens, have been shown to be effective for inducing transmission-blocking immunity (8, 17, 20, 31). Ookinete surface antigens, Pfs25 in P. falciparum and Pvs25 in P. vivax, have been tested in phase I clinical trials, and a positive correlation of TBV efficacy with antibody titers has been demonstrated (18, 22, 44). However, P25 is not expressed by blood stage parasites and hence it is not exposed to the human immune response (7, 27); therefore, the anti-Pfs25 immune response will not be boosted by natural malaria infection. In contrast to ookinete surface antigens, antigens that are expressed on male and female gametes, such as Pfs48/45 and Pfs230 (prefertilization antigens), may be boostable by natural malaria infection, as they are also expressed on gametocytes during infection and are thus exposed to the human immune system (7, 27). The major proteins found on the surface of both male and female gametocytes/gametes belong to a family of six cysteine-containing domain proteins designated cysteine motif (CM) domains that include Pfs48/45 (21) and Pfs230 (41). Pfs230 is a 360-kDa protein that contains 7 CM domains (Fig. 1A) (6, 11, 36) and elicits a humoral immune response in infected individuals that can mediate transmission-blocking immunity (12, 16). Consequently, Pfs230 as a component of a vaccine offers the advantage that it will elicit an immune response that is boosted by natural infection and will thus provide long-lasting immunity (3, 28).
Fig. 1.
Pfs230 primary structure and design of constructs. (A) Schematic representation of predicted structural motifs for Pfs230. SP represents a signal peptide. The cleavage site at amino acid (aa) 443 represents the site at which processing of Pfs230 occurs during gamete formation. The region comprising amino acids 443 to 588 refers to the pro domain. CM1 through CM7 (regions shaded in gray) represent CM domains as described by Williamson et al. (43). Amino acid positions (arrows) 589, 918, 1285, and 3135 represent the starts of CM1, CM2, and CM3 and the end of CM7, respectively. Also, domains I through XIV, as described by Gerloff et al. (11), are in parentheses. (B) Construct Pfs230C spans the pro domain through domain III and comprises amino acids 443 to 1132. Construct Pfs230C2 spans the pro domain through domain II and comprises amino acids 443 to 915. Construct Pfs230C1 spans the pro domain and domain I and comprises amino acids 443 to 715. Construct Pfs230C0 spans the pro domain only and comprises amino acids 443 to 588.
The monoclonal antibodies (MAbs) recognizing the CM domain of Pfs230 potently block the infectivity of the P. falciparum parasites to mosquitoes (26, 28, 29, 37). Several regions of Pfs230 have been expressed as maltose-binding protein (MBP) fusions in Escherichia coli (designated r230/MBP) (43). Antibodies raised against an N-terminal 76-kDa fragment of Pfs230 designated r230/MBP.C (amino acids 443 to 1132) were bound to the surfaces of gametes and reduced the infectivity of P. falciparum to mosquitoes in the presence of complement (43). This was the first demonstration of the transmission-blocking activity of antibodies against a recombinant Pfs230 antigen. However, r230/MBP.C protein elicited incomplete oocyst reduction. Attempts to increase the efficacy of Pfs230C-based vaccines have included yeast expression systems (38), DNA vaccine (9), and vaccinia virus expression systems (40). Disappointingly, these attempts to produce a more efficacious vaccine have failed, with r230/MBP.C appearing to be the most potent Pfs230 TBV identified to date (40). Recently, we reported that among the various protein synthesis systems, the wheat germ cell-free protein synthesis system is permissive for the production of correctly folded malaria proteins (30, 32). The wheat germ cell-free system was demonstrated to produce malaria proteins without any codon optimization (33). With the expectation that the wheat germ cell-free system would produce higher-quality Pfs230 proteins more effective in eliciting transmission-blocking activity than the E. coli-expressed r230/MBP.C protein, we decided to express recombinant Pfs230C and its truncated forms and to characterize the minimal region that contains the immunodominant epitope(s) of Pfs230C sufficient to induce malaria transmission-blocking activity.
MATERIALS AND METHODS
Cloning of fragments encoding truncated forms of Pfs230.
Williamson et al. (43) predicted the CM domains, CM1 through CM7, in Pfs230 (PFB0405w) (Fig. 1A). Gerloff et al. (11) also described cysteine-rich domains I through XIV in Pfs230. Based on those predictions, we synthesized four different truncated forms of Pfs230, i.e., Pfs230C (pro domain through domain III, amino acids 443 to 1132 with 13 cysteines), Pfs230C2 (pro domain through domain II, amino acids 443 to 915 with 8 cysteines), Pfs230C1 (pro domain and domain I, amino acids 443 to 715 with 4 cysteines), and Pfs230C0 (pro domain, amino acids 443 to 588 without any cysteines) (Fig. 1B). Genomic DNA encoding each of the truncated forms of Pfs230C was amplified by PCR from P. falciparum 3D7 DNA and cloned between the XhoI and NotI sites of plasmid pEU-E01-GST-TEV (a vector with an N-terminal glutathione S-transferase tag followed by a tobacco etch virus protease cleavage site; CellFree Sciences, Matsuyama, Japan). The inserted nucleotide sequences were confirmed using the ABI PRISM 3130 Genetic Analyzer and the BigDye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA).
Production and purification of truncated Pfs230 proteins.
Recombinant proteins Pfs230C, Pfs230C2, Pfs230C1, and Pfs230C0 were produced with the wheat germ cell-free protein expression system by the bilayer translation reaction method described previously (33, 34). After their synthesis, the truncated forms of Pfs230C were affinity purified by passage through a glutathione-Sepharose 4B column (GE Healthcare, Camarillo, CA) and eluted by on-column cleavage with AcTEV protease (Invitrogen, Carlsbad, CA) after extensive washing of the column with phosphate-buffered saline (PBS). Concentrations of purified proteins were determined using the Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and the bands were visualized with Coomassie brilliant blue (Fig. 2). Purified protein samples were stored in aliquots at −80°C until further use.
Fig. 2.
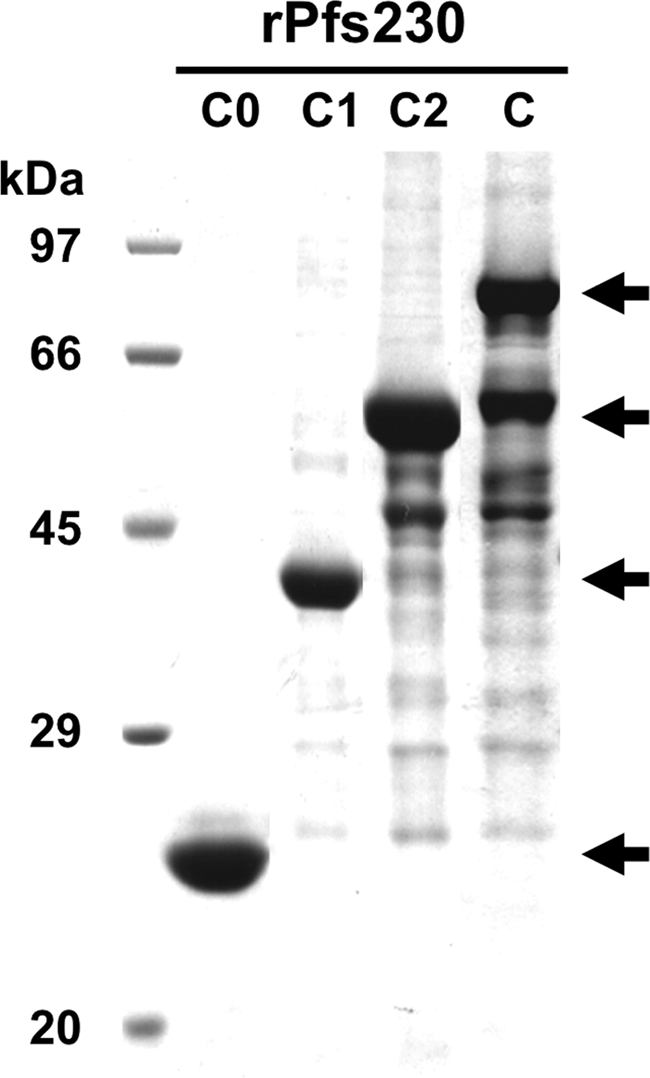
SDS-PAGE analysis of the purified truncated recombinant Pfs230 (rPfs230) proteins expressed. The different truncated Pfs230 proteins, Pfs230C0 (C0), Pfs230C1 (C1), Pfs230C2 (C2), and Pfs230C (C), were expressed in the wheat germ cell-free system and separated on an SDS-12.5% polyacrylamide gel under reducing condition and stained with Coomassie brilliant blue (arrows indicate the expected truncated Pfs230 proteins). The extra bands other than those indicated by arrows in lanes C2 and C are translation products due to premature termination of translation.
Preparation of rabbit antisera.
A Japanese White rabbit for each antigen was immunized subcutaneously 3 times at 3-week intervals with 250 μg of each purified truncated Pfs230 protein emulsified with complete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO) for the prime and with incomplete Freund's adjuvant (Sigma-Aldrich) for the first and the second boosts. Blood was collected before immunization and 2 weeks after the third immunization. Antisera were prepared as previously described (25). Preimmune sera from individual rabbits were used as controls.
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Ehime University, and the experiments were conducted according to the Ethical Guidelines for Animal Experiments of Ehime University.
Enzyme-linked immunosorbent assay (ELISA).
The titers of the IgG in the rabbit antisera raised against different truncated Pfs230 proteins were measured by ELISA. Briefly, 96-well MaxiSorp ELISA plates (Nunc, Rochester, NY) were coated with 0.5 μg/ml recombinant truncated forms of Pfs230 in 20 mM borate buffer (pH 8.9) and incubated overnight at 4°C. After blocking with 2 mg/ml gelatin in 20 mM borate buffer, serum samples were plated in duplicate at a starting dilution of 1:100 with PBS containing 0.01% Tween 20 and 2 mg/ml gelatin and titrated in 10-fold dilutions. Target-specific IgG was detected using horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG at 1:2,000 (Biosource, Camarillo, CA) and visualized using 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Wako, Osaka, Japan) as the substrate. The reaction was then stopped with 0.1 M citric acid, and the optical density values were measured. Reciprocal serum dilutions that gave a mean absorbance value of 0.5 at 415 nm were determined as the endpoint titers. In order to measure the antibody titers against native Pfs230 protein, stage V gametocyte of the P. falciparum NF54 line was extracted with PBS with 1% Triton X-100 containing Complete Proteinase Inhibitor Cocktail (Roche, Indianapolis, IN) and centrifuged at 21,900 × g for 20 min. The collected supernatant was diluted to a final concentration of 1.0 μg protein/ml and used as a capture antigen, and ELISA was performed as described above.
Immunofluorescence assay (IFA).
An indirect IFA was performed with cultured gametocytes or macrogametes/zygotes after the induction of gametogenesis from the cultured gametocytes of the P. falciparum NF54 line (43). Air-dried thin smears of the parasites were prepared on glass slides and stored at −80°C until use. The smears were thawed, fixed with ice-cold acetone for 3 min, and blocked with PBS containing 5% nonfat milk (PBS milk) at 37°C for 30 min. They were then incubated with either rabbit immune serum (1:500 dilution) or matched preimmune serum as a negative control at 37°C for 1 h, followed by incubation with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen) as a secondary antibody (1:500) at 37°C for 30 min. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 2 μg/ml) mixed with a secondary antibody solution. Slides were mounted in ProLong Gold Antifade reagent (Invitrogen) and observed under a 63× oil immersion lens. High-resolution image capture and processing were performed using a confocal scanning laser microscope (LSM5 PASCAL; Carl Zeiss MicroImaging, Thornwood, NY). Images were processed in Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Western blot analysis.
Proteins of the cultured gametocytes were extracted in nonreducing SDS-PAGE loading buffer and boiled at 98°C for 3 min, and extract from approximately 105 gametocytes per lane was subjected to electrophoresis on a 12.5% polyacrylamide gel (ATTO, Tokyo, Japan). Proteins were then transferred to a 0.2-μm polyvinylidene fluoride (PVDF) membrane (GE Healthcare). The proteins were immunostained with either preimmune or immune serum as the primary antibody. To ensure that equal amounts of the protein samples were loaded in each lane for Western blot analysis, the membranes were simultaneously probed with anti-PfHSP70 mouse MAb (4C9) as a quantitative marker for parasite proteins (35). The membranes were then probed by HRP-conjugated goat anti-rabbit IgG antibody (GE Healthcare) together with HRP-conjugated goat anti-mouse IgG antibody (GE Healthcare) and visualized with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA) on a LAS 4000 mini luminescent image analyzer (GE Healthcare). The relative molecular masses of the proteins were estimated with reference to Precision Plus Protein Standards (Bio-Rad, Hercules, CA).
Transmission-blocking assays.
The transmission-blocking activity of the sera was tested by an ex vivo standard membrane feeding assay (SMFA) as described previously (44). Briefly, an in vitro gametocyte culture of P. falciparum (NF54 line) was evaluated for the percentage of stage V gametocytes (>0.5%), and the vitality of exflagellation centers was observed at ×400 magnification. The gametocyte pellet was diluted with normal O+ red blood cells (Interstate Blood Bank, Memphis, TN) and a normal heat-inactivated (complement minus) or unheated (complement plus) AB+ human serum pool (Interstate Blood Bank, Memphis, TN) to achieve a 0.15% ± 0.05% concentration of stage V gametocytes and a hematocrit of 50%. This infected blood mixture was kept at 37°C and aliquoted into 200-μl portions prior to feeding. One 200-μl aliquot of “infected blood” was mixed with 60 μl of the test serum diluted with a normal heat-inactivated O+ human serum pool at 1:1, 1:2, or 1:4 for final dilution of the sera in feeders at 1:5.3, 1:11, and 1:21, respectively. The mixture was immediately fed to 3- to 8-day-old Anopheles stephensi (Nijmegen strain) mosquitoes prestarved for 24 to 30 h through a membrane feeding apparatus using a thin stretched Parafilm membrane. Mosquitoes were kept for 7 to 8 days after feeding at 26°C under high-humidity conditions to allow parasites to develop into oocysts. Infectivity was measured by dissecting at least 20 mosquitoes per sample, staining the midguts with a 0.05% merbromin (Mercurochrome) solution in water for at least 20 min, and counting the oocysts in each midgut. The percent reduction of the oocyst count per mosquito was determined by the formula 100 × (mean oocyst no.negative control − mean oocyst no.test)/mean oocyst no.negative control, where the negative-control feeding used preimmune serum from the same rabbit. All samples or diluted samples were tested in replicate. To verify whether the difference in oocyst numbers between preimmune and immune groups is statistically significant, data were analyzed by comparing the medians of two groups using the Mann-Whitney U test. Oocyst prevalence was statistically analyzed for all vaccination regimens by Fisher's exact test. Probability (P) values of less than 0.05 were considered statistically significant in both analyses.
RESULTS
Synthesis of soluble Pfs230C proteins in a wheat germ cell-free system.
In order to evaluate the transmission-blocking activities elicited by the wheat germ cell-free expressed truncated forms of Pfs230C and to define the minimal region of Pfs230 sufficient to induce the malaria transmission-blocking activity, we designed different Pfs230C constructs, i.e., Pfs230C (pro domain through domain III, amino acids 443 to 1132 with 13 cysteines), Pfs230C2 (pro domain through domain II, amino acids 443 to 915 with 8 cysteines), Pfs230C1 (pro domain and domain I, amino acids 443 to 715 with 4 cysteines), and Pfs230C0 (pro domain, amino acids 443 to 588 without any cysteines), based on schematic diagrams of Pfs230 (Fig. 1A and B) without codon optimization and expressed them in a wheat germ cell-free system (Fig. 2). Figure 2 shows the different truncated Pfs230 proteins resolved in a 12.5% SDS-polyacrylamide gel. Almost all of the truncated Pfs230 proteins were recovered in the supernatant fraction and easily purified as a single dominant band (Fig. 2, arrows) along with other, nonspecific, faint bands by affinity chromatography in lanes C2 and C. The yields of purified Pfs230C0, Pfs230C1, Pfs230C2, and Pfs230C proteins were 62, 54, 65, and 75 μg/5.0 ml of the reaction mixture, respectively. These results demonstrate that the wheat germ cell-free system is able to translate the native Pfs230 gene sequences and produce soluble proteins.
Rabbit antibodies against truncated forms of Pfs230C recognized native parasite proteins.
To evaluate the immunogenicity of the recombinant truncated forms of Pfs230, rabbit antisera were collected on study days 0 (pre) and 56 (post). ELISA analysis of the IgG responses elicited by the four truncated forms of Pfs230C (Fig. 3 A) showed that all of the postimmune sera contained high titers of antibodies, with no discernible difference among the four recombinant protein formulations, i.e., titers against all of the recombinant proteins, Pfs230C0 (105.8), Pfs230C1 (106.1), Pfs230C2 (105.8), and Pfs230C (106.0) (Fig. 3A). All of the negative-control preimmune sera showed no IgG responses (Fig. 3A).
Fig. 3.
IgG responses elicited by immunization with different truncated forms of Pfs230 in rabbits. (A). Serum IgG titers in samples collected before antigen administration (pre) and on day 56 postimmunization using recombinant Pfs230C0, -C1, -C2, and -C as ELISA capture antigens. (B). Serum IgG titers in samples collected on day 56 postimmunization using native, parasite-derived Pfs230 as the plate antigen. Reciprocal serum dilutions that gave a mean absorbance at 415 nm of 0.5 were determined as the endpoint titers. OD, optical density.
To evaluate the immunoreactivity of the antisera against parasite-derived native proteins, Western blot analysis was performed. Extract from stage V gametocytes of the P. falciparum NF54 line was separated by 12.5% SDS-PAGE, and specific bands with the expected mobility of native Pfs230 protein (Fig. 4, arrow) were detected under nonreducing conditions (Fig. 4, left panel) using antiserum against each recombinant Pfs230 protein. Preimmune serum failed to recognize the native Pfs230 protein (Fig. 4, right panel). These results suggest that the recombinant truncated forms of Pfs230C prepared by the cell-free system as soluble proteins retained native epitopes. To ensure that the same amount of each gametocyte protein sample was loaded in each lane for Western blot analysis, the membranes were also probed with anti-PfHSP70 MAb (4C9) (35). The intensities of the PfHSP70 bands indicated that the amounts of samples loaded in the lanes were comparable (Fig. 4, arrowhead). Importantly, ELISA analysis of the IgG responses elicited by the four truncated forms of Pfs230C showed that the postimmune serum against each recombinant protein, Pfs230C0 (titer, 103.5), Pfs230C1 (titer, 103.8), Pfs230C (titer, 103.7), or Pfs230C2 (titer, 102.4) contained significant levels of antibodies with no discernible difference in the levels of antibodies against the parasites' native Pfs230 protein extracted from the gametocytes of P. falciparum (Fig. 3B).
Fig. 4.
Western blot analysis using antisera against different truncated forms of Pfs230, Pfs230C0 (C0), Pfs230C1 (C1), Pfs230C2 (C2), and Pfs230C (C). Extracts prepared from stage V gametocytes of the P. falciparum NF54 line were separated on SDS-12.5% polyacrylamide gels under nonreducing condition and transferred onto PVDF membrane. Proteins on PVDF membranes were immunostained with either rabbit anti-Pfs230C0, -C1, -C2, and -C sera (lanes C0, C1, C2, and C in the left panel) or the corresponding preimmune (Pre) sera (right panel) (arrow). The relative molecular masses of the proteins were estimated with reference to Precision Plus Protein Standards (Bio-Rad, Hercules, CA). To ensure that equal amounts of the protein samples were loaded into the lanes for Western blot analysis, the membranes were simultaneously probed with anti-PfHSP70 mouse MAb (4C9) as a quantitative marker of parasite protein (35) (arrowhead).
To confirm the specificity of the antisera for the truncated forms of Pfs230, IFA was performed against stage V gametocytes and gametes of P. falciparum (Fig. 5). Antisera against the recombinant truncated forms of Pfs230 specifically stained the surfaces of both gametocytes and gametes (Fig. 5). All of the negative-control preimmune sera showed no staining of the parasites (data not shown).
Fig. 5.
Reactivity of antisera against Pfs230C0, -C1, and -C2 and Pfs230C in immunofluorescence microscopy. Samples prepared from stage V gametocytes (upper panels) and gametes (lower panels) of the P. falciparum NF54 line were immunostained with the antisera indicated above the panels. Immunostained images were visualized with Alexa Fluor 488-conjugated goat anti-rabbit IgG (green). Nuclei were stained with DAPI (blue). Scale bars, 5 μm.
Antibodies against truncated forms of Pfs230 reduce parasite transmission to the mosquito vector in the presence of complement.
The transmission-blocking activity of the rabbit antisera raised against recombinant Pfs230C was evaluated by SMFA. The antisera were diluted to minimize nonspecific effects of sera on transmission-blocking activity, and the preimmune sera from individual animals were used as negative controls. The multiple assays were performed on different days using the cultured P. falciparum NF54 line (Table 1). The number of oocysts formed in the presence of complement in experiment 1 was significantly lower (P < 0.0001), by 86%, than that obtained with the preimmune serum (with complement, experiment 1). A similar result (88% reduction) was obtained (P < 0.0001) in experiment 2 (with complement, experiment 2). In the third experiment (Table 1), a further reduction was observed (P < 0.0001, 99.0%) when a 1:5.3 dilution of anti-Pfs230C antiserum was tested in the presence of complement. The number of oocysts was inversely proportional to the concentration of antiserum added (experiment 3 in Table 1), and the differences between pre- and postimmune groups, even at the lowest concentration of the antiserum (1:21) tested were still significant (P < 0.0001).
Table 1.
Evaluation of sera from rabbits immunized with Pfs230Cs by SMFAa
| Antigen and expt no. (dilution) | Sampleb | With complement |
Without complement |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. inf./diss.c | Median (IQRe) | % Reductionf | Pg | No. inf./diss. | Median (IQR) | % Reduction | P | ||
| Pfs230C | |||||||||
| 1 (1:5.3) | Rabbit 2 pre | 24/25 | 14.0 (8.5–33.0) | 22/24 | 12.5 (5.0–41.5) | ||||
| 1 (1:5.3) | Rabbit 2 post | 18/23 | 2.0 (1.0–4.0) | 86 | <0.0001 | 23/25 | 11.0 (5.5–26.0) | 34 | NSh |
| Pfs230C | |||||||||
| 2 (1:5.3) | Rabbit 2 pre | 23/23 | 21.0 (11.0–28.0) | 23/24 | 17.0 (8.0–20.0) | ||||
| 2 (1:5.3) | Rabbit 2 post | 21/23 | 2.0 (1.0–3.5) | 88 | <0.0001 | 23/25 | 8.0 (5.5–15.5) | 33 | NS |
| Pfs230C | |||||||||
| 3 (1:5.3) | Rabbit 2 pre | 21/22 | 57.0 (30.0–76.0) | ||||||
| 3 (1:5.3) | Rabbit 2 post | 6/23d | 0.0 (0.0–1.0) | 99 | <0.0001 | ||||
| 3 (1:11) | Rabbit 2 pre | 22/22 | 62.0 (29.5–88.0) | ||||||
| 3 (1:11) | Rabbit 2 post | 20/21 | 3.0 (1.0–6.5) | 94 | <0.0001 | ||||
| 3 (1:21) | Rabbit 2 pre | 22/22 | 55.0 (37.0–73.0) | ||||||
| 3 (1:21) | Rabbit 2 post | 23/24 | 16.5 (12.0–24.0) | 70 | <0.0001 | ||||
| Pfs230C0 | |||||||||
| 4 (1:5.3) | Rabbit 100 pre | 21/21 | 111.0 (78.5–134.5) | 22/22 | 58.0 (39.5–105.0) | ||||
| 4 (1:5.3) | Rabbit 100 post | 20/23 | 20.0 (13.0–28.0) | 82 | <0.0001 | 20/21 | 53.0 (33.0–82.5) | 8 | NS |
| Pfs230C1 | |||||||||
| 4 (1:5.3) | Rabbit 101 pre | 21/21 | 81.0 (66.0–120.0) | 20/21 | 84.0 (68.0–101.0) | ||||
| 4 (1:5.3) | Rabbit 101 post | 22/22 | 30.5 (14.5–45.5) | 63 | <0.0001 | 24/24 | 53.5 (16.5–83.5) | 38 | <0.01 |
| Pfs230C2 | |||||||||
| 4 (1:5.3) | Rabbit 102 pre | 21/21 | 120.0 (80.0–166.5) | 21/21 | 67.0 (39.5–90.0) | ||||
| 4 (1:5.3) | Rabbit 102 post | 22/22 | 33.5 (20.5–52.5) | 71 | <0.0001 | 26/26 | 38.0 (16.0–63.5) | 46 | <0.05 |
| Pfs230C0 | |||||||||
| 5 (1:5.3) | Rabbit 100 pre | 18/22 | 2.0 (1.0–5.0) | 23/23 | 15.0 (10.0–21.0) | ||||
| 5 (1:5.3) | Rabbit 100 post | 6/28d | 0.0 (0.0–0.0) | 92 | <0.0001 | 24/24 | 5.0 (3.0–7.5) | 62 | <0.0001 |
| Pfs230C1 | |||||||||
| 5 (1:5.3) | Rabbit 101 pre | 22/24 | 6.0 (3.0–8.0) | 22/24 | 14.0 (8.5–16.0) | ||||
| 5 (1:5.3) | Rabbit 101 post | 8/23d | 0.0 (0.0–1.0) | 92 | <0.0001 | 22/24 | 6.5 (2.5–10.0) | 41 | <0.05 |
| Pfs230C2 | |||||||||
| 5 (1:5.3) | Rabbit 102 pre | 18/24 | 2.5 (0.5–4.0) | 28/28 | 9.0 (5.5–15.5) | ||||
| 5 (1:5.3) | Rabbit 102 post | 8/23d | 0.0 (0.0–1.0) | 79 | <0.001 | 25/25 | 6.0 (3.5–8.0) | 48 | <0.01 |
One 200-μl aliquot of “infected blood” with a hematocrit of 50% was mixed with 60 μl of the test serum diluted with a normal heat-inactivated O+ human serum pool in a 1:1, 1:2, or 1:4 final dilution of the sera in feeders at 1:5.3, 1:11, and 1:21, respectively.
The serum sample from an individual rabbit was used. Numbers such as 2, 100, 101, and 102 are animal identification numbers.
Oocyst prevalence, expressed as the number of oocyst-infected mosquitoes over the total number of mosquitoes dissected (inf./diss.), was statistically analyzed between pre- and postimmune serum groups for all vaccination regimens by Fisher's exact test.
Statistically significant, P < 0.05.
IQR, interquartile range.
Percent reduction in the oocyst count per mosquito was determined by the formula 100 × (mean oocyst no.negative control − mean oocyst no.test)/mean oocyst no.negative control, where the negative-control feeding used preimmune serum from the same rabbit.
The median number of oocysts was statistically analyzed by comparing the day 56 immune serum with the matched preimmune rabbit serum (Mann-Whitney U test), and P values were obtained.
NS, not significant.
To identify the minimal functional transmission-blocking domains within Pfs230C, the complement-dependent transmission-blocking activity was also evaluated in sera raised against Pfs230C0, -C1, and -C2 (Fig. 1B). As shown in Table 1, the antisera raised against Pfs230C0, -C1, and -C2 (with complement, experiment 4) significantly reduced the number of oocysts per mosquito if complement was present in the SMFA. Surprisingly, the rabbit antisera (other than anti-Pfs230C0) reduced oocyst formation significantly in the mosquito, even in the absence of complement (without complement, experiment 4). Consistent with observations in SMFAs in general, the apparent transmission-blocking efficacy was lower when the oocyst load of the preimmune control was higher (Table 1, experiment 4), and it was higher when the oocyst load of the preimmune control was lower (Table 1, experiment 5).
The oocyst prevalence, expressed as the number of oocyst-positive mosquitoes over the total number of mosquitoes dissected (numbers infected/dissected in Table 1), was statistically analyzed for all vaccination regimens by Fisher's exact test. Both the number of oocyst and the oocyst prevalence in mosquitoes were significantly reduced by the Pfs230C (1:5.3) antiserum in experiment 3 and the Pfs230C0 (1:5.3), Pfs230C1 (1:5.3), and Pfs230C2 (1:5.3) antisera in experiment 5.
DISCUSSION
We have demonstrated that the wheat germ cell-free system supported the production of soluble recombinant Pfs230C (comprising amino acids 443 to 1132 of Pfs230), which induced transmission-blocking antibodies (Table 1). In previous studies (4, 43), transmission-blocking antibodies against Pfs230C were effective only at a higher concentration (i.e., a 1:2 dilution). However, in this study, the transmission-blocking antibodies induced by Pfs230C were effective even at a lower concentration (i.e., a 1:5.3 dilution). The specific IgG titers of these immune sera were comparable when they were tested against the immunizing antigens; the IgG titers of the anti-Pfs230C2 serum was slightly lower than those of the other sera when they were tested against native, parasite-derived Pfs230. The transmission-blocking efficacy is complement dependent, as previously reported (40). Moreover, even when the antiserum was further diluted (1:21 dilution in Table 1, experiment 3), a 70% reduction of the oocyst number was still achieved (P < 0.0001). These results suggest that the transmission-blocking efficacy seen is comparable to that of E. coli-produced recombinant Pfs230C, i.e., r230/MBP.C comprising amino acids 443 to 1132.
Evidence from previous studies and the data presented herein that recombinant Pfs230C has transmission-blocking activity, defining a minimal region of Pfs230 sufficient to induce the malaria transmission-blocking activity, will facilitate the development of candidate Pfs230 TBV into a vaccine. Bustamante et al. (4) expressed the regions of recombinant truncated Pfs230 proteins in E. coli as MBP fusions, i.e., r230/MBP.C (amino acids 443 to 1132), r230/MBP.C5′ (amino acids 443 to 791), r230/MBP.CM1 (amino acids 583 to 913), r230/MBP.C1·6 (amino acids 453 to 913), and r230/MBP.C2 (amino acids 914 to 1268), and used them to generate antibodies in mice. All of the antisera recognized native Pfs230 on the surfaces of the gametes, but only antibodies against r230/MBP.C, and not antibodies against other truncated forms, reduced the number of oocysts. The authors therefore concluded that the entire Pfs230C protein is required to produce the transmission-blocking epitope (4). However, our results demonstrate that antibodies against all of the recombinant truncated forms of Pfs230 reduced the number of oocysts not only in the presence but even in the absence of active complement (Table 1). The reason for the transmission-blocking activity in the absence of active complement may be due to the presence of antibodies that block biological function such as blocking of the fertilization of gametes besides the antibodies that are involved in the complement-mediated lysis of gametes in polyclonal antibodies. These results demonstrate that the topology of the native Pfs230 domains may be better retained in the recombinant truncated forms of Pfs230 synthesized in the eukaryotic wheat germ cell-free system than in the proteins expressed in E. coli. Importantly, there is no obvious difference in the transmission-blocking efficacy of antibodies raised against Pfs230C0, -C1, or -C2. This suggests that the transmission-blocking epitope is confined to the N-terminal cysteine-free pro domain (Pfs230C0). Therefore, the entire Pfs230C protein is not required for immunization to induce complement-dependent transmission-blocking activity against P. falciparum, but the minimal N-terminal cysteine-free pro domain (Pfs230C0) is sufficient (Table 1, experiments 4 and 5).
Several TBV candidates have complex structures and multiple disulfide bonds, which hamper their production, evaluation, and development as recombinant vaccine candidates (18, 19). However, the N-terminal pro domain of Pfs230 (Pfs230C0) is cysteine free and hence not constrained by disulfide bonds. The absence of disulfide bonds is expected to facilitate the production and development of Pfs230C0 as a TBV candidate. Future vaccine development efforts should focus on vaccine formulation or delivery methods to increase the immunogenicity of Pfs230C0.
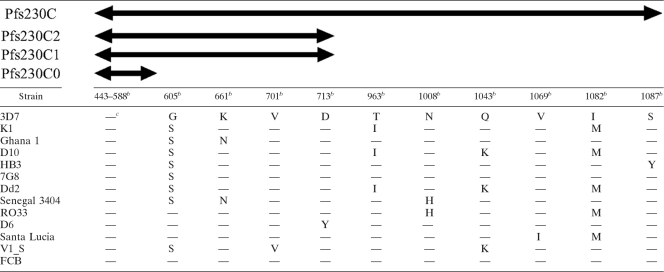
It is critical to design a vaccine based on sequences with minimum polymorphisms to avoid parasite evasion of vaccine-induced immunity. The native Pfs230 protein is expressed on the surface of gametocytes, and field studies have found that antibodies against Pfs230 are generated during natural infection (12, 16). Exposure to host immune pressure has resulted in Pfs230 sequence polymorphisms observed in field isolates (11, 24, 42). A compilation of single nucleotide polymorphisms (SNPs) in Pfs230 sequences from 13 laboratory strains deposited in PlasmoDB (http://plasmodb.org/plasmo/) showed that Pfs230C contains a total of 10 nonsynonymous SNPs. Pfs230C2 and Pfs230C1 contain 4 of them, and, importantly, Pfs230C0 does not contain any nonsynonymous SNPs (Table 2). These data further support the notion that Pfs230C0 is a promising TBV candidate.
Table 2.
Nonsynonymous SNP sites of Pfs230 deposited in PlasmoDBa
The data were obtained from the PlasmoDB version 7.1 (http://plasmodb.org/plasma).
Amino acid position(s) in the Pfs230 amino acid sequence based on the 3D7 sequence used as a reference.
—, same amino acid residue as in the 3D7 sequence.
Production of functionally active truncated forms of Pfs230 will also facilitate understanding of the dynamics of naturally acquired transmission-blocking immunity and assist with the future evaluation and deployment of TBVs in populations living in areas where malaria is endemic. Recently Bousema et al. (3) reported that the antibody response to Pfs230 is boosted by exposure to gametocytes. To date, most of those studies used the sandwich ELISA method using anti-Pfs230 MAbs in combination with cultured P. falciparum gametocyte crude lysates (2). In the case of TBV application in future field trials, seroepidemiological surveillance of such naturally existing transmission-reducing immunity seems to be essential; however, the availability of crude parasite antigen for such a large-scale study is one of the hurdles to be overcome. Our study has proven that we could synthesize properly folded soluble truncated forms of Pfs230 in large quantities using the wheat germ cell-free system. Therefore, the availability of antigens for such a large-scale seroepidemiological surveillance will no longer be limiting.
The present study demonstrates the potential of wheat germ cell-free protein synthesis for the production of Pfs230C for clinical studies. However, to date, there is no cGMP facility using the cell-free system for the production of recombinant proteins for clinical studies. Therefore, expression trials based on the plant-based transient protein expression system applicable to the downstream cGMP (23) are under way toward the development of Pfs230-based TBV (10).
ACKNOWLEDGMENTS
We thank Thangavelu U. Arumugam for critical reading of the manuscript.
This research was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (21022034, 21249028, 21406010) and by the Ministry of Health, Labor, and Welfare, Japan (H20-Shinkou-ippan-013, H21-Chikyukibo-ippan-005). This work was also supported in part by a grant from The Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Birkett A. J. 2010. PATH malaria vaccine initiative (MVI): perspectives on the status of malaria vaccine development. Hum. Vaccin. 6:139–145 [DOI] [PubMed] [Google Scholar]
- 2. Bousema J. T., et al. 2006. Rapid onset of transmission-reducing antibodies in javanese migrants exposed to malaria in Papua, Indonesia. Am. J. Trop. Med. Hyg. 74:425–431 [PubMed] [Google Scholar]
- 3. Bousema T., et al. 2010. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PLoS One 5:e14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bustamante P. J., et al. 2000. Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol. 22:373–380 [DOI] [PubMed] [Google Scholar]
- 5. Carter R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309–2314 [DOI] [PubMed] [Google Scholar]
- 6. Carter R., Coulson A., Bhatti S., Taylor B. J., Elliott J. F. 1995. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol. Biochem. Parasitol. 71:203–210 [DOI] [PubMed] [Google Scholar]
- 7. Carter R., Graves P. M., Quakyi I. A., Good M. F. 1989. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 169:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duffy P. E., Kaslow D. C. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fanning S. L., et al. 2003. A glycosylphosphatidylinositol anchor signal sequence enhances the immunogenicity of a DNA vaccine encoding Plasmodium falciparum sexual-stage antigen, Pfs230. Vaccine 21:3228–3235 [DOI] [PubMed] [Google Scholar]
- 10. Farrance C. E., et al. 2011. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin. Vaccine Immunol., 18:1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerloff D. L., Creasey A., Maslau S., Carter R. 2005. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 102:13598–13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. 1988. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 10:209–218 [DOI] [PubMed] [Google Scholar]
- 13. Greenwood B. 2009. Can. malaria be eliminated? Trans. R. Soc. Trop. Med. Hyg. 103(Suppl. 1):S2–S5 [DOI] [PubMed] [Google Scholar]
- 14. Greenwood B., Targett G. 2009. Do we still need a malaria vaccine? Parasite Immunol. 31:582–586 [DOI] [PubMed] [Google Scholar]
- 15. Greenwood B. M., et al. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Healer J., McGuinness D., Carter R., Riley E. 1999. Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119:425–433 [DOI] [PubMed] [Google Scholar]
- 17. Hisaeda H., et al. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaslow D. C. 2002. Transmission-blocking vaccines. Chem. Immunol. 80:287–307 [DOI] [PubMed] [Google Scholar]
- 19. Kaslow D. C., et al. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62:5576–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaslow D. C., et al. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76 [DOI] [PubMed] [Google Scholar]
- 21. Kocken C. H., et al. 1993. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol. Biochem. Parasitol. 61:59–68 [DOI] [PubMed] [Google Scholar]
- 22. Malkin E. M., et al. 2005. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23:3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Musiychuk K., et al. 2007. A launch vector for the production of vaccine antigens in plants. Influenza Other Respi. Viruses 1:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niederwieser I., Felger I., Beck H. P. 2001. Limited polymorphism in Plasmodium falciparum sexual-stage antigens. Am. J. Trop. Med. Hyg. 64:9–11 [DOI] [PubMed] [Google Scholar]
- 25. Otsuki H., et al. 2009. Single amino acid substitution in Plasmodium yoelii erythrocyte ligand determines its localization and controls parasite virulence. Proc. Natl. Acad. Sci. U. S. A. 106:7167–7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quakyi I. A., et al. 1987. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139:4213–4217 [PubMed] [Google Scholar]
- 27. Quakyi I. A., et al. 1989. Differential non-responsiveness in humans of candidate Plasmodium falciparum vaccine antigens. Am. J. Trop. Med. Hyg. 41:125–134 [PubMed] [Google Scholar]
- 28. Read D., et al. 1994. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 16:511–519 [DOI] [PubMed] [Google Scholar]
- 29. Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 158:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeo S., Arumugam T. U., Torii M., Tsuboi T. 2009. Wheat germ cell-free technology for accelerating the malaria vaccine research. Expert Opin. Drug Discov. 4:1191–1199 [DOI] [PubMed] [Google Scholar]
- 31. Tsuboi T., et al. 1998. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med. 4:772–782 [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuboi T., Takeo S., Arumugam T. U., Otsuki H., Torii M. 2010. The wheat germ cell-free protein synthesis system: a key tool for novel malaria vaccine candidate discovery. Acta Trop. 114:171–176 [DOI] [PubMed] [Google Scholar]
- 33. Tsuboi T., et al. 2008. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 76:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsuboi T., Takeo S., Sawasaki T., Torii M., Endo Y. 2010. An efficient approach to the production of vaccines against the malaria parasite. Methods Mol. Biol. 607:73–83 [DOI] [PubMed] [Google Scholar]
- 35. Tsuji M., Mattei D., Nussenzweig R. S., Eichinger D., Zavala F. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80:16–21 [DOI] [PubMed] [Google Scholar]
- 36. van Dijk M. R., et al. 2010. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 6:e1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vermeulen A. N., et al. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162:1460–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vincent A. A., Fanning S., Caira F. C., Williamson K. C. 1999. Immunogenicity of malaria transmission-blocking vaccine candidate, y230.CA14 following crosslinking in the presence of tetanus toxoid. Parasite Immunol. 21:573–581 [DOI] [PubMed] [Google Scholar]
- 39. WHO 2010. World malaria report 2010. WHO Press, Geneva, Switzerland [Google Scholar]
- 40. Williamson K. C. 2003. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 25:351–359 [DOI] [PubMed] [Google Scholar]
- 41. Williamson K. C., Criscio M. D., Kaslow D. C. 1993. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol. Biochem. Parasitol. 58:355–358 [DOI] [PubMed] [Google Scholar]
- 42. Williamson K. C., Kaslow D. C. 1993. Strain polymorphism of Plasmodium falciparum transmission-blocking target antigen Pfs230. Mol. Biochem. Parasitol. 62:125–127 [DOI] [PubMed] [Google Scholar]
- 43. Williamson K. C., Keister D. B., Muratova O., Kaslow D. C. 1995. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol. Biochem. Parasitol. 75:33–42 [DOI] [PubMed] [Google Scholar]
- 44. Wu Y., et al. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]