Abstract
Recombinant Plasmodium falciparum merozoite surface protein 3 (PfMSP3F) and a 24-kDa fragment from its N terminus (MSP3N) that includes the essential conserved domain, which elicits the maximum antibody (Ab)-dependent cellular inhibition (ADCI), were expressed as soluble proteins in Escherichia coli. Both proteins were found to be stable in both soluble and lyophilized forms. Immunization with MSP3F and MSP3N formulated separately with two human-compatible adjuvants, aluminum hydroxide (Alhydrogel) and Montanide ISA 720, produced significant antibody responses in mice and rabbits. Polyclonal Abs against both antigens recognized native MSP3 in the parasite lysate. These two Abs also recognized two synthetic peptides, previously characterized to possess B cell epitopes from the N-terminal region. Antibody depletion assay showed that most of the IgG response is directed toward the N-terminal region of the full protein. Anti-MSP3F and anti-MSP3N rabbit antibodies did not inhibit merozoite invasion or intraerythrocytic development but significantly reduced parasitemia in the presence of human monocytes. The ADCI demonstrated by anti-MSP3N antibodies was comparable to that exhibited by anti-MSP3F antibodies (both generated in rabbit). These results suggest that the N-terminal fragment of MSP3 can be considered a vaccine candidate that can form part of a multigenic vaccine against malaria.
INTRODUCTION
Malaria, caused by the intracellular parasite of the genus Plasmodium, is a global health problem resulting in up to 2.5 million deaths annually. Of the four species, Plasmodium falciparum is the causative agent for the severity of the disease that leads to sequestration of parasite-infected red blood cells (RBCs) in the brain, lung, and placenta. Cerebral malaria is the most severe manifestation of P. falciparum infection. Due to the massive burden of the disease in the developing world, the global spread of drug resistance, and difficulty in achieving sustainable control of the mosquito vector, there is an urgent need to develop vaccines and new drugs against malaria. The clinical symptoms of malarial infection are observed during the asexual blood stages in the life cycle of the malaria parasite. After repeated exposures to the infection for several years, people living in areas of endemicity acquire clinical protection against the disease. Several proteins on the merozoite surface have been shown to play a role in the initial recognition, binding, and invasion of parasites into the red blood cells (10, 13). Antibody (Ab) responses to merozoite surface proteins have been shown to be associated with protective immunity against malaria (4). On the other hand, some merozoite proteins seem to mediate their protective role through complement-mediated lysis or through cooperation of Fc receptor-bearing cells (17). In a few instances, cytophilic antibodies (like IgG1 and IgG3) have been shown to facilitate the phagocytosis of merozoite through opsonization or mediate antibody-dependent cellular inhibition (ADCI) by cooperating with blood monocytes (1). The ADCI effect triggered by merozoite surface components is mediated by the soluble components released by the monocytes which inhibit intraerythrocytic development of the parasite (2). This mechanism led to identification of merozoite surface protein 3 (MSP3) as a major target of ADCI-effective antibodies (15, 18). Antibodies to other antigens such as glutamate-rich protein (GLURP) and serine repeat protein (SERP-SERA) have also exhibited an ADCI effector activity. These proteins are also not anchored to the merozoite surface but instead are associated with the merozoite surface, possibly through formation of complexes with other surface molecules (15, 23, 24). Recently, MSP1 block 2-specific antibodies have also been demonstrated to be involved in ADCI in an allele-specific manner (9).
MSP3 is abundantly expressed on the surface of merozoites and is released as a soluble protein (14). Recently suggested nomenclature has placed MSP3 in a new MSP3 multigene family and termed it MSP3.1. MSP3.1 has been shown to be the least cross-reactive among the members of the MSP3 family (22). Affinity-purified MSP3 antibodies from the sera of P. falciparum-exposed individuals and antibodies from mice vaccinated with MSP3 peptides exhibited ADCI activity (15, 21). Aotus monkeys vaccinated with yeast (Pichia pastoris) expressed full-length MSP3 (MSP3F) were partially protected from a challenge with P. falciparum parasites (12). Antigenicity and functional assays have identified a 70-amino-acid conserved domain in the N-terminal region of MSP3 to be a target of biologically active antibodies (21). Long synthetic peptides based on the conserved N-terminal sequences, including the 70-amino-acid sequence, have been developed for vaccine trials in humans (6, 7). Structurally, MSP3 is a highly conserved protein that consists of 12 copies of a degenerate heptad repeat (AXXAXXX) in three blocks in the N-terminal region with a glutamic acid-rich domain and a leucine zipper motif in the C-terminal region (14). While the C-terminal region has been implicated in oligomerization of the protein, its role in the generation of a protective antibody response is not clear (3, 11).
Previous studies have indicated that naturally occurring antibodies to both conserved and polymorphic regions of MSP3 were associated with protection and that the C terminus of MSP3 antigen (glutamic acid stretch and leucine zipper-like motif) was not significantly associated with a reduced risk of malaria (16). The objective of the present work was to compare immune responses to MSP3F and its N-terminal fragment (MSP3N) and to assess if MSP3N is suitable for development as a vaccine candidate. In the present work, MSP3F and an N-terminal polypeptide construct that includes well-characterized B and T cell epitopes but lacks the glutamate-rich domain and the leucine zipper region located in the C terminus were expressed and characterized. Here we report that while Abs to these constructs do not block red cell invasion, they exhibit potent ADCI activity that results in significantly reduced parasitemia in cultures supplemented with human monocytes.
MATERIALS AND METHODS
Cloning, expression, and purification of recombinant MSP3F and MSP3N.
The full-length-codon-optimized gene of MSP3F with a C-terminal His tag (GeneScript) was cloned in the pET-28a(+) expression vector (Novagen) using the restriction sites NcoI and XhoI. MSP3N, encoding the N-terminal region (21 to 238 amino acids), was PCR amplified with primers 5′-GGCCATGGGCAACAATGTTGCTAGCAAAGAAA-3′ (forward primer) and 5′-CCGCTCGAGTTAGTGGTGGTGGTGGTGGTGTTCCTCCTTCTCGTCCAGAACATCGTC-3′ (reverse primer) using MSP3F as a template. The PCR products were cloned into the pGEM-T Easy vector, and the cloned fragments were sequenced. The pGEM-T Easy vector containing the correct MSP3N insert was excised with the restriction enzymes NcoI and XhoI and cloned in the pET28a vector (Novagen). The MSP3F and MSP3N clones were sequenced and transformed in the expression host strain Escherichia coli BL21(DE3).
E. coli BL21(DE3) cells containing the recombinant plasmids pET28a(+)MSP3F and pET28a(+)MSP3N were grown in LB medium-kanamycin (25 μg/ml) at 37°C with shaking at an optical density at 600 nm (OD600) of 0.6 to 0.7. The culture was induced by addition of isopropyl-beta-d-thiogalactopyranoside (IPTG; Sigma) at a final concentration of 0.5 mM. The induced cultures were further grown at 37°C for 4 h and then harvested by centrifugation and stored at −70°C. E. coli cell pellets were thawed, resuspended in sonication buffer (20 mM Tris, pH 8.0, and 500 mM NaCl containing 1 mM benzamidine hydrochloride and 1% Tween 20) at 4°C, and sonicated using five sonication cycles, each consisting of 10-s pulses at 10-s intervals. Bacterial lysates were centrifuged at 15,000 × g for 30 min at 4°C. Supernatants were passed over nickel-nitrilotriacetic acid-agarose (Qiagen) 5-ml columns preequilibrated with sonication buffer at 4°C. The columns were washed with 10 column volumes of wash buffer (20 mM Tris-HCl, pH 8.0, 250 mM NaCl, 5 mM imidazole), and the bound proteins were eluted with 15 ml of elution buffer (20 mM Tris and 250 mM NaCl) containing 50 mM imidazole. The fractions containing MSP3F and MSP3N were pooled and extensively dialyzed against 20 mM Tris-20 mM NaCl (pH 8.0) buffer at 4°C. The recombinant proteins were further purified by ion-exchange chromatography with a Q-Sepharose column equilibrated with 20 mM Tris (pH 8.0) and eluted with a linear 20 to 500 mM NaCl gradient in Tris-HCl buffer. All purification processes were carried out with buffers prepared with pyrogen-free water. Eluates were analyzed by SDS-PAGE, and the fractions containing the recombinant protein with a clear single band were pooled. Concentration of proteins was determined using the bicinchoninic acid method, taking bovine serum albumin as a standard (Bio-Rad protein assay system). MSP3F and MSP3N were tested for homogeneity and purity by reverse-phase high-pressure liquid chromatography (RP-HPLC) using a C8 column (Supelco). For lyophilization, both antigens were taken in 100 μl of 1 mg/ml concentration in buffer (10 mM Tris, 10 mM NaCl, pH 8.0), frozen to −80°C, and lyophilized in microcentrifuge tubes covered with Parafilm (needle pierced) under vacuum (Lab Conco lyophilizer).
The purities of the recombinant MSP3F and MSP3N were evaluated on a 12% SDS-polyacrylamide gel run under reducing and nonreducing conditions, with 5 μg of protein loaded per lane. For Western blot analysis, proteins were transferred onto a nitrocellulose membrane and were probed with polyclonal mouse sera as well as human hyperimmune sera (collected from areas of endemicity in Orissa, India). The blots were developed using hydrogen peroxide as the substrate and diaminobenzidine (DAB) as the chromogen. The stabilities of MSP3F and MSP3N were determined by SDS-PAGE of protein samples drawn monthly from aliquots stored at −80°C, −20°C, 4°C, 19°C, and 37°C. Purified MSP3F and MSP3N were lyophilized and stored for further analysis. Endotoxin levels were measured with a Limulus amebocyte lysate (LAL) gel clot assay (Charles River Endosafe). Endotoxin levels for MSP3N and MSP3F were according to the standard protocol.
Synthesis of B cell epitope peptides based on MSP3 N-terminal region of MSP3.
Two MSP3 peptides, MSP3b184-210 (peptide b; AKEASSYDYILGWEFGGGVPEHKKEEN) and MSP3c203-230 (peptide c; PEHKKEENMLSHLYVSSKDKENISKENE), corresponding to the conserved regions of MSP3 that have been reported to elicit maximum ADCI, were synthesized (21). These peptides were synthesized on Rinkamide 4-methylbenzhydrylamine (MBHA) resin (0.75 mmol/g) using the 9-fluorenylmethoxy carbonyl (Fmoc) methodology. Couplings were performed by using carbodiimide. Fmoc deprotection was performed with piperidine (20% in dimethyl formamide [DMF]). After addition of the final residue, the resin was rinsed with DMF-dichloromethane (DCM)-methanol and dried. The final peptide deprotection and cleavage from the resin were achieved with 10 ml of trifluoroacetic acid-triisopropylsilane-H2O-Phenol (88:2:5:5) for 2 h. The crude peptide was precipitated with cold ether, filtered, lyophilized, and purified by preparative reverse-phase HPLC under CH3CN-water conditions. The purified fractions were pooled as the eluent, lyophilized, and stored at −20°C as dry powder. Peptide identity was confirmed by mass spectrometry.
Immunization of mice with recombinant MSP3F and MSP3N formulated with different adjuvants.
Recombinant MSP3F and MSP3N were formulated with three different adjuvants: aluminum hydroxide (Alhydrogel; Brenntag, Germany), Freund's complete adjuvant (CFA) and Freund's incomplete adjuvant (IFA; both from Sigma), and Montanide ISA 720 (Seppic, Paris, France) according to the manufacturers' instructions. A control formulation was prepared with phosphate-buffered saline (PBS) with all three adjuvants. After the aluminum hydroxide formulation was prepared, antigen adsorption was determined as follows: antigens were incubated with aluminum hydroxide with gentle mixing overnight at 4°C, the formulation was gently spun at 2,000 rpm for 2 min, and the supernatant was analyzed for any unbound protein by measuring the OD at 280 nm. More than 90% protein was adsorbed to aluminum hydroxide. Montanide ISA 720 and the Freund's formulations were prepared fresh before each immunization (i.e., they were not stored for a long time). All formulations for both antigens were prepared using bulk antigen (in solution).
Mice used in this study were procured from the Animal Facility of the International Centre for Genetic Engineering and Biotechnology, New Delhi, India. Animals were handled according to the guidelines of the ICGEB Institutional Animal Care and Use Committee. Groups of five BALB/c mice (male) at 5 to 6 weeks of age were immunized intraperitoneally with 25 μg of purified recombinant MSP3F or MSP3N (final volume, 100 μl) formulated with different adjuvants. Control animals were immunized with adjuvant in saline. Mice were boosted twice with the same doses of antigen and adjuvant on day 28 and on day 42, according to the schedule (data not shown). Mice were bled at 7- to 14-day intervals. The sera were obtained and stored at −80°C.
Rabbits were immunized intramuscularly with 200 μg of MSP3F or MSP3N formulated in Montanide ISA 720 (final volume, 500 μl for each rabbit) for generating large amounts of antibodies that were used in the ADCI assay. Rabbits were immunized on days 0, 28, and 56 and bled on days 42 and 72.
IgG purification from rabbit and mouse sera.
Total IgG was purified from sera obtained from immunized groups of mice and rabbits as well as from the control groups by using a protein G-Sepharose column (Pharmacia) according to the manufacturer's instructions. Briefly, a serum sample, after equal dilution with binding buffer (20 mM sodium phosphate buffer, pH 7.0), was loaded onto a protein G-Sepharose column preequilibrated with binding buffer. The column was washed with 10 column volumes of the binding buffer. The bound IgG was eluted with 0.2 M glycine-HCl (pH 3.0), and eluted fractions were collected in tubes already containing 100 μl of 1 M Tris at pH 8.0. Purified IgGs were analyzed by SDS-PAGE. The IgGs were pooled and dialyzed against PBS (pH 7.4). For ADCI experiments, pooled fractions were concentrated using Amicon filters by centrifugation for 2 h at 4°C and dialyzed against RPMI medium. The purified IgG was sterilized using a 0.22-μm-pore-size filter, and its concentration was estimated at A280.
Human immune sera from a region where malaria is endemic.
Forty-two serum samples were collected from healthy individuals residing in a region of Orissa, India, where P. falciparum is endemic. These individuals did not have any blood-stage infection at the time of sample collection and had no recent history of any P. falciparum infection. Consent from these individuals and approval from the Human Volunteers Research Ethical Committee of the International Centre for Genetic Engineering and Biotechnology were obtained prior to the study. Serum samples were also collected from individuals who had no known history of malaria and who had never visited a region where malaria is endemic.
ELISA.
The IgG titers against MSP3F and MSP3N and their IgG isotypes in mouse sera were determined using enzyme-linked immunosorbent assay (ELISA). Sera collected at different time points from each mouse were diluted 1:50,000 and tested for recognition of MSP3F and MSP3N. Preimmunization sera and adjuvant control sera tested at a 1:2,500-fold dilution yielded A490 values that were comparable to the background and were used as controls. Briefly, 96-well flat-bottom microtiter ELISA plates (MaxiSorp; Nunc) were coated overnight at 4°C with 100 μl carbonate buffer (0.159% Na2CO3, 0.293% NaHCO3, pH 9.6) containing either MSP3F (0.2 μg/well) or MSP3N (0.2 μg/well). The plates were washed with PBS containing 0.05% Tween 20 (PBST), and the unbound sites were blocked with 5% skim milk in phosphate-buffered saline for 2 h at 37°C. Test sera were diluted 1:50,000 in 0.25% skim milk in PBST and were incubated in duplicate (100 μl/well) overnight at 4°C. Sera from control groups were diluted 1:200. The wells were washed three times with PBST. Anti-mouse IgG goat antibodies conjugated to horseradish peroxidase (HRP; Sigma) were diluted 1:10,000 and added to the wells, and the plates were incubated for 1.5 h at 37°C. The enzyme reaction was developed with hydrogen peroxide as the substrate and o-phenylenediamine dihydrochloride (OPD) as the chromogen. The reaction was stopped with 2.0 N sulfuric acid. The A490 was measured using an ELISA plate reader (Molecular Devices). Mean A490 values and standard errors of the means for each group were calculated. Sera from individual mice in each group were pooled for determination of endpoint titers, defined as the OD value below the mean OD plus 2 standard deviations for the preimmunization sera. Relative ELISA units were expressed as the reciprocal of the dilution. ELISAs were also performed with the synthetic peptides MSP3b and MSP3c. Both peptides were coated on 96-well titer plates separately and together as a mixture of the two. The plates were then incubated with sera raised against MSP3F and MSP3N at a 1:500 dilution. The preimmune and control (adjuvant alone) sera were included separately for each set of peptides. An unrelated peptide (an HIV-derived 17-residue peptide) was used to assess the nonspecific reactivities of both anti-MSP3F and anti-MSP3N Abs.
To identify the IgG subclasses among the anti-MSP3F and anti-MSP3N mouse antibodies, an ELISA was performed as described above but with secondary antibodies specific for mouse IgG1, IgG2a, IgG2b, and IgG3 (Sigma) at dilutions of 1:1,000. The differences in IgG titers between different groups were statistically determined by the Student t test. Differences were considered statistically significant when P was <0.05.
Immunoprecipitation assay.
Purified merozoites were lysed in 1% deoxycholate buffer-0.05% SDS with protease inhibitor cocktail (tablets from Roche). The merozoite suspension was then centrifuged at 15,000 × g for 20 min. The supernatant was removed, incubated with protein A-Sepharose for 30 min at 4°C, and further centrifuged at 2,000 rpm for 2 min. The precleared supernatant was then incubated at 4°C overnight with 2.5 μg of anti-MSP3F and anti-MSP3N rabbit IgGs. A control experiment was set up with 2.5 μg of preimmune rabbit IgG. Twenty microliters of washed preequilibrated (in PBS) protein A-Sepharose beads was added, and the mixture was incubated further for 2 h at 4°C. The suspension was centrifuged, and the beads were washed three times with PBS and then boiled in SDS-gel loading buffer. The test and control samples were then immunoblotted using anti-MSP3F and anti-MSP3N purified antibodies raised in mice. Bands were detected using enhanced chemiluminescence (ECL; Amersham).
Immunofluorescence assay.
The recognition of native proteins in P. falciparum 3D7 schizonts by anti-MSP3F and anti-MSP3N was assessed by indirect immunofluorescence assay. Thin smears of parasite-infected erythrocytes were made and fixed with a mixture of acetone-methanol (9:1) at −20°C for 40 min. Fixed smears were incubated with the respective antibodies at a 1:200 dilution for 2 h at room temperature. Slides were washed with PBS-Tween 20 and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma) at 1:200 dilutions in 0.5% fetal calf serum for 1 h at room temperature in the dark. Later, slides were stained with 4′,6′-diamino2-phenylindole (DAPI) for 30 min at 37°C at a final concentration of 2 μg/ml and then washed twice with PBS- 0.05% Tween 20 and once with PBS and mounted on a coverslip in the presence of antifade reagent (Invitrogen). Slides were viewed in a Nikon SE300 microscope under a ×100 oil immersion objective. Colocalization of MSP3F and MSP3N with P. falciparum MSP119 (PfMSP119) was also investigated.
Detection of native protein with antibodies against recombinant MSP3.
Late-stage schizonts from the 3D7 parasite lines were lysed in a standard reducing SDS-PAGE sample buffer and on a 10% SDS-polyacrylamide gel, and immunoblotting was done using anti-MSP3 Abs. A schizont extract equivalent to 5 × 105 schizonts was run in each gel. For immunoblotting, rabbit polyclonal antisera (1:1,000 dilution) were first used, followed by secondary goat anti-rabbit IgG HRP conjugate at a 1:1,000 dilution (Pierce, Rockford, IL). The immunoblot was developed with DAB as chromogen and hydrogen peroxide as substrate.
Antibody depletion assay.
An antibody depletion assay was used to determine the relative abundance of antibodies specific for MSP3F and MSP3N in polyclonal mouse sera (20). Flat-bottom Immunolon-2 plates were coated with 100 μl of MSP3N antigen (10 μg/ml). The wells were blocked with 5% low-fat milk in PBS (pH 7.2) for 1 h at room temperature and washed with 0.05% Tween 20 in PBS, followed by a wash with PBS (pH 7.2). The first row of wells was incubated with anti-MSP3F sera at a dilution of 1: 50,000 in PBS, while the remaining wells were left in PBS. After a 1-hour incubation, the serum dilution from the first row was transferred to the second row and the plate was incubated for another hour. This serial incubation was carried out until antibodies with respect to the particular antigen were depleted, as determined by decreasing OD readings at 490 nm. An ELISA subsequently analyzed the antibody-depleted sera for their reactivity with MSP3F antigen on a separate plate coated with MSP3F antigen. This was similarly repeated with MSP3N sera using the same dilutions incubated in a 96-well ELISA plate coated with MSP3F. The reactivities of the recombinant proteins with the depleted and undepleted sera were compared to analyze the relative contribution of each antigen.
ADCI assay.
The ADCI assay was done with purified rabbit IgG according to a procedure described previously by Oeuvray et al. (15). Briefly, human monocytes were purified from blood samples of healthy donors by using CD14 (monocyte/macrophage) microbeads (Miltenyi Biotech) according to the manufacturer's protocol. The study was approved by the Human Volunteers Research Ethical Committee of the International Centre for Genetic Engineering and Biotechnology prior to the study. Tightly synchronized Plasmodium falciparum 3D7 schizont-stage parasites (0.5% parasitemia and 1% hematocrit) were cocultured in a 96-well flat-bottom microculture plate with adherent human monocytes (2 × 105 monocytes/well) in complete medium (RPMI medium containing 10% human serum). MSP3F and MSP3N IgGs were added to these cultures at different final concentrations (12.5, 25, 50 μg/well), and the plates were incubated at 37°C for 48 h. Starting at 0.5%, parasitemia generally reached 1 to 3% in one erythrocytic cycle. After 48 h of growth, parasitemia was estimated using a microscope with a thin Giemsa-stained smear of RBCs from each well by counting the parasitized erythrocytes in more than 10,000 erythrocytes. Control wells consisted of (i) parasite alone, (ii) parasite and control IgG (purified from naïve mouse sera), (iii) parasites and monocytes, (iv) parasites and purified IgG without monocytes, and (v) parasites, control IgG, and monocytes.
The specific growth inhibition index (SGI; in percent) was calculated as follows: 1 − [(percent parasitemia with monocytes and test antibodies/percent parasitemia with test antibodies)/(percent parasitemia with monocytes and control IgG/percent parasitemia with control IgG)] × 100. The direct inhibition of purified IgG (DI; in percent) on parasite growth, i.e., in the absence of monocytes, was calculated by the following formula: {[number of parasites alone − (number of parasites + IgG titer)]/(number of parasites alone)} × 100.
RESULTS
Expression and characterization of recombinant MSP3F and MSP3N.
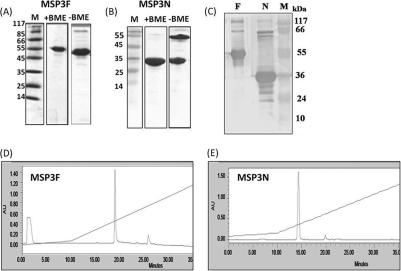
Recombinant polyhistidine-tagged forms of MSP3F and MSP3N (Fig. 1B) were expressed as soluble proteins in E. coli and purified by immobilized metal affinity chromatography (IMAC), followed by ion-exchange chromatography. Purified MSP3F and MSP3N migrated on a reducing SDS-polyacrylamide gel with the expected mobilities of ∼48 kDa and ∼35 kDa, respectively (Fig. 2A and B). While there was no significant difference in the mobility of MSP3F under reducing and nonreducing conditions, MSP3N under nonreducing conditions migrated as two distinct bands, corresponding to 35 kDa and 78 kDa, suggesting the presence of a disulfide-linked dimer species. MSP3F and MSP3N eluted as single symmetric peaks in RP-HPLC (Fig. 2D and E) and were further characterized by mass spectrometry (for MSP3F, expected mass of 38,705.8 and observed mass of 38,674.12; for MSP3N, expected mass of 24,942.3 and observed mass of 24,948.68). Both proteins were stable for 30 days at 4°C in solution and for 60 days at room temperature in a lyophilized state (data not shown). The endotoxin contents in the case of MSP3F and MSP3N described in this study ranged from 2 to 5 endotoxin units/25 μg of protein, measured in several different preparations and determined by the standard LAL assay.
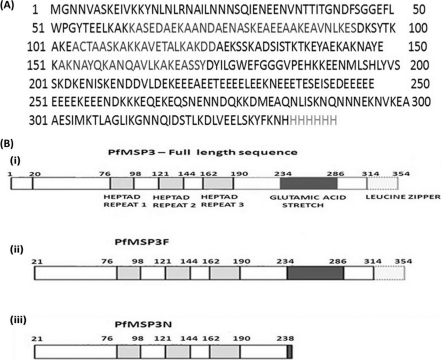
Fig. 1.
(A) Amino acid sequence of the full-length P. falciparum MSP3 (3D7) minus 19 residues from the N terminus; this sequence represents MSP3F, including the hexahistidine tag at the C terminus. (B) (i) Schematic diagram of full-length MSP3 showing alanine heptad repeats (light gray), glutamic acid-rich region (dark gray), and leucine zipper-like motif (dotted border); (ii and iii) schematic structures of MSP3F (ii) and MSP3N (iii).
Fig. 2.
(A and B) Coomassie blue-stained SDS-polyacrylamide gel showing purified MSP3F and MSP3N under reducing and nonreducing conditions. Molecular mass markers (in kDa) are shown. BME, β-mercaptoethanol. (C) Western blot by human hyperimmune sera shows recognition of both recombinant proteins MSP3F (lane F) and MSP3N (lane N). Lanes M, molecular size markers. (D and E) RP-HPLC profiles of purified MSP3F (D) and MSP3N (E). AU, absorbance units.
Both MSP3F and MSP3N were recognized in immunoblots by hyperimmune sera collected from individuals living in areas where P. falciparum is endemic as well as by ELISA (data not shown), suggesting that both recombinant proteins were recognized by the naturally acquired antibodies (Fig. 2C).
Immune response of MSP3F and MSP3N with different adjuvants in mice.
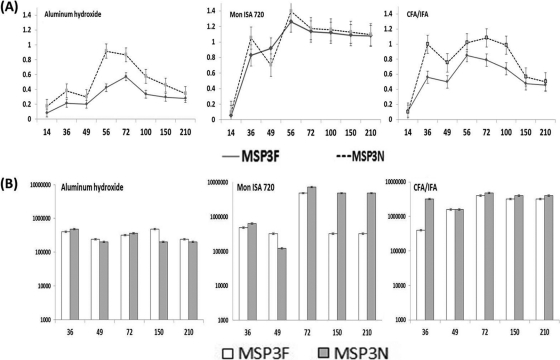
Significant antibody titers were induced in mice immunized with either MSP3F or MSP3N formulated with CFA, IFA, Montanide ISA 720, or aluminum hydroxide (data not shown). The time course for the antibody response showed that antibody titers increased significantly after the first boost with either antigen (Fig. 3A) and peaked after the second boost, and the titers were maintained for more than 6 months. Sera from adjuvant control mice showed no MSP3F- or MSP3N-specific antibodies (data not shown). Antibodies raised against MSP3F in different adjuvant formulations reacted with MSP3N as effectively as with MSP3F and vice versa (data not shown). Since the A490 values for individual mice from the same test groups determined by ELISA showed minimal variation and small standard errors of the means (data not shown), sera from mice in the same group were pooled for determination of endpoint titers (Fig. 3B) and use in the other assays described below.
Fig. 3.
(A) Immune responses in BALB/c mice immunized with MSP3F (solid line) and MSP3N (dotted line) formulated in aluminum hydroxide, Montanide ISA 720, and CFA/IFA adjuvant at a dilution of 1:50,000 (x axis represents number of days, and y axis represents OD at 490 nm). (B) Endpoint titers of the pooled mouse sera for MSP3F and MSP3N with different adjuvants at days 36, 49, 72, 150, and 210 are shown (x axis represents number of days, and y axis represents log10 endpoint titers).
Further, IgG isotype profiles for both antigens with different adjuvant formulations were compared. Antibodies of all IgG subtypes were detected in the pooled sera of mice immunized with both proteins, with a predominance of IgG1, followed by IgG3 and IgG2b. This antibody subtype profile was observed with each formulation, irrespective of the adjuvant used in the formulation (data not shown).
Antibodies to MSP3F and MSP3N recognize native parasite protein.
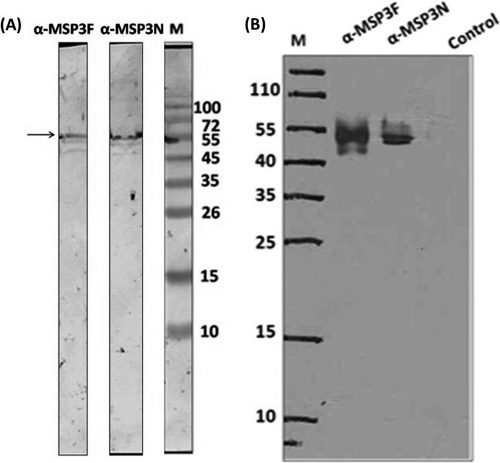
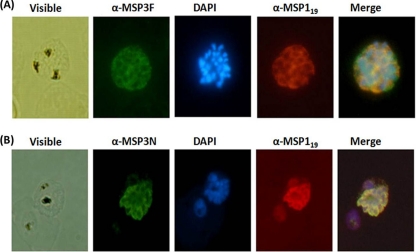
Polyclonal antibodies generated against both MSP3F and MSP3N proteins in mice and rabbits were affinity purified and used to detect native antigen. Western blot analysis carried out with schizont-stage parasite lysates showed a band of ∼55 kDa using both anti-MSP3N and anti-MSP3F antibodies raised in mice (Fig. 4A). Similarly, in the immunoprecipitation experiment, a clear band from the purified merozoite lysates was observed at ∼55 kDa (Fig. 4B). Anti-MSP3N and anti-MSP3F antibodies also recognized the native parasite protein in schizont-stage P. falciparum 3D7 parasites by immunofluorescence assay. Anti-MSP3F and anti-MSP3N antibodies recognized proteins on the merozoite membrane, exhibiting a grape-like pattern of rimmed fluorescence around the merozoites (Fig. 5), whereas preimmune sera did not show any reactivity (data not shown). Further, confirmation of MSP3 localization at the merozoite surface was done by dual-staining immunofluorescence with polyclonal rabbit anti-MSP19 antibodies and polyclonal anti-MSP3F and anti-MSP3N antibodies. The fluorescence staining revealed a strict colocalization with MSP119 upon merging of the images (Fig. 5).
Fig. 4.
(A) Recognition of native MSP3 (∼55 kDa) from parasite lysate in a Western blot, using antibodies against recombinant MSP3F and MSP3N. (B) Immunoprecipitation assay showing the ∼55-kDa band from parasite lysate by ECL using anti-MSP3F and anti-MSP3N antibodies. Preimmune IgGs were used as controls. Lanes M, molecular mass markers.
Fig. 5.
Immunofluorescence assay with P. falciparum 3D7 parasites using anti-MSP3F (A) (green) and anti-MSP3N (B) (green). Both panels also include immunostaining with anti-MSP119 antibodies (red). Shown are fluorescence and bright-field images of acetone-methanol-fixed P. falciparum 3D7 parasites at the schizont stage. Parasite nuclei were stained with DAPI (blue).
Determination of focus of antibody response to immunization with MSP3F and MSP3N.
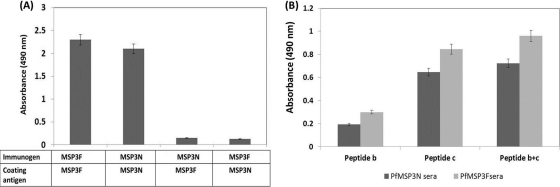
A depletion ELISA with pooled sera from mice immunized with MSP3F was used to determine the contribution of MSP3N-specific epitopes in the generation of the immune response. Ninety-six-well ELISA plates coated with MSP3N were incubated with MSP3F polyclonal sera and serially transferred to fresh wells until the OD at 490 nm attained a constant value. The concentration of polyclonal antibodies gradually decreased for MSP3F sera, and after seven serial transfers, the OD value of depleted sera was comparable to that of preimmune sera. Similarly, in a reciprocal experiment, MSP3N polyclonal serum was completely depleted of antibodies when it was used against MSP3F as the coating antigen (Fig. 6A).
Fig. 6.
(A) Immunodepletion assay showing relative contributions of MSP3N and MSP3F epitopes. OD values of the MSP3F sera depleted of MSP3N-specific Abs and vice versa were compared with those of the undepleted sera of MSP3F and MSP3N at similar dilutions of 1:50,000. (B) Detection of antibodies specific for epitopic peptide b and peptide c. Absorbance at 490 nm in both MSP3F and MSP3N sera incubated with peptide b and peptide c separately and together in a 96-well ELISA plate at dilutions of 1:500.
Two specific epitopes were used to determine their relative contribution in generating an immune response against MSP3F and MSP3N antigen in mice. Previous studies had characterized a 70-amino-acid region in MSP3 as the target for naturally occurring protective ADCI responses (21). Out of the three peptides overlapping in this region as described previously, MSP3b184-210 (AKEASSYDYILGWEFGGGVPEHKKEENML) and MSP3c203-230 (PEHKKEENMLSHLYVSSKDKENISKENE) were randomly selected to detect the presence of protective antibodies generated against MSP3F and MSP3N. Both peptides were coated on an ELISA plate either individually or in combination, and sera against MSP3F or MSP3N were added to the wells at a 1:500 dilution. Compared to the preimmune serum, Abs against MSP3F andMSP3N recognized both peptides (Fig. 6B). The absorbance at 490 nm was enhanced for both test sera when they were incubated with peptide b and peptide c coated together (Fig. 6B). Neither anti-MSP3F nor anti-MSP3N antibodies recognized an unrelated peptide of similar length as peptides b and c synthesized by same procedures (data not shown).
Biological effect of anti-MSP3F and anti-MSP3N antibodies.
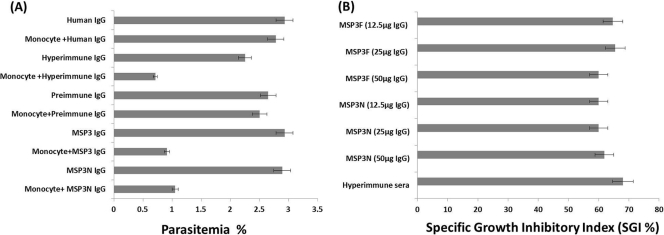
The ADCI assay was performed to assess the functional role of antibodies generated against recombinant MSP3F and MSP3N. DIs (which take into account the inhibitory effects of IgG alone) for both anti-MSP3F and anti-MSP3N purified IgGs were statistically insignificant compared with the DI for the preimmune IgGs. These results were comparable with the direct inhibition calculated for normal human IgG and the positive control (hyperimmune sera), indicating a nonsignificant effect on the parasitemia in the absence of effector cells, i.e., monocytes (data not shown). All three concentrations of IgG antibodies against MSP3F and MSP3N exhibited a strong inhibitory effect on the parasite growth in the presence of human monocytes, as determined by the SGI. The preimmune or normal human IgGs did not promote parasite killing, indicating that the observed ADCI effects were due to the presence of specific antibodies to MSP3F and MSP3N (Fig. 7A). SGIs (which take into account the possible nonspecific effect of monocytes as well as the antibodies alone) calculated for anti-MSP3F and anti-MSP3N antibodies were 61% and 60%, respectively, which were comparable to the SGI of the hyperimmune sera taken as a control, i.e., 67%. However, no correlation was observed between the inhibitory effect and IgG concentration over the range tested (Fig. 7B).
Fig. 7.
ADCI assay. (A) Parasites at the schizont stage were cocultured with human monocytes in the presence of purified antibodies (12.5, 25, and 50 μg/well of culture), and parasite growth was monitored after 48 h. Antibodies purified from preimmune sera, normal human serum (negative control), and hyperimmune serum (positive control) were taken as controls. (B) SGI for the three concentrations (12.5, 25, 50 μg/well) for anti-MSP3F and anti-MSP3N antibodies in the same ADCI assay.
DISCUSSION
The two primary aims of the present work were (i) to develop convenient methods to produce the full-length MSP3 (MSP3F) and its large N-terminal fragment MSP3N in an easily accessible bacterial expression system and (ii) to characterize and compare antibody responses in small animals upon immunization with the two recombinant proteins formulated with human-compatible adjuvants.
Both MSP3F and MSP3N expressed and purified in reasonably good yields were found to be stable under standard storage conditions, both in solution and in the lyophilized form. Although preparation of the full-length recombinant MSP3 has been described earlier using bacterial and yeast expression systems (11, 12), the stability of recombinant MSP3 has been a matter of concern. For example, it was found to be necessary to mutate tyrosine to Ala (Y223A of the K1 isolate of P. falciparum) to produce a stable version of MSP3 (3). Similarly, in another recent report, expression of MSP3 required deletion of 43 residues from the N terminus and a few mutations in the C terminus (25).
The immune response to the two recombinant proteins was evaluated in mice using aluminum hydroxide and Montanide ISA 720, along with Freund's adjuvant. Antibody responses for both antigens formulated with Montanide ISA 720 were as high as those for the antigens formulated with CFA or IFA, but interestingly, high levels of antibodies were also obtained with aluminum hydroxide formulations of both antigens. This is encouraging, given that most P. falciparum recombinant antigens are usually poorly immunogenic in aluminum hydroxide formulations and that the nonavailability of easily accessible human-compatible adjuvants is a major hurdle in the development of subunit vaccines against malaria (5).
Results of antibody depletion experiments using MSP3N as the coating antigen suggested that the major focus of the Ab response in immunization with MSP3F was primarily directed toward the N terminus, which has been mentioned earlier to contain several dominant B and T cell epitopes. We found that sera against MSP3F and MSP3N recognized two synthetic peptide-based non-cross-reactive B cell epitopes (21). Immunofluorescence assay and immunoprecipitation assay further provided evidence that Abs against both MSP3F and MSP3N could recognize the native antigen.
In earlier studies, MSP3 has been reported to contain B cell epitopes that are targeted by an ADCI mechanism (15). Since direct binding of rabbit IgGs with human monocytes via Fc receptors has earlier been quantitatively characterized, we performed the ADCI assay with antibodies for both antigens generated in rabbits (19). Abs against MSP3F and MSPN did not significantly inhibit parasite growth on their own; rather, they demonstrated monocyte-mediated parasite killing in three independent assays.
In a recent immunogenicity study of MSP3, it has been demonstrated that the extension of an N-terminal flanking region to the immunologically relevant 69 amino acids (MSP3184-252) enhanced the antibody response. However, addition of sequences from the leucine zipper region in the C terminus proved to be detrimental (8). Therefore, the N-terminal region of MSP3 might be more relevant for generating a protective immune response, and the C terminal region, including the leucine zipper, is not effective in this regard. An interesting observation in the present study was that the immunization with MSP3F produces a robust immune response with human-compatible adjuvants like aluminum hydroxide, which is largely focused on the N-terminal region of the protein. Also significant is the fact that antibody responses against the N-terminal fragment (MSP3N) not only are strong but also exert an ADCI effect no less than the responses produced by anti-MSP3F antibodies. Our work in this study has demonstrated that although immunizations with the full-length MSP3 leads to a substantial antibody response to the epitopes present in the N-terminal region, the N-terminal polypeptide fragment itself elicits a strong and effective immune response that makes it a strong vaccine candidate.
ACKNOWLEDGMENTS
We are grateful to Asif Muhammad and Shams Yazdani for discussion on expression of MSP3 and Paushali Mukherjee for her assistance in immunological characterization. We also thank the human volunteers for donating the serum samples; A. Kumar, R. Shakri, N. Negi, and S. Sachdeva for their help; and Rakesh Singh and Ashok Das for assistance in handling animals at the animal house.
CSIR is deeply acknowledged for the financial assistance.
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Bouharoun-Tayoun H., Druilhe P. 1992. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 60:1473–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouharoun-Tayoun H., Oeuvray C., Lunel F., Druilhe P. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgess B. R., Schuck P., Garboczi D. N. 2005. Dissection of merozoite surface protein 3, a representative of a family of Plasmodium falciparum surface proteins, reveals an oligomeric and highly elongated molecule. J. Biol. Chem. 280:37236–37245 [DOI] [PubMed] [Google Scholar]
- 4. Chauhan V. S., Yazdani S. S., Gaur D. 13 September 2010. Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum. Vaccin. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 5. Coler R. N., Carter D., Friede M., Reed S. G. 2009. Adjuvants for malaria vaccines. Parasite Immunol. 31:520–528 [DOI] [PubMed] [Google Scholar]
- 6. Corradin G., Kajava A. V. 2010. Malaria vaccine: why is it taking so long? Expert Rev. Vaccines 9:111–114 [DOI] [PubMed] [Google Scholar]
- 7. Corradin G., Kajava A. V., Verdini A. 2010. Long synthetic peptides for the production of vaccines and drugs: a technological platform coming of age. Sci. Transl. Med. 2:50–53 [DOI] [PubMed] [Google Scholar]
- 8. Demanga C. G., et al. 2010. Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of antigenicity studies in humans. Infect. Immun. 78:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galamo C. D., Jafarshad A., Blanc C., Druilhe P. 2009. Anti-MSP1 block 2 antibodies are effective at parasite killing in an allele-specific manner by monocyte-mediated antibody-dependent cellular inhibition. J. Infect. Dis. 199:1151–1154 [DOI] [PubMed] [Google Scholar]
- 10. Girard M. P., Reed Z. H., Friede M., Kieny M. P. 2007. A review of human vaccine research and development: malaria. Vaccine 25:1567–1580 [DOI] [PubMed] [Google Scholar]
- 11. Gondeau C., et al. 2009. The C-terminal domain of Plasmodium falciparum merozoite surface protein 3 self-assembles into alpha-helical coiled coil tetramer. Mol. Biochem. Parasitol. 165:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hisaeda H., et al. 2002. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657–664 [DOI] [PubMed] [Google Scholar]
- 13. Holder A. A., et al. 1994. Malaria parasites and erythrocyte invasion. Biochem. Soc. Trans. 22:291–295 [DOI] [PubMed] [Google Scholar]
- 14. McColl D. J., et al. 1994. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 68:53–67 [DOI] [PubMed] [Google Scholar]
- 15. Oeuvray C., et al. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594–1602 [PubMed] [Google Scholar]
- 16. Osier F. H., et al. 2007. Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol. 29:387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perignon J. L., Druilhe P. 1994. Immune mechanisms underlying the premunition against Plasmodium falciparum malaria. Mem. Inst. Oswaldo Cruz 89(Suppl. 2):51–53 [DOI] [PubMed] [Google Scholar]
- 18. Polley S. D., et al. 2007. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J. Infect. Dis. 195:279–287 [DOI] [PubMed] [Google Scholar]
- 19. Rasmussen J. M., Brandslund I., Leslie R. G., Svehag S. E. 1983. Quantitative studies of Fc receptors on human monocytes: characterization by binding of homologous and heterologous monomeric IgG and soluble immune complexes of different composition. Immunology 49:537–544 [PMC free article] [PubMed] [Google Scholar]
- 20. Sachdeva S., Ahmad G., Malhotra P., Mukherjee P., Chauhan V. S. 2004. Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kilodalton fragments expressed in Escherichia coli. Infect. Immun. 72:5775–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh S., et al. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010–1018 [DOI] [PubMed] [Google Scholar]
- 22. Singh S., et al. 2009. A conserved gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4:e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soe S., Singh S., Camus D., Horii T., Druilhe P. 2002. Plasmodium falciparum serine repeat protein, a new target of monocyte-dependent antibody-mediated parasite killing. Infect. Immun. 70:7182–7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soe S., Theisen M., Roussilhon C., Aye K. S., Druilhe P. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsai C. W., et al. 2009. Characterization of a protective Escherichia coli-expressed Plasmodium falciparum merozoite surface protein 3 indicates a non-linear, multi-domain structure. Mol. Biochem. Parasitol. 164:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]