Abstract
Plasmodium falciparum is transmitted to a new host after completing its sexual cycle within a mosquito. Developing vaccines against the parasite sexual stages is a critical component in the fight against malaria. We are targeting multiple proteins of P. falciparum which are found only on the surfaces of the sexual forms of the parasite and where antibodies against these proteins have been shown to block the progression of the parasite's life cycle in the mosquito and thus block transmission to the next human host. We have successfully produced a region of the Pfs230 antigen in our plant-based transient-expression system and evaluated this vaccine candidate in an animal model. This plant-produced protein, 230CMB, is expressed at approximately 800 mg/kg in fresh whole leaf tissue and is 100% soluble. Administration of 230CMB with >90% purity induces strong immune responses in rabbits with high titers of transmission-blocking antibodies, resulting in a greater than 99% reduction in oocyst counts in the presence of complement, as determined by a standard membrane feeding assay. Our data provide a clear perspective on the clinical development of a Pfs230-based transmission-blocking malaria vaccine.
INTRODUCTION
Malaria is a severe, and at times fatal, mosquito-borne disease caused by a protozoan parasite. The most severe form of the disease is caused by Plasmodium falciparum. Hundreds of millions of malaria cases occur around the world each year, with close to one million deaths (http://www.who.int/mediacentre/factsheets/fs094/en/index.html). The development of vaccines against malaria is a critical component in the control of the infection and elimination of the disease, as parasites and mosquitoes are growing resistant to the current methods of chemical intervention (10, 14). The focus is on developing vaccines targeting different stages of the parasite's life cycle, including transmission-blocking (TB) vaccines (TBVs) that can inhibit the sexual stages of parasite development in the mosquito midgut, thus alleviating the transmission pressure and the disease burden at the population level (3, 45). After a blood meal on infected humans, mosquitoes become infected by ingesting a sexual form of the malaria parasite called gametocytes (reviewed in reference 1). Subsequent sporogenic development in the mosquito can be prevented by the presence of antimalarial TB antibodies in the ingested blood meal. Thus, TBVs are directed against the parasite's sexual stages in the mosquito and are designed to halt the development of oocysts and the subsequent production of infective sporozoites (4). It has been proposed that TBVs should be considered a major part of a malaria eradication program aimed at combining vaccines against multiple stages of the parasite's life cycle to maximize efficacy (reviewed in reference 20).
Development of TBVs has been explored for over 20 years, with the candidate targets falling into two classes: (i) antigens that are present on the surface of P. falciparum gametocytes and gametes (such as Pfs230 and Pfs48/45) and are expressed in the human host, where immunity against them could potentially be boosted by natural infection, and (ii) antigens that are expressed on the zygote and ookinete stages (such as Pfs25 and Pfs28) and are not expressed in the human host (32, 43, 47). In the 1980s, incidents of serum-mediated TB activity were reported in individuals infected with Plasmodium vivax (25) and serum samples collected during field studies contained antibodies against the gametocyte surface proteins Pfs230 and Pfs48/45 (12, 15, 17, 27, 29, 33, 36). Additionally, Graves et al. (15) and Healer et al. (17) showed a positive correlation between the abilities of sera to immunoprecipitate 125I-labeled Pfs230 and to block P. falciparum transmission in a standard membrane feeding assay (SMFA). Antibodies in these sera recognize Pfs230 only under nonreducing conditions, suggesting that recognition is conformation dependent, and further studies revealed that Pfs230-associated TB activity is complement dependent (18, 34, 35, 37). Thus, Pfs230 has been targeted as a potential candidate for TBV development. Furthermore, the immune response to a Pfs230 TBV may be boosted by natural infection, potentially providing long-lasting immunity, and may be advantageous to a Pfs230-containing vaccine (34, 47, 48).
Pfs230 is a 363-kDa protein that contains 70 cysteine residues. Carter et al. (5) predicted that Pfs230 has seven paired domains and that the TB target epitopes are located within these motif-defined domains. Preceding the conserved paired domains are a region of 25 glutamic acids (amino acids [aa] 280 to 304) and a region of 16 tandem repeats of the tetramer EEVG (aa 379 to 442). Both of these regions are processed from the N-terminal end of the protein upon release of the gametocyte from the erythrocyte, and antibodies generated against these glutamate-rich regions do not bind to gametes (2). Gerloff et al. (13) defined repeated structures of cysteine motifs from aa 589 to aa 3135 and predicted the complex disulfide bonding necessary for correct folding and conformation. Recombinant expression of full-length Pfs230, due to its complexity (13), has not been accomplished, but different regions have been expressed as fusions to the maltose-binding protein (MBP) in Escherichia coli (48). Antibodies against one such region of Pfs230, designated C (aa 443 to 1132), are able to immunoprecipitate radiolabeled Pfs230, bind to the surface of gametes, and, in the presence of complement, reduce the infectivity of P. falciparum to mosquitoes. This was the first report of induction of TB antibodies against a recombinant Pfs230 antigen that is immunogenic during natural malaria infection and the first step in TBV development (48). However, this r230/MBP.C construct elicits only partial oocyst reduction. The MBP fusion was produced in the cytosol of E. coli in the absence of disulfide bond formation; thus, it is possible that only a small fraction of the recombinant protein correctly displays the native epitopes, therefore resulting in low levels of TB activity.
In an effort to dissect functional regions within the sequences corresponding to r230/MBP.C, Tachibana et al. (40a) produced a recombinant Pfs230 domain C (aa 443 to 1132) and its truncated forms Pfs230C0 (aa 443 to 588), Pfs230C1 (aa 443 to 715), and Pfs230C2 (aa 443 to 915) in a wheat germ cell-free expression system (41). Rabbit antibodies raised against these recombinant proteins displayed significant TB activities in the SMFA (40a). However, the wheat germ cell-free expression system presents a challenge for large-scale manufacturing of recombinant proteins for clinical applications, and to date there is no facility compliant with current good manufacturing practices (cGMP) guidelines. The plant-based transient protein expression platform developed by the Fraunhofer Center for Molecular Biotechnology (CMB) allows for manufacturing of recombinant vaccine antigens under cGMP, and the ongoing clinical testing of two vaccine candidates (H1N1 and H5N1 influenza viruses) produced at CMB's pilot plant facility supports the feasibility of this system.
In our study, we have engineered and produced a portion of Pfs230 domain C, 230CMB, corresponding to aa 444 to 730 of Pfs230, using our plant-based expression system (28). The protein was purified and characterized by biophysical methods, and its immunogenicity and TB activity were assessed in rabbits. Results showed that serum antibodies generated against 230CMB were able to bind to the parasite with high efficiency in immunofluorescence assays (IFAs) and showed a greater than 99% reduction in oocyst counts in the presence of complement. These data indicate that the 230CMB antigen has great potential as a TBV candidate.
MATERIALS AND METHODS
Cloning and expression analysis.
After the region comprising aa 444 to 730 of Pfs230 was optimized for plant expression (GeneArt, Regensburg, Germany), it was cloned into the launch expression vector pGRD4 (Fig. 1C) (28, 38) for expression in plants. The target construct included sequences encoding the signal peptide of plant pathogenesis-related protein 1a (PR-1a) (24) for entry into the secretory pathway, the KDEL sequence for retention in the endoplasmic reticulum (ER), and a 6-histidine (6-His) tag for ease of purification and detection. The expression constructs were then electroporated into Agrobacterium tumefaciens strain GV3101, and the resulting bacteria were grown overnight in minimal medium (16). The optical density (OD) of the cultures was determined, and the protein expression strain was mixed with an Agrobacterium strain expressing a suppressor of silencing, p19 protein (46). The Agrobacterium solution was introduced by manual infiltration into the leaves of 6-week-old, soil-grown Nicotiana benthamiana plants as described previously (16). Plant leaf tissue samples were collected 3 to 7 days postinfiltration to determine the levels of expression and solubility of the target protein. Samples were weighed and extracted in three volumes of a phosphate-based buffer containing 0.5% Triton X-100 for extraction of total soluble protein or with the addition of gel loading buffer (50 mM Tris HCl [pH 6.8], 2% sodium dodecyl sulfate, 0.1% bromophenol blue, 10% glycerol, 100 mM dithiothreitol) for extraction of total protein. Proteins were resolved by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Target protein expression levels were assessed by Western blot analysis using an anti-His mouse monoclonal antibody (MAb; Roche, Indianapolis, IN) or a target-specific MAb (LMIV; NIH) as the primary antibody and a horseradish peroxidase (HRP)-labeled anti-mouse antibody as the secondary antibody (Jackson ImmunoResearch, West Grove, PA). Results were analyzed using the GeneGnome system (Syngene, Frederick, MD). Peak days of expression and solubility profiles were determined. Constructs of interest were vacuum infiltrated on a larger scale into hydroponically grown N. benthamiana for protein purification (16).
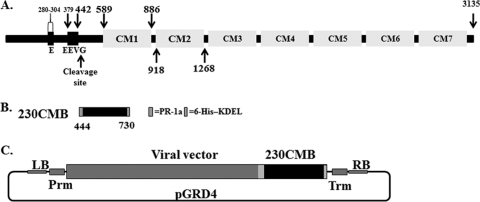
Fig. 1.
Pfs230 genetic organization and construct design. (A) Schematic representation of predicted structural motif for Pfs230, including the approximate amino acid range of each relevant region or domain. The cleaved portion of Pfs230 contains the 25 glutamic acid repeat region, E, and the 16 tandem EEVG repeat region and precedes the seven paired cysteine motif domains, CM1 to CM7. (B) 230CMB includes aa 444 to 730 and contains the PR-1a signal peptide at the N terminus and a 6-His tag and the KDEL ER retention signal at the C terminus (light gray boxes). (C) Diagram of the launch vector for target expression containing the Agrobacterium left border (LB) and right border (RB) sequences, the plant promoter (Prm) and terminator (Trm) regulatory elements, the viral vector launch sequences, and the Pfs230CMB target sequence.
Purification.
Aerial plant tissue (500 g) was harvested, homogenized in extraction buffer containing 20% glycerol, and incubated for 20 min at 4°C in the presence of 0.5% Triton X-100. Following incubation, the plant homogenate was clarified by centrifugation (15,000 × g for 45 min). The supernatant was then filtered through Miracloth (Calbiochem, Gibbstown, NJ) and passed through a 10-in. BioLife filtration capsule (Cuno; 3M, Meriden, CT) and a 0.2-μm Sartopore filter cartridge (Sartorius, Bohemia, NY). The filtrate was loaded onto an XK 26 column with Chelating Sepharose Big Beads resin (70-ml column volume [CV]) using the ÄKTA Purifier 100 (GE Healthcare, Piscataway, NJ) at a flow rate of 25 ml/min. The column was washed with 5 CVs of 50 mM sodium phosphate (pH 7.5)–0.5 M NaCl–20 mM imidazole–0.5% Triton X-100 and then with 10 CVs of 50 mM sodium phosphate (pH 7.5)–0.5 M NaCl–20 mM imidazole. The target was eluted with 50 mM sodium phosphate (pH 7.5)–0.5 M NaCl–20% glycerol–300 mM imidazole at a flow rate of 3.5 ml/min. Immobilized metal affinity chromatography (IMAC) eluant was dialyzed into 10 mM sodium phosphate (pH 7.0)–10% glycerol, and the resulting material was centrifuged at 78,000 × g for 10 min to remove the potential insoluble material. The clarified and dialyzed sample was loaded at a flow rate of 3 ml/min onto three 5-ml HiTrap Capto Q ion-exchange chromatography columns attached in tandem, and the target was eluted with 300 mM NaCl at a flow rate of 3 ml/min. The purified target was dialyzed into saline (0.9% NaCl) and filtered through a 25-mm 0.2-μm nylon syringe filter (Fisher Scientific, Pittsburgh, PA) prior to freezing and storage at −70°C.
Protein characterization.
SDS-PAGE was performed using 10% acrylamide gels stained with Coomassie (Gel Code Blue, Pierce, Rockford, IL) as described previously (21). When Western blot assays were performed (40), samples were transferred to PVDF membranes and blocked with I-Block (Applied Biosystems, Carlsbad, CA), and the target protein was detected using anti-6-His (Qiagen, Valencia, CA or Roche, Indianapolis, IN) or anti-Pfs230 MAb (2G5 or 2B10; NIH) as the primary antibody and HRP-labeled anti-mouse (Jackson ImmunoResearch, West Grove, PA) antibody as the secondary antibody. N-terminal sequencing was performed by Edman degradation (Proteos, Inc., Kalamazoo, MI). Recognition of plant-produced 230CMB by Pfs230-specific TB MAbs was determined by enzyme-linked immunosorbent assay (ELISA) using 96-well MaxiSorp plates (Nunc, Rochester, NY) coated with 1 μg/ml 230CMB in phosphate-buffered saline (PBS) and incubated overnight at 4°C. After blocking with 0.5% I-Block in PBS with 0.1% Tween 20, MAbs 2G5 and 2B10 were plated in triplicate at starting concentrations of 5 μg/ml and 2 μg/ml, respectively, and titrated in 5-fold dilutions. The MAbs were detected using HRP-conjugated goat anti-mouse IgG at 1:7,500 (Jackson ImmunoResearch, West Grove, PA) and visualized using o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich, St. Louis, MO) as a substrate with an acid stop. Size exclusion chromatography (SEC)-multiangle laser light scattering (MALLS) experiments were performed using a Superdex 200 column (10 by 300 mm; 13-μm particle size) with inline UV (GE) and MALLS (Wyatt) detectors to determine the molecular weight of the purified target. The purified 230CMB protein was analyzed at a 1-mg/ml concentration (100 μg) and injected with 50% sample loop overfill. The dilution (where needed) and running buffer was PBS (pH 7.5). The SEC-MALLS flow rate was 0.5 ml/min at room temperature. Notably, when determining the molar extinction coefficient (ε), we use the method of Edelhoch (8). The sum of the specific contributions leading toward total absorption at 280 nm for each residue (Trp, Tyr, and Cys) is determined based on the nominal amino acid sequence.
Rabbit immunogenicity study.
Plant-produced 230CMB protein was evaluated for immunogenicity and TB potential in rabbits. Groups of 5 rabbits were immunized with 100 μg of 230CMB on study days 0 and 28 either subcutaneously emulsified in complete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO) for the prime followed by incomplete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO) for the boost or intramuscularly adsorbed to Alhydrogel (Brenntag-Biosector, Frederikssund, Denmark). Control animals were immunized with PBS plus adjuvant. Serum samples from immunized rabbits were collected on study days 0, 28, 42, and 56 and assessed for total IgG titers by ELISA. The presence of gametocyte-binding antibodies in sera from study day 42 was determined using an IFA on fixed gametocytes. If results from the IFA were positive, the samples were then tested in a suspension IFA (SIFA) using live macrogametes/zygotes. Following positive SIFA results, the day 42 serum samples were then tested for the presence of TB antibodies in an SMFA. All animal protocols were approved by the Institutional Animal Care and Use Committee at Covance Research Products, Inc. (Denver, PA).
ELISA.
230CMB-specific serum IgG titers were measured by ELISA using 96-well MaxiSorp plates (Nunc, Rochester, NY) coated with 1 μg/ml 230CMB in PBS and incubated overnight at 4°C. After blocking with 0.5% I-Block in PBS with 0.1% Tween 20, serum samples were plated in duplicate at a starting dilution of 1:100 and titrated in 5-fold dilutions. Target-specific IgGs were detected using HRP-conjugated goat anti-rabbit IgG at 1:7,500 (Jackson ImmunoResearch, West Grove, PA) and visualized using OPD (Sigma-Aldrich, St. Louis, MO) as a substrate with an acid stop. Reciprocal serum dilutions that gave a mean absorbance value four times greater than the background were determined as the endpoint titers.
IFA.
An indirect IFA was performed with a mixture of cultured sexual and asexual stage P. falciparum (isolate NF54) parasites air dried onto a multispot slide as previously described (30). Briefly, parasites were incubated with a 1:100 dilution of the test sera in PBS, rinsed with PBS, and incubated with Alexa Fluor 488-labeled chicken anti-rabbit IgG (H+L; Invitrogen). The slides were rinsed, washed, mounted under a coverslip, and examined under UV illumination with a Leitz Ortholux fluorescence microscope (×500 magnification).
SIFA.
As described previously for SIFA analysis (30, 37), gametocytes were allowed to undergo gametogenesis. Subsequently, a suspension of 106 macrogametes/zygotes (100 μl) was mixed with 100 μl of a 1:100 dilution of the sera in PBS and incubated for 20 min on ice. Parasites were washed with 1 ml of PBS, collected by centrifugation for 3 min at 3,000 rpm (benchtop centrifuge), and incubated with 50 μl of Alexa Fluor 488-labeled chicken anti-rabbit IgG (H+L; Invitrogen). After a wash with PBS, the cells were examined as described for the IFA.
SMFA.
Antisera obtained from rabbits immunized with the 230CMB antigen were assessed for TB activity by SMFA as previously described (22, 31, 37). Briefly, 90 μl of rabbit serum was mixed with 30 μl of naïve human serum (as a source of complement) and 150 μl of in vitro gametocyte culture of P. falciparum (NF54 line). The mixture was fed to Anopheles stephensi mosquitoes through a membrane feeding apparatus. Preimmune sera, sera from rabbits immunized with PBS alone, and MAb 63F2A2 (37) served as the controls. All serum samples were tested separately at a final dilution of 1:3 in the feeder assay in the presence of active or heat-inactivated (56°C, 30 min) human complement. Complement was collected from type AB blood from Dutch blood bank donors with no previous malaria exposure as described previously (37). Fully engorged mosquitoes were separated and held at 26°C. Seven days later, midguts of 20 mosquitoes were examined for oocysts. The observed TB activity of serum was determined as the percentage reduction in the arithmetic mean oocyst number in test samples compared to that in paired controls (42). The experiment was considered valid when at least 70% of the mosquitoes feeding on control sera were infected. For comparison of groups and to assess for a statistically significant difference between the groups, data were analyzed by a nonparametric test (not normally distributed) by comparing the medians of two groups using the Mann-Whitney test or by comparing three or more groups using the Kruskal-Wallis test followed by post tests. If significance was indicated, Dunn's analysis was used for comparison with the control group.
RESULTS
230CMB is expressed in N. benthamiana as a soluble protein.
Schematic diagrams of the Pfs230 antigen and the expression vector are shown in Fig. 1A and B. Region C of Pfs230 (aa 443 to 1132) starts in the EEVG repeat region and continues to the middle of the second cysteine motif domain of the protein. We have engineered the N-terminal portion of region C spanning aa 444 to 730, which we designate 230CMB (Fig. 1C), into our launch vector system (28, 38) and transformed the resulting plasmid into A. tumefaciens GV3101 for expression in plants. The target gene sequence, prior to cloning, was optimized for plant expression by GeneArt and included sequences encoding the PR-1a signal peptide (24) at the N terminus and the ER retention sequence (KDEL) and a 6-His tag, to aid in purification, at the C terminus. Following infiltration of N. benthamiana plants with the Agrobacterium strain harboring 230CMB, expression (in the range of 800 mg/kg of fresh leaf tissue) and solubility (100%) of 230CMB in leaf tissue were confirmed (Fig. 2A). Other constructs designed for expression of different Pfs230 region C fragments (aa 444 to 1132, 444 to 886, and 585 to 700) have also been tested but were down-selected due to a low target expression level or poor target solubility.
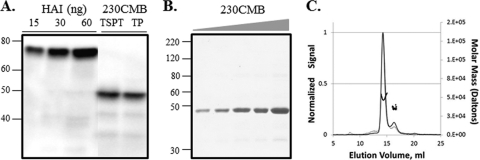
Fig. 2.
Expression analysis and in vitro characterization of the purified 230CMB antigen produced for immunological evaluation. (A) Crude plant extract consisting of total soluble protein with 0.5% Triton X-100 (TSPT) and total protein (TP) were resolved by SDS-PAGE, transferred to PVDF membrane, detected with anti-4-His MAb, and compared to different amounts of the standard (plant-produced hemagglutinin from the A/Indonesia/05/05 strain of influenza virus [HAI]). (B) Increasing amounts of purified 230CMB were resolved by SDS-PAGE and stained with Gel Code Blue. Size comparisons to MagicMark XP (A) or BenchMark (B) molecular size markers were made. The values to the left of panels A and B are molecular sizes in kilodaltons. (C) SEC of 230CMB followed by UV and light scattering detection. The solid black trace is UV280, the thin dotted trace is the light scatter signal (Rayleigh ratio), and the markers indicate the calculated molar mass over the given elution volume.
Purification and biophysical characterization of 230CMB produced in N. benthamiana.
Vacuum infiltration of hydroponically grown N. benthamiana was performed for purification of 230CMB for further studies. Protein was purified as described in Materials and Methods. Following IMAC, 230CMB was approximately 75% pure, a value that was improved to 95% purity, as determined by Coomassie staining of an SDS-PAGE gel, after anion-exchange chromatography. A single predominant band was visible on a Coomassie-stained SDS-PAGE gel (Fig. 2B) and was also detected using an anti-6-His tag-specific MAb in a Western blot assay (data not shown). The nominal molecular mass of 230CMB is 34 kDa; however, it is resolved at approximately 48 kDa under reducing denaturing conditions. The greater molecular mass of 230CMB determined under reducing denaturing conditions can potentially be explained by the glycosylated nature of the product. Indeed, upon treatment with peptide N-glycosidase F, 230CMB has been shown to migrate at a slightly lower molecular weight (unpublished data). This, however, does not exclude the possibility of other posttranslational modifications or O-linked glycosylation leading to the larger-than-expected-size molecule.
The 230CMB preparation was further characterized to determine the molecular weight and solution state profile of the product by SEC-MALLS. SEC of 230CMB, followed by UV and light scattering detection, indicated one primary peak with a molar mass distribution of 48 kDa, while the small signal at the 8-ml column void volume indicates a very small amount of a high-molecular-weight species in the protein sample (Fig. 2C).
To confirm correct processing of the signal peptide, the protein was subjected to N-terminal sequencing performed by Edman degradation and was shown to have the expected N terminus (YVDEK). In addition, plant-produced 230CMB reacted with two anti-Pfs230 MAbs, 2B10 and 2G5, which were shown to be TB antibodies. 230CMB was detected by these two MAbs with endpoint titers, determined at a level 4-fold over the background, of greater than 50,000 for each MAb, indicating that the TB epitopes in our plant-produced protein are recognized by these antibodies.
230CMB elicits TB antibodies in rabbits.
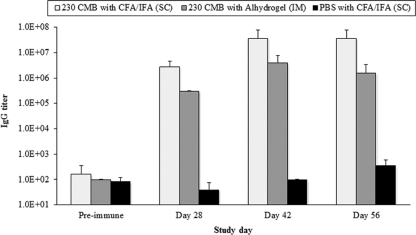
To evaluate the immunogenicity and TB activity of 230CMB, rabbits were immunized with 100 μg of 230CMB with Freund's adjuvant (subcutaneously) or with Alhydrogel (intramuscularly) using a prime-boost regimen on days 0 and 28. Serum samples were collected on study days 0, 28, 42, and 56. ELISA analysis of the IgG responses elicited by the two 230CMB vaccine formulations showed that protein emulsified in Freund's adjuvant generated a 9-fold higher IgG titer than that adsorbed onto Alhydrogel. However, the kinetics of the response was the same for both formulations, with antibody titers peaking on study day 42 and remaining elevated through day 56 (Fig. 3). Importantly, day 42 sera from animals immunized with both formulations contained a high titer of antibodies that bound to parasites in both IFA and SIFA. Sera from the Freund's adjuvant-treated group of animals had a higher serum IFA titer (≥12,800) than sera from the group of animals receiving antigen adsorbed on Alhydrogel (= 800), and both serum samples were positive in the SIFA at a dilution of 1:250.
Fig. 3.
IgG responses elicited by the 230CMB vaccine in rabbits using either complete Freund's adjuvant (CFA) followed by incomplete Freund's adjuvant (IFA) subcutaneously (SC) or Alhydrogel intramuscularly (IM). Serum 230CMB-specific IgG titers were measured by ELISA using samples collected before (Preimmune) antigen administration, and on days 28, 42, and 56 postvaccination. Reciprocal serum dilutions that gave a mean OD value greater than 4-fold over the background were determined as the endpoint titers. Data are shown as mean serum IgG endpoint titers ± standard deviations.
Further evaluation of sera in the SMFA in the presence of complement demonstrated that all animals immunized with 230CMB emulsified in Freund's adjuvant or adsorbed on Alhydrogel generated antibodies that resulted in ≥99% TB (Table 1). As was anticipated, in the absence of complement, the levels of mean oocyst reduction were less pronounced, with the group receiving antigen emulsified in Freund's adjuvant exhibiting a 55% reduction, while the group receiving antigen adsorbed on Alhydrogel showed an 80% reduction. These findings were confirmed by the data from an independent laboratory using the same rabbit sera (see Table S1 in the supplemental material). The experimental sera were compared to known TB MAb 63F2A2 (IgG2a isotype) (37) and were shown to perform as well as the control, which achieved 99% TB in the presence of complement (Table 1).
Table 1.
Evaluation of sera from rabbits immunized with 230CMB in the standard membrane feeding assay
| Vaccine candidate, adjuvant, and sample | With complement |
Without complement |
||||||
|---|---|---|---|---|---|---|---|---|
| No. inf./diss.d | Median (IQR)e | % Reductionb | P valueb | No. inf./diss. | Median (IQR) | % Reductionb | P valueb | |
| 230CMB, Freund'sa | ||||||||
| Pre | 20/20 | 60.5 (31.5–80.0) | 19/20 | 52.0 (22.0–59.0) | ||||
| Final | 5/50 | 0.0 (0.0–0.5) | 99 | <0.001 | 19/20 | 16.5 (6.5–31.5) | 55 | >0.05 |
| 230CMB, Alhydrogel | ||||||||
| Pre | 20/20 | 67.5 (39.0–75.5) | 19/20 | 49.0 (16.0–62.5) | ||||
| Final | 3/20 | 0.0 (0.0–0.0) | 100 | <0.001 | 16/20 | 8.0 (3.5–13.5) | 80 | <0.05 |
| NA,f NA, 63F2A2c | 7/20 | 0.0 (0.0–1.0) | 99 | <0.001 | 19/19 | 44.0 (33.0–64.0) | 0 | >0.05 |
The immunization strategy consisted of complete Freund's adjuvant (prime) followed by incomplete Freund's adjuvant (boost).
Percent reductions in oocyst counts and P values were obtained by comparing the day 42 immune pooled rabbit sera (Final) with the preimmune pooled rabbit sera (Pre).
63F2A2 (IgG2a isotype) is a MAb (37) that was used as a positive control.
No. inf./diss., numbers of infected/dissected mosquitoes.
IQR, interquartile range.
NA, not applicable.
DISCUSSION
TBVs represent a unique opportunity to limit the spread of the malaria parasite and are viewed as critical for the successful eradication of the parasite. Multiple targets present on the sexual stages of Plasmodium parasites show initial promise in producing antibodies capable of disrupting development of the parasite in the mosquito. Pfs230, a large and complex protein found on the surface of the gametocytes, which has an important function during fertilization (1, 9), is among these targets. Recombinant expression of full-length Pfs230 for TBV development has not been successful due to the size and complexity of the protein. Additionally, production of subdomains of Pfs230 in early studies using both E. coli (48) and Saccharomyces cerevisiae (44) has not been successful in forming a correctly folded molecule capable of consistently eliciting a high level of TB antibodies. However, expression of recombinant Pfs230C with TB activity has been achieved by Tachibana et al. (40a), who demonstrated TB activity of Pfs230C produced in the wheat germ cell-free expression system. The wheat germ cell-free expression system, however, presents a challenge for large-scale manufacturing.
In this study, we have successfully used a plant-based transient-expression system (28) to produce a number of target proteins with desired biological activity and with levels of target expression ranging from 100 mg to over 1 g/kg of fresh whole leaf tissue (7, 19, 23, 26, 40). Due to the complexity of the Pfs230 protein, we endeavored to use our transient-expression system in N. benthamiana to express the portion of Pfs230C (230CMB) that was shown to have TB activity using an in vitro translation system (40a). In this study, we expressed, purified, and characterized 230CMB (aa 444 to 730). The 230CMB antigen described here is similar to r230/MBP.C5 (aa 443 to 791) produced by Bustamante et al. (2), which comprises the region of Pfs230 positioned just after the N-terminal cleaved domain until midway through the first cysteine motif domain and contains four cysteine residues. However, unlike the MBP fusion, 230CMB is a stand-alone soluble antigen which is recognized in both Western blot assays and ELISA by two different anti-Pfs230 MAbs possessing TB activity. SEC-MALLS data indicated that the purified protein was in a monomeric state and had a molecular mass of 48 kDa. When administered to rabbits in the presence of adjuvant, plant-produced 230CMB elicited high titers of anti-Pfs230 antibodies. Differences between the antibody titers elicited by the two adjuvants tested were detected, with the complete/incomplete Freund's adjuvant combination eliciting higher antibody titers than Alhydrogel. This enhanced immunogenicity is likely to be a result of the presence of inactivated mycobacteria in the complete Freund's adjuvant used for the primary immunization. However, antibodies generated using either adjuvant were shown to bind specifically to the native protein of P. falciparum on the surfaces of gametes/zygotes, resulting in significant (>99%) TB activity. This, in turn, indicates that plant-produced 230CMB is correctly folded and antibodies generated against it are able to fix complement, resulting in destruction of the parasite in the mosquito.
The capacity of our subdomain, 230CMB, to produce effective TB antibodies indicates that targeting of this complex protein domain to the ER of a plant cell is sufficient to confer proper folding and results in an antigen that reflects the native conformation. Additional process development and formulation studies are under way to improve target yields and establish methodologies for cGMP production, as well as examine the potential for enhancement of immunogenicity. The demonstrated success of this potential TBV consisting of a target protein from the gametocyte stage of the parasite also lends itself to the investigation of a combination vaccine consisting of an additional target from a later sexual stage of the parasite, such as Pfs25, which is present on gametes/zygotes, retorts, and ookinetes. Combining Pfs230 and Pfs25 targets in a single vaccine may lead to a highly effective TBV. Also, combining Pfs230 and Pfs48/45 (involved in the fertilization process) can lead to a more effective TBV.
The efficacy of plant-produced 230CMB in eliciting TB antibodies not only shows the potential of this vaccination approach as an essential part of a malaria eradication program but also further supports the potential of expressing complex recombinant proteins in plants. The transient-expression technology used here is both time efficient and easily scalable (6, 3d9).
ACKNOWLEDGMENTS
We thank Hong Bi, Robert Stevens, Susan Underkoffler, Shama Satinover, Sandra Gibbs, and Emily Damon for technical assistance, Rebecca Snow and Matt Sarlo for providing plant material, and Natasha Kushnir for editorial assistance.
This work was funded by a grant from The Bill and Melinda Gates Foundation, Seattle, WA.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Aly A. S. I., Vaughan A. M., Kappe S. H. I. 2009. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63:195–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bustamante P. J., et al. 2000. Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol. 22:373–380 [DOI] [PubMed] [Google Scholar]
- 3. Butler D. 2009. Initiative targets malaria eradication. Nature 462:19. [DOI] [PubMed] [Google Scholar]
- 4. Carter R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309–2314 [DOI] [PubMed] [Google Scholar]
- 5. Carter R., Coulson A., Bhatti S., Taylor B. J., Elliot J. F. 1995. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol. Biochem. Parasitol. 71:203–210 [DOI] [PubMed] [Google Scholar]
- 6. Chichester J. A., Haaheim L. R., Yusibov V. 2009. Using plant cells as influenza vaccine substrates. Expert Rev. Vaccines 8:493–498 [DOI] [PubMed] [Google Scholar]
- 7. Chichester J. A., et al. 2009. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine 27:3471–3474 [DOI] [PubMed] [Google Scholar]
- 8. Edelhoch H. 1967. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6:1948–1954 [DOI] [PubMed] [Google Scholar]
- 9. Eksi S., et al. 2006. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 61:991–998 [DOI] [PubMed] [Google Scholar]
- 10. Enserink M. 2010. Malaria's drug miracle in danger. Science 328:844–846 [DOI] [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12. Foo A., et al. 1991. Conserved and variant epitopes of target antigens of transmission-blocking antibodies among isolates of Plasmodium falciparum from Malaysia. Am. J. Trop. Med. Hyg. 44:623–631 [DOI] [PubMed] [Google Scholar]
- 13. Gerloff D. L., Creasey A., Maslau S., Carter R. 2005. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 102:13598–13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Good M. F., Kaslow D. C., Miller L. M. 1998. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 16:57–87 [DOI] [PubMed] [Google Scholar]
- 15. Graves P. M., Carter R., Burkpt T. R., Quakyi I. A., Kumar N. 1988. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 10:209–218 [DOI] [PubMed] [Google Scholar]
- 16. Green B. J., et al. 2009. Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol. J. 4:230–237 [DOI] [PubMed] [Google Scholar]
- 17. Healer J., McGuinness D., Carter R., Riley E. 1999. Transmission blocking immunity to Plasmodium falciparum in malaria immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119:425–433 [DOI] [PubMed] [Google Scholar]
- 18. Healer J., et al. 1997. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect. Immun. 65:3017–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hull A. K., et al. 2005. Human-derived, plant-produced monoclonal antibody for the treatment of anthrax. Vaccine 23:2082–2086 [DOI] [PubMed] [Google Scholar]
- 20. Kappe S. H., Vaughan A. M., Boddey J. A., Cowman A. F. 2010. That was then but this is now: malaria research in the time of an eradication agenda. Science 328:862–866 [DOI] [PubMed] [Google Scholar]
- 21. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 22. Lensen A., et al. 1996. Measurement by membrane feeding of reduction in Plasmodium falciparum transmission induced by endemic sera. Trans. R. Soc. Trop. Med. Hyg. 90:20–22 [DOI] [PubMed] [Google Scholar]
- 23. Massa S., et al. 2007. Anticancer activity of plant-produced HPV16 E7 vaccine. Vaccine 25:3018–3021 [DOI] [PubMed] [Google Scholar]
- 24. Matsuoka M., et al. 1987. Classification and structural comparison of full-length cDNAs for pathogenesis-related proteins. Plant Physiol. 85:942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendis K. N., Munesinghe Y. D., de Silva Y. N., Keragalla I., Carter R. 1987. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect. Immun. 55:369–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mett V., et al. 2008. A plant-produced influenza subunit vaccine protects ferrets against virus challenge. Influenza Other Respi. Viruses 2:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulder B., et al. 1994. Malaria transmission-blocking activity in experimental infections of Anopheles gambiae from naturally infected Plasmodium falciparum gametocyte carriers. Trans. R. Soc. Trop. Med. Hyg. 88:121–125 [DOI] [PubMed] [Google Scholar]
- 28. Musiychuk K., et al. 2007. A launch vector for the production of vaccine antigens in plants. Influenza Other Respi. Viruses 1:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ong C. S., Zhang K. Y., Eida S. J. 1990. The primary antibody response of malaria patients to Plasmodium falciparum sexual stage antigens which are potential transmission blocking vaccine candidates. Parasite Immunol. 12:447–456 [DOI] [PubMed] [Google Scholar]
- 30. Outchkourov N., et al. 2007. Epitope analysis of the malaria surface antigen Pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J. Biol. Chem. 282:17148–17156 [DOI] [PubMed] [Google Scholar]
- 31. Ponnudurai T., et al. 1989. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98(Pt. 2):165–173 [DOI] [PubMed] [Google Scholar]
- 32. Pradel G. 2007. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology 134:1911–1929 [DOI] [PubMed] [Google Scholar]
- 33. Premawansa S., et al. 1994. Plasmodium falciparum malaria transmission-blocking immunity under conditions of low endemicity as in Sri Lanka. Parasite Immunol. 16:35–42 [DOI] [PubMed] [Google Scholar]
- 34. Read D., et al. 1994. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 16:511–519 [DOI] [PubMed] [Google Scholar]
- 35. Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. 1983. Target antigens of transmission blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 158:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roeffen W., et al. 1995. Plasmodium falciparum: a comparison of the activity of Pfs230-specific antibodies in an assay of transmission-blocking immunity and specific competition ELISAs. Exp. Parasitol. 80:15–26 [DOI] [PubMed] [Google Scholar]
- 37. Roeffen W., et al. 1995. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230-specific antibodies is isotype dependent. Infect. Immun. 63:467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shoji Y., et al. 2009. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 27:1087–1092 [DOI] [PubMed] [Google Scholar]
- 39. Shoji Y., et al. 2011. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum. Vaccines 7:41–50 [DOI] [PubMed] [Google Scholar]
- 40. Shoji Y., et al. 2009. Immunogenicity of hemagglutinin from A/Bar-headed Goose/Qinghai/1A/05 and A/Anhui/1/05 strains of H5N1 influenza viruses produced in Nicotiana benthamiana plants. Vaccine 27:3467–3470 [DOI] [PubMed] [Google Scholar]
- 40a. Tachibana M., et al. 2011. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin. Vaccine Immunol. 18:1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuboi T., et al. 2008. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 76:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Kolk M., et al. 2005. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology 130(Pt. 1):13–22 (Erratum, Parasitology 131(Pt. 4):578, 2005. Sauerwein, W [corrected to Sauerwein, RW].) [DOI] [PubMed] [Google Scholar]
- 43. Vermeulen A. N., et al. 1985. Sequential expression of antigens of sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162:1460–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vincent A. A., Fanning S., Caira F. C., Williamson K. C. 1999. Immunogenicity of malaria transmission-blocking vaccine candidate, y230. CA14 following crosslinking in the presence of tetanus toxoid. Parasite Immunol. 21:573–581 [DOI] [PubMed] [Google Scholar]
- 45. Vogel G. 2010. The ‘do unto others’ malaria vaccine. Science 328:847–848 [DOI] [PubMed] [Google Scholar]
- 46. Voinnet O., Rivas S., Mestre P., Baulcombe D. 2003. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33:949–956 [DOI] [PubMed] [Google Scholar]
- 47. Williamson K. C., Criscio M. D., Kaslow D. C. 1993. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking antigen, Pfs230. Mol. Biochem. Parasitol. 58:355–358 [DOI] [PubMed] [Google Scholar]
- 48. Williamson K. C., Keister D. B., Muratova O., Kaslow D. C. 1995. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol. Biochem. Parasitol. 75:33–45 [DOI] [PubMed] [Google Scholar]