Abstract
Herpes simplex virus 1 (HSV-1) and varicella-zoster virus (VZV) cause serious central nervous system (CNS) diseases that are diagnosed with PCR using samples of cerebrospinal fluid (CSF) and, during later stages of such infections, with assays of intrathecal IgG antibody production. However, serological diagnoses have been hampered by cross-reactions between HSV-1 and VZV IgG antibodies and are commonly reported in patients with herpes simplex encephalitis (HSE). In this study we have evaluated VZV glycoprotein E (gE) as a new antigen for serological diagnosis of VZV-induced CNS infections. Paired samples of CSF and serum from 29 patients with clinical diagnosis of VZV CNS infection (n = 15) or HSE (n = 14), all confirmed by PCR, were analyzed. VZV gE and whole VZV were compared as antigens in enzyme-linked immunosorbent assays (ELISAs) for serological assays in which the CSF/serum sample pairs were diluted to identical IgG concentrations. With the gE antigen, none of the HSE patients showed intrathecal IgG antibodies against VZV, compared to those shown by 11/14 patients using whole-VZV antigen (P < 0.001). In the patients with VZV infections, significantly higher CSF/serum optical density (OD) ratios were found in the VZV patients using the VZV gE antigen compared to those found using the whole-VZV antigen (P = 0.001). These results show that gE is a sensitive antigen for serological diagnosis of VZV infections in the CNS and that this antigen was devoid of cross-reactivity to HSV-1 IgG in patients with HSE. We therefore propose that VZV gE can be used for serological discrimination of CNS infections caused by VZV and HSV-1.
INTRODUCTION
Herpes simplex encephalitis (HSE) and varicella-zoster virus (VZV) infections of the central nervous system (CNS) are serious diseases with risk of fatality and neurological sequels despite adequate antiviral treatment (14, 19, 21). PCR, with its high sensitivity and specificity, has improved diagnostics of both of these conditions (1, 19, 20) and is the standard diagnostic procedure, together with detection of a specific intrathecal antibody response (17). The antibody response gradually increases in parallel with the disappearance of viral DNA in the cerebrospinal fluid (CSF). In HSE patients, the PCR has been shown to be positive in up to 27 days after onset of disease, but the majority are negative after 14 days (1, 25). In patients with VZV CNS infection, the PCR might be positive in up to 26 days, but many patients are negative after 7 days (7). A considerable number of patients with VZV CNS infection and a few patients with HSE are diagnosed after viral DNA has vanished from the CSF (7). At this stage, detection of intrathecal antibody response against the specific virus is required to confirm the diagnosis (6, 25). For this purpose, the use of specific and sensitive antigens is a prerequisite.
Serological cross-reactivity in HSE patients with findings of intrathecal antibodies to both herpes simplex virus 1 (HSV-1) and VZV have been reported (22–24, 26, 28), most likely due to shared epitopes on proteins expressed by these two viruses (4, 15). Another possible interpretation of the presence of antibodies to both HSV-1 and VZV in CSF samples would be a response to dual infections. This was suggested in a study of 46 patients with suspected HSE in which 7/46 patients had both VZV DNA and HSV 1-DNA detected in the CSF samples by qualitative PCR (3).
To detect antibodies against VZV, either whole-VZV-infected cell lysates or purified glycoproteins are used as antigens (12). The major viral antigens of VZV are glycoprotein E (gE), gB, gH, and gL (16), which are structural components of the viral envelope. The use of whole-VZV-infected cell lysates increases serological cross-reactivity since VZV and HSV-1 expose common epitopes on gB and maybe some other proteins (15). VZV gE is the most abundant viral glycoprotein expressed in VZV-infected cells (18) and has been demonstrated to be highly immunogenic (9). Moreover, in contrast to gB and some other proteins, gE has a relatively low degree of genetic similarity between VZV and HSV-1. Here, we have utilized VZV gE as an enzyme-linked immunosorbent assay (ELISA) antigen for serological diagnoses of VZV infection in the CNS. This antigen was devoid of cross-reaction with HSV-1 antibodies in the CSF, as judged from samples from patients with HSE. We propose that a VZV gE ELISA is a novel tool for serological discrimination of VZV and HSV-1 CNS infections.
MATERIALS AND METHODS
Patients, their serum and CSF samples, and PCR.
Twenty-nine patients with a clinical picture of CNS infection, consecutively sampled at the Virological Laboratory of Sahlgren's University Hospital and all PCR positive in CSF samples against HSV-1 (n = 14) or VZV (n = 15), were included. From these patients, paired serum and CSF samples showing the presence of intrathecal antibodies (for criteria, see below) against VZV and/or HSV-1 in the routine serology were selected for analysis of antibodies to VZV gE. These serum and CSF samples were in most cases collected at later time points in relation to the initial, PCR-positive CSF samples. Clinical data on these patients and samples, including their diagnoses, are presented in Table 1.
Table 1.
VZV DNA detected by PCR and ELISA antibody titers in serum and CSF samples from 15 VZV patients and 14 HSE patients with CNS infection
| Patient | Sexc | Age (yr) | Diagnosisd | Days from onset | VZV DNA in CSF (copies/ml) | Titer |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole-VZV antigen |
VZV gE antigen |
HSV-1 |
|||||||||
| CSF | Serum | CSF | Serum | CSF | Serum | ||||||
| VZV | |||||||||||
| 1 | M | 45 | Encephalitis | 48 | Negb | 160 | 6,400 | 40 | 3,200 | 160 | 25,600 |
| 2 | M | 68 | Encephalitis | 113 | Neg | 320 | 12,800 | 320 | 12,800 | 320 | 102,400 |
| 3 | F | 82 | Encephalitis | 16 | Neg | 5,120 | 102,400 | 320 | 12,800 | 5,120 | 12,800 |
| 4 | M | 82 | Encephalitis | NDa | 2,700 | 640 | 6,400 | 640 | 3,200 | Neg | Neg |
| 5 | F | 64 | Encephalitis | 20 | 50 | 5,120 | 12,800 | 640 | 3,200 | 2,560 | 51,200 |
| 6 | F | 38 | Meningitis | 6 | 25,000 | 20,480 | 102,400 | 10,240 | 102,400 | 20 | 100 |
| 7 | F | 70 | Meningitis | 11 | 2,000 | 640 | 25,600 | 160 | 6,400 | 320 | 51,200 |
| 8 | M | 67 | Meningitis | 18 | Neg | 5,120 | 25,600 | 2,560 | 25,600 | 40 | 800 |
| 9 | F | 81 | Encephalitis | 2 | 200 | 5,120 | 102,400 | 2,560 | 102,400 | 80 | 6,400 |
| 10 | M | 78 | Meningitis | 16 | 74,000 | 2,560 | 12,800 | 1,280 | 6,400 | 320 | 25,600 |
| 11 | F | 58 | R-H | 1 | 50 | 1,280 | 102,400 | 1,280 | 102,400 | 80 | 25,600 |
| 12 | M | 3 | Vasculitis, left-sided hemiparesis | 0 | 300 | 640 | 12,800 | 160 | 3,200 | Neg | Neg |
| 13 | F | 81 | Encephalitis | 5 | 130 million | 20,480 | 102,400 | 10,240 | 51,200 | 1,280 | 25,600 |
| 14 | F | 63 | R-H | 8 | 15,300 | 2,560 | 6,400 | 2,560 | 6,400 | 160 | 6,400 |
| 15 | F | 36 | Encephalitis | 358 | Neg | 80 | 3,200 | 10 | 400 | 640 | 204,800 |
| HSE | |||||||||||
| 16 | F | 63 | Encephalitis | 88 | ND | 1,280 | 12,800 | 10 | 100 | 81,920 | 819,200 |
| 17 | F | 32 | Encephalitis | 6 | ND | 320 | 12,800 | 20 | 400 | 2,560 | 12,800 |
| 18 | M | 60 | Encephalitis | 30 | ND | 12,800 | 10,240 | 10 | 800 | 81,920 | 204,800 |
| 19 | F | 65 | Encephalitis | 6 | ND | 320 | 6,400 | 10 | 400 | 2,560 | 12,800 |
| 20 | M | 37 | Encephalitis | 46 | ND | 5,120 | 51,200 | 10 | 400 | 20,480 | 204,800 |
| 21 | F | 72 | Encephalitis | 51 | ND | 40,960 | 102,400 | 10 | 200 | 5,120 | 12,800 |
| 22 | M | 67 | Encephalitis | 37 | ND | 2,560 | 6,400 | 20 | 400 | 81,920 | 102,400 |
| 23 | F | 63 | Encephalitis | 16 | ND | 20,480 | 51,200 | 10 | 200 | 81,920 | 409,600 |
| 24 | M | 66 | Encephalitis | 37 | ND | 40 | 800 | 10 | 400 | 5,120 | 25,600 |
| 25 | F | 64 | Encephalitis | 30 | ND | 640 | 6,400 | 80 | 3,200 | 40,960 | 102,400 |
| 26 | F | 79 | Encephalitis | 30 | ND | 2,560 | 12,800 | 40 | 400 | 40,960 | 409,600 |
| 27 | M | 53 | Encephalitis | 34 | ND | 2,560 | 6,400 | 20 | 800 | 81,920 | 204,800 |
| 28 | F | 65 | Encephalitis | 46 | ND | 5,120 | 6,400 | 10 | 400 | 81,920 | 102,400 |
| 29 | M | 67 | Encephalitis | 26 | ND | 20,480 | 25,600 | 10 | 800 | 81,920 | 204,800 |
ND, not done.
Neg, negative result.
M, male; F, female.
R-H, Ramsay Hunt syndrome.
In all 29 patients, CSF samples were PCR positive for VZV DNA (19) (n = 14) or HSV-1 DNA (n = 14) 0 to 4 months before detection of intrathecal antibodies, except 1 patient who was sampled 1 year after VZV DNA positivity. None of the CSF samples revealed the dual finding of VZV DNA and HSV-1 DNA. In addition to PCR analysis of the CSF samples at first lumbar puncture, the later CSF samples showing intrathecal antibodies of the patients with VZV CNS disease were also analyzed for VZV DNA.
Preparations of VZV glycoprotein E and whole-VZV antigen.
The VZV gE antigen was prepared as recently described (27). Briefly, the coding sequence of the extracellular domain (ED) of gE from VZV was cloned into a mammalian expression vector and transfected into CHO-K1 cells. Stable clones were generated, ad production of recombinant VZV gE was screened among the clones, and one good producer was expanded and adapted to serum-free suspension growth and then cultured in a 3-liter stirred-tank bioreactor for production. VZV gE was purified from the cultured supernatant by affinity chromatography, using the His tag.
To compare the purified VZV gE antigens, we used a conventional whole-VZV antigen prepared from virus-infected cellular membranes as previously described (5). For assessment of virus-specific HSV antibodies, membrane antigen achieved after infection of baby hamster kidney (BHK) cells (HSV-1) was used (11). HSV-1 and HSV-2 antibodies in sera were typed using glycoprotein G-1 (gG-1) and gG2 antigens (HerpeSelect ELISA IgG; Focus Diagnostics, Cypress, CA).
Immunofluorescence.
Analysis of HSV-1 IgM antibodies by immunofluorescence was performed using HSV-1-infected GMK cells according to routine in-house diagnostic procedures of the Virological Laboratory, Sahlgren's University Hospital. Serum samples showing specific fluorescence of infected foci at a dilution of 4 or higher were determined to be positive.
Enzyme-linked immunosorbent assay.
Briefly, the ELISAs were performed with different antigen dilutions depending on the type of antigen (1:3,200 of antigen VZV gE, 1:3,000 of whole-VZV antigen, 1:1,000 of HSV type-common antigen). Wells were coated with the given antigen and then incubated with the serum and CSF samples diluted in 2-fold steps followed by goat antibody to human IgG. Next, a substrate solution and diethanolamine buffer were added to each well. The plates were read once per 10 min (20 to 80 min) on a spectrophotometer (Emax Precision microplate reader; Molecular Devises, Sunnyvale, CA) to determine optical density (OD) levels at A450 to A650. The cutoff rate was set as a negative serum control (diluted 1:200) plus 0.200 for all ELISAs. Standard dilutions of defined antibody-positive and antibody-negative sera were included in each experiment.
Assessment of intrathecal antibody production by sample titration.
The presence of intrathecal antibodies to HSV-1 or VZV, one of the inclusion criteria of this study, was determined by the routine method used at our laboratory. Thus, we calculated the serum/CSF sample ratio by measuring the IgG titers of the specific virus by ELISA in serum and CSF samples from each patient, whereafter this ratio was compared with the ratio of corresponding IgG titers against a reference virus (17) in the form of morbilli. Evidence of intrathecal antibody production were CSF findings of IgG VZV or IgG HSV at ≥80 using whole-VZV antigen or type-common HSV antigen, respectively, and a serum/CSF sample ratio of HSV or VZV 4 times lower than the serum/CSF sample ratio of morbillus-reactive IgG. IgG VZV of ≥20 in the CSF samples was the lower limit when VZV gE was used as an antigen (Table 1).
In addition, the CSF/serum albumin ratio and IgG index, (CSF/serum IgG ratio)/(CSF/serum albumin ratio), were calculated to estimate blood-brain barrier damage and intrathecal antibody production.
Assessment of intrathecal antibody production against VZV by sample dilution to an identical IgG concentration.
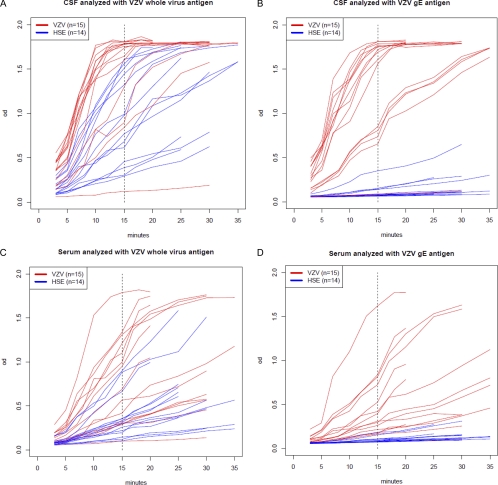
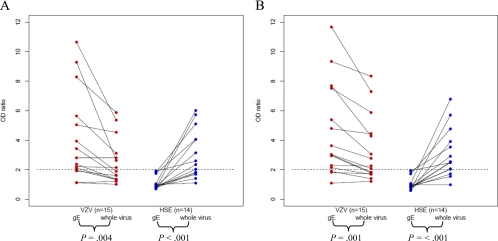
The CSF and serum sample pairs from the patients with HSE (n = 14) and the patients with VZV CNS infection (n = 15) were further analyzed by diluting CSF and serum samples to an identical IgG concentration. First, IgG concentrations were determined in all CSF/serum paired samples using a human IgG ELISA kit (Novakemi AB, Handen, Sweden). Next, the CSF and serum samples were diluted to an identical concentration of 1 μg of total IgG/ml, and 100 μl was added to each well for determination of viral IgG by ELISA. All CSF and serum samples were tested in triplicate in plates coated with VZV gE antigen (dilution, 1:2,000) or whole-VZV antigen (dilution, 1:3,000) and tests with standard dilutions of defined antibody-positive and antibody-negative sera were included in each experiment. The plates were read on a spectrophotometer (same as above) at A450 to A650, and the signal was recorded every 2 or 3 min for kinetics and determination of Vmax (observation range 3 to 30 min) (Fig. 1 and 2). At 15 min, most of the expected positive samples had reached their maximum levels (Fig. 1). Therefore, we choose to compare the OD levels of all samples at this time point. In addition, we assessed the intrathecal antibody production by means of using the formula antibody index of ODCSF/ODserum at 15 min and at Vmax. An intrathecal antibody production was assumed, with an antibody index of ≥2.0 (8, 24) (Fig. 2).
Fig. 1.
OD levels at different time points of IgG analysis by ELISA of CSF (A and B) and serum (C and D) samples from 15 patients with VZV CNS infection and 14 patients with HSE. The IgG response was analyzed with whole-VZV antigen versus VZV gE antigen. All samples of serum and CSF were diluted to an identical concentration of total IgG (1 μg/ml).
Fig. 2.
Intrathecal antibody production illustrated by the CSF/serum OD ratios at 15 min (A) and at Vmax (B) of 15 VZV patients with CNS infection and 14 patients with HSE. The total IgG in serum and CSF samples is diluted to identical concentrations (1 μg/ml) in all samples and analyzed with VZV gE antigen versus whole-virus antigen by ELISA. The paired t test was used for analysis between VZV gE and whole-VZV antigen. Significantly higher CSF/serum OD ratios were found in the VZV patients at both 15 min and at Vmax using VZV gE antigen. In the HSE patients, the CSF/serum OD ratios were significantly lower using VZV gE antigen at both 15 min and at Vmax.
Statistics.
Paired continuous data (measurements by VZV gE versus whole VZV) were analyzed with paired t test, while paired categorical data were analyzed with the McNemar test using the binomial distribution. Differences in proportions between HSE and VZV patients were tested with the Fisher exact test.
RESULTS
PCR detection of HSV-1 DNA and VZV DNA.
All 29 patients with VZV CNS infection (n = 15) and HSE (n = 14) were included based on PCR positivity for their respective virus at the time of diagnosing their disease. The CSF and serum samples that revealed intrathecal antibody production were collected after various numbers of days after the onset of disease. These later samples of the patients with VZV CNS infection were also analyzed with quantitative PCR for detection of VZV DNA. Only a few of them (5/15) were negative, which is in accordance with earlier findings (7), as most of these samples were collected relatively close in time to the first diagnostic lumbar puncture. In the positive samples, quantities of VZV DNA varied between 50 copies/ml and 13 million copies/ml (median, 2,350 copies/ml) (Table 1).
VZV and HSV-1 IgG reactivity and assessment of intrathecal antibody production by serum/CSF titer ratios.
The results, expressed as reciprocal titers, of the ELISAs using whole HSV-1, VZV, and morbillus antigen are presented in Table 1. These titers, when calculated as serum/CSF titer ratios, were all <4 compared to those of morbilli IgG, confirming the presence of intrathecal antibodies to the respective viral agent in all paired samples.
To investigate the eventual cross-reactivity of IgG to the VZV gE antigen in patients with HSE, ELISAs were performed on the CSF and serum samples from such patients and compared to corresponding results with the whole-VZV antigen. Only 4/14 HSE patients had intrathecal production of antibodies with VZV gE antigen, in comparison to production by 11/14 patients when the whole-VZV antigen was used (P = 0.021). In contrast, in the patients with VZV-induced CNS infections, the VZV gE antigen showed sensitivity comparable to that of the whole-VZV antigen, as 14/15 patients had positive results with the former antigen. The patient with a negative result in CSF was sampled 1 year after the onset of VZV disease and PCR positivity, suffering from systemic lupus erythematosus (SLE) with suspected CNS involvement. When the patients with VZV CNS infection were analyzed regarding antibodies to HSV-1, 3/15 patients had intrathecal antibodies. One of these three patients was gG1 negative and gG2 positive in serum samples and had an HSV IgM titer of 80 (i.e., indicative of HSV-2 infection). The two others were HSV gG1 positive and gG2 negative in serum samples. In total, 11/15 patients with VZV CNS infection and 13/14 patients with HSE were HSV gG1 positive in serum samples.
When the IgG index was calculated, 6/15 patients with VZV CNS infection (patients 2, 7, 8, 10, 11, and 13) and 0/14 HSE patients (patients 16, 18, and 22 were not analyzed) showed no intrathecal antibody production. A total of 7/15 VZV patients and 5/14 HSE patients (1 patient was not analyzed) showed no blood-brain barrier damage by the CSF/serum albumin ratio.
Assessment of VZV IgG antibodies in CSF and serum samples by sample dilution to an identical IgG concentration.
To further evaluate VZV gE as a specific antigen for detection of CSF antibodies to VZV, CSF and serum samples from all 29 patients were diluted to an IgG concentration of 1 μg/ml and analyzed kinetically with both of the VZV antigens (Fig. 1). Clearly, antibody reactivity to the VZV gE antigen was almost absent in the HSE patients at all observation time points, compared to analysis using the whole-VZV antigen. The lack of reactivity was more pronounced for the CSF samples than the serum samples (Fig. 1). The highest OD levels recorded at 15 min with the CSF and serum samples of the HSE patients were as low as 0.350 and 0.184, respectively. All other CSF and serum samples showed even lower OD levels (< = 0.155). In contrast, when samples from the HSE patients were analyzed with whole-VZV antigen, the lowest OD level at 15 min for CSF samples was 0.294, and all the other CSF OD levels were higher (Table 2).
Table 2.
OD values at 15 min for CSF and serum samples from patients with HSE or VZV CNS infectiona
| Antigen | Mean OD (SE) |
|||
|---|---|---|---|---|
| HSE (n = 14) |
VZV (n = 15) |
|||
| CSF | Serum | CSF | Serum | |
| VZV gE | 0.15 (0.05) | 0.10 (0.01) | 1.36 (0.15) | 0.47 (0.10) |
| Whole VZV | 0.92 (0.13) | 0.37 (0.07) | 1.50 (0.13) | 0.78 (0.13) |
The total IgG in serum and CSF samples is diluted to an identical concentration of 1 μg/ml in all samples and analyzed with VZV gE antigen versus whole-VZV antigen by ELISA (data illustrated in Fig. 1).
When samples from the patients with VZV CNS infection were analyzed with the VZV gE antigen at 15 min (Table 2), the OD levels of CSF and serum samples were similar to the reactivities recorded using the whole-VZV antigen. Only the patient with samples taken 1 year after PCR positivity and SLE (patient 15) showed very low OD levels in both CSF and serum samples. In addition, 4 more CSF samples revealed lower OD levels than the others (patients 1, 2, 3, and 4) when analyzed with VZV gE antigen (in addition, patients 7 and 12 showed low reactivity when estimating titers in the CSF samples [Table 1]). One of these four patients (mentioned above), with only 200 copies of VZV DNA in CSF, was gG1 negative and gG2 positive and showed an HSV IgM titer of 80, serological findings compatible with an HSV-2 CNS infection. Two of these patients were sampled at 113 days and 48 days after onset of disease, respectively, while the sampling date of the last patient was unknown.
Assessment of intrathecal VZV antibody production at 15 min and at Vmax at an identical IgG concentration.
With an antibody index (ODCSF/ODserum) of ≥2.0 (24) at Vmax using the VZV gE antigen, no intrathecal antibody production was detected (0/14) in the HSE patients, in comparison to detection in 11/14 patients using whole-VZV antigen. This difference was confirmed with the paired t test (P < 0.001). Furthermore, the VZV gE antigen demonstrated satisfying sensitivity in the VZV group at this dilution, with 12/15 patients revealing intrathecal antibody production compared to that revealed by 9/15 patients with the whole-VZV antigen, which was confirmed with the paired t test (P = 0.001). The results at 15 min corresponded to the ones received at Vmax (Fig. 2). Taken together, we interpret these findings in the following way: the VZV gE antigen was superior to the whole-VZV antigen in detecting intrathecal VZV antibodies in patients with VZV CNS infection, without signs of HSV-1 cross-reactivity in the HSE patients.
DISCUSSION
A difficulty with serological diagnosis of viral CNS infections is that the methodology must combine specificity with high sensitivity, since the amount of IgG against the infecting virus in the CSF is relatively small even during convalescence. For this reason, a standard reference method for VZV IgG in serum samples such as using fluorescent antibody to membrane antigen (FAMA) (2) is not suitable. To boost sensitivity, complex ELISA antigens containing a plethora of viral and cellular proteins have often been used, conferring a risk of cross-reactivity between related viruses. Such serological cross-reactions between HSV-1 and VZV CNS infections are well-recognized phenomena (22, 23, 28). The finding has been described in a substantial proportion of CSF samples from suspected herpesvirus-infected patients using independent methods such as CSF/serum antibody index in ELISA or isoelectric focusing followed by antigen blotting (24). Here, using patient samples from PCR-confirmed VZV or HSV-1 CNS infections, we present data indicating that purified gE, an immune-dominant VZV envelope glycoprotein, is a highly sensitive and specific ELISA antigen for the detection of intrathecal antibody production against this virus. This was evident when serum/CSF titer ratios and ODCSF/ODserum ratios at identical IgG concentrations were used and was also valid for the five patients that had become VZV PCR negative.
Why does VZV gE provide such specificity as a serological antigen? The reason behind cross-reactions between antibodies reactive with VZV and HSV-1 CSF antibodies when using whole-VZV antigen is most likely due to shared epitopes between VZV gB and HSV-1 gB (4, 13, 15). When using VZV gE as an ELISA antigen, the problem is probably diminished due to a relatively low degree of similarity to HSV-1 gE (33% identity as demonstrated by a search by BLAST [http://blast.ncbi.nlm.nih.gov/Blast.cgi]). To the best of our knowledge, VZV gE shares no epitopes with any HSV-1 protein.
In serology, the cost of specificity may be a loss of sensitivity. Narrowing down an ELISA antigen from a mixture of viral proteins to a single protein such as gE might carry such a risk. In contrast, the use of the VZV gE antigen for the purpose of diagnosing VZV CNS infections showed good sensitivity (14/15 PCR-positive patients [93%]), as assayed by serum/CSF IgG titers compared to IgG titers of a reference antibody. Furthermore, when we assayed intrathecal antibody production through a dilution of serum and CSF samples to an identical concentration of IgG and then calculated the CSF/serum OD ratio at Vmax, samples from 12/15 VSV patients (80% sensitivity) showed positive results. Of the 3 remaining patients, 1 patient was sampled 1 year after VZV PCR positivity during an attack of SLE with suspected CNS engagement, and another (with only 200 VZV DNA copies/ml in the CSF sample) had serological signs of an HSV-2 CNS infection. In addition to these maybe correctly analyzed VZV CSF antibody-negative patients, 3 of the 15 VZV patients revealed distinct lower OD levels in the CSF than the other patients when analyzed with VZV gE (the group represented by the four red lines in the middle of Fig. 1B). In 2 of these patients, a delay from the onset of disease until the CSF sampling (48 days and 113 days, respectively) might have resulted in lower avidity for the IgG antibodies (10).
We also noticed that 3/15 of the patients with PCR-verified VZV CNS infection showed intrathecal antibodies against HSV, using a whole-virus antigen. One of these patients, as mentioned above, had serological indications of a concurrent HSV-2 CNS infection that was not verified by PCR. Although we cannot rule out serological cross-reaction also in the direction of VZV antibodies binding to the HSV antigen, the finding of intrathecal antibodies to HSV-1 and HSV-2 in patients suffering from VZV CNS infection seems to be a much less common problem compared to the VZV-cross-reacting antibodies regularly detected in HSE patients.
To further evaluate the intrathecal antibody production, in addition to the conventional method comparing titers to those obtained with a morbillus antigen as mentioned above, we here diluted the serum and CSF samples to an identical IgG concentration (8) and then calculated the CSF/serum sample ratios at Vmax. The purpose of this method was to assay the antibody reaction at its most dynamic phase. At this time point, the signal differentiated the most between the CSF and serum samples, probably best reflecting intrathecal antibody production. In our opinion, this may be a more sensitive and specific method than calculating the serum/CSF sample ratios of endpoint titers obtained in ELISAs and comparing them to titers of a reference antibody (17). However, further studies of serological methods on PCR-verified cases of CNS infection seems warranted before a clear recommendation can be made.
In conclusion, VZV gE appears to be a sensitive and specific antigen for the assessment of intrathecal antibody production against VZV, being devoid of cross-reactivity to HSV-1 antibodies in patients with HSE. In addition, we suggest that determining the intrathecal antibodies as a CSF/serum OD ratio at Vmax after dilution of CSF and serum samples to an identical IgG concentration may be a more precise method than that of calculating the CSF/serum ratios of endpoint titers and comparing the titers to those of a reference virus. Lastly, our results are compatible with a higher avidity of the CSF IgG antibodies to the gE antigen, compared with those reactive with the conventional whole-VZV membrane antigen, and further studies will address the phenomena.
ACKNOWLEDGMENTS
This work was supported by the Swedish Medical Research Council, the LUA-ALF and R&D Foundations of Sahlgren's University Hospital, and the A. Lundgren Foundation.
We also thank Maria Johansson at the Virological Laboratory, Sahlgren's University Hospital, for skillful technical assistance.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Aurelius E., Johansson B., Skoldenberg B., Staland A., Forsgren M. 1991. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337:189–192 [DOI] [PubMed] [Google Scholar]
- 2. Breuer J., Schmid D. S., Gershon A. A. 2008. Use and limitations of varicella-zoster virus-specific serological testing to evaluate breakthrough disease in vaccinees and to screen for susceptibility to varicella. J. Infect. Dis. 197(Suppl. 2):S147–S151 [DOI] [PubMed] [Google Scholar]
- 3. Casas I., Tenorio A., de Ory F., Lozano A., Echevarria J. M. 1996. Detection of both herpes simplex and varicella-zoster viruses in cerebrospinal fluid from patients with encephalitis. J. Med. Virol. 50:82–92 [DOI] [PubMed] [Google Scholar]
- 4. Edson C. M., Hosler B. A., Respess R. A., Waters D. J., Thorley-Lawson D. A. 1985. Cross-reactivity between herpes simplex virus glycoprotein B and a 63,000-dalton varicella-zoster virus envelope glycoprotein. J. Virol. 56:333–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forghani B., Schmidt N. J., Dennis J. 1978. Antibody assays for varicella-zoster virus: comparison of enzyme immunoassay with neutralization, immune adherence hemagglutination, and complement fixation. J. Clin. Microbiol. 8:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilden D., Cohrs R. J., Mahalingam R., Nagel M. A. 2009. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 8:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gregoire S. M., van Pesch V., Goffette S., Peeters A., Sindic C. J. 2006. PCR analysis and oligoclonal antibody in the cerebrospinal fluid from 34 patients with varicella-zoster virus infection of the nervous system. J. Neurol. Neurosurg. Psychiatry 77:938–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen K., Cruz M., Link H. 1990. Oligoclonal Borrelia burgdorferi-specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 161:1194–1202 [DOI] [PubMed] [Google Scholar]
- 9. Haumont M., et al. 1996. Purification, characterization and immunogenicity of recombinant varicella-zoster virus glycoprotein gE secreted by Chinese hamster ovary cells. Virus Res. 40:199–204 [DOI] [PubMed] [Google Scholar]
- 10. Hedman L., Soderlund-Venermo M., Jartti T., Ruuskanen O., Hedman K. 2010. Dating of human bocavirus infection with protein-denaturing IgG-avidity assays—secondary immune activations are ubiquitous in immunocompetent adults. J. Clin. Virol. 48:44–48 [DOI] [PubMed] [Google Scholar]
- 11. Jeansson S., Forsgren M., Svennerholm B. 1983. Evaluation of solubilized herpes simplex virus membrane antigen by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 18:1160–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keller P. M., Lonergan K., Neff B. J., Morton D. A., Ellis R. W. 1986. Purification of individual varicella-zoster virus (VZV) glycoproteins gpI, gpII, and gpIII and their use in ELISA for detection of VZV glycoprotein-specific antibodies. J. Virol. Methods 14:177–188 [DOI] [PubMed] [Google Scholar]
- 13. Kitamura K., Namazue J., Campo-Vera H., Ogino T., Yamanishi K. 1986. Induction of neutralizing antibody against varicella-zoster virus (VZV) by VZV gp3 and cross-reactivity between VZV gp3 and herpes simplex viruses gB. Virology 149:74–82 [DOI] [PubMed] [Google Scholar]
- 14. Koskiniemi M., et al. 2002. Acute central nervous system complications in varicella zoster virus infections. J. Clin. Virol. 25:293–301 [DOI] [PubMed] [Google Scholar]
- 15. Kuhn J. E., Klaffke K., Munk K., Braun R. W. 1990. HSV-1 gB and VZV gp-II crossreactive antibodies in human sera. Arch. Virol. 112:203–213 [DOI] [PubMed] [Google Scholar]
- 16. Kutinova L., Hainz P., Ludvikova V., Maresova L., Nemeckova S. 2001. Immune response to vaccinia virus recombinants expressing glycoproteins gE, gB, gH, and gL of varicella-zoster virus. Virology 280:211–220 [DOI] [PubMed] [Google Scholar]
- 17. Linde A., et al. 1997. Specific diagnostic methods for herpesvirus infections of the central nervous system: a consensus review by the European Union Concerted Action on Virus Meningitis and Encephalitis. Clin. Diagn. Virol. 8:83–104 [DOI] [PubMed] [Google Scholar]
- 18. Montalvo E. A., Parmley R. T., Grose C. 1985. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J. Virol. 53:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Persson A., Bergstrom T., Lindh M., Namvar L., Studahl M. 2009. Varicella-zoster virus CNS disease—viral load, clinical manifestations and sequels. J. Clin. Virol. 46:249–253 [DOI] [PubMed] [Google Scholar]
- 20. Puchhammer-Stockl E., Popow-Kraupp T., Heinz F. X., Mandl C. W., Kunz C. 1991. Detection of varicella-zoster virus DNA by PCR in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J. Clin. Microbiol. 29:1513–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raschilas F., et al. 2002. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin. Infect. Dis. 35:254–260 [DOI] [PubMed] [Google Scholar]
- 22. Roberg M., Forsberg P., Tegnell A., Ekerfeldt K. 1995. Intrathecal production of specific IgA antibodies in CNS infections. J. Neurol. 242:390–397 [DOI] [PubMed] [Google Scholar]
- 23. Schmidt N. J., Lennette E. H., Magoffin R. L. 1969. Immunological relationship between herpes simplex and varicella-zoster viruses demonstrated by complement-fixation, neutralization and fluorescent antibody tests. J. Gen. Virol. 4:321–328 [DOI] [PubMed] [Google Scholar]
- 24. Schultze D., et al. 2004. Diagnostic significance of intrathecally produced herpes simplex and varizella-zoster virus-specific antibodies in central nervous system infections. Swiss Med. Wkly. 134:700–704 [DOI] [PubMed] [Google Scholar]
- 25. Skoldenberg B. 1991. Herpes simplex encephalitis. Scand. J. Infect. Dis. Suppl. 80:40–46 [PubMed] [Google Scholar]
- 26. Studahl M., Bergstrom T., Hagberg L. 1998. Acute viral encephalitis in adults—a prospective study. Scand. J. Infect. Dis. 30:215–220 [DOI] [PubMed] [Google Scholar]
- 27. Thomsson E., et al. 2011. Recombinant glycoprotein E produced in mammalian cells in large-scale as an antigen for varicella-zoster-virus serology. J. Virol. Methods 175:53–59 [DOI] [PubMed] [Google Scholar]
- 28. Vandvik B., et al. 1985. Long-term persistence of intrathecal virus-specific antibody responses after herpes simplex virus encephalitis. J. Neurol. 231:307–312 [DOI] [PubMed] [Google Scholar]