Abstract
The indirect hemagglutination assay (IHA) is the most frequently used serological test to confirm exposure to Burkholderia pseudomallei. Patients with culture-confirmed disease often have a nonreactive IHA at presentation and occasionally fail to seroconvert on serial testing. We investigated whether using antigens derived from the cultured isolates of persistently IHA-nonreactive patient sera improved the sensitivity of the IHA. In addition, we assessed the antigen-specific lymphocyte response in this group of patients to a panel of B. pseudomallei antigens, including those derived from their own cultured isolates. Eleven patients with culture-proven melioidosis were identified as having persistently IHA-nonreactive sera. A modified IHA using erythrocytes sensitized with patient isolate-derived antigen tested against convalescent-phase serum was performed. The majority (82%) of sera showed a negative (≤1:5) result, one was borderline (1:20), and one was positive at the cutoff value (1:40). IHA-nonreactive sera were also tested by enzyme immunoassay (EIA), with 73% (8/11) demonstrating IgG positivity. In addition, lymphocytes isolated from persistently IHA-nonreactive patient sera demonstrated significantly increased proliferation in response to B. pseudomallei antigens compared to controls. These studies demonstrate the presence of B. pseudomallei-specific antibody by EIA and B. pseudomallei-specific lymphocytes in patient sera categorized as persistently nonreactive according to the IHA. New immunoassays are required and should incorporate B. pseudomallei antigens that are immunoreactive for this subset of IHA-nonreactive patient sera.
INTRODUCTION
Melioidosis, a disease endemic to northern Australia and southeast Asia, is caused by the Gram-negative soil saprophyte Burkholderia pseudomallei. It causes significant morbidity and mortality, with a wide spectrum of clinical presentations (15, 19). The gold standard for the diagnosis of melioidosis is culture from clinical specimens. However, serological and possibly cellular assays may have an adjunctive role in certain situations, such as screening travelers returning from areas of endemicity with a febrile illness or aiding diagnosis in unusual presentations (e.g., chronic disease) or where specimens for culture may be unavailable (e.g., deep brain abscesses). It may also provide supportive information if melioidosis is suspected but the organism fails to grow. Simple, rapid, and reliable serological tests for melioidosis hold the possibility of identifying cases earlier and thereby improving outcomes, given that culture and identification of B. pseudomallei can be delayed. Furthermore, early diagnosis and selection of appropriate antimicrobial therapy may help reduce the significant mortality associated with the disease.
The indirect hemagglutination assay (IHA) has been the mainstay of serological testing for melioidosis over many years, and the technique has remained largely unchanged since first described over 40 years ago (12). Despite variable sensitivity and specificity, it remains the most commonly employed serological test, with titers of 1:40 or greater considered reactive in Australia (2). High background rates of seropositivity have been described in areas of endemicity and can reduce the utility of the test (16). Several patterns of serological responses have been described in previous studies that examined serial IHA testing over time, including late seroconversion and persistently reactive and persistently nonreactive tests as well as seroreversion (8, 11). The range of titers in seropositive specimens is often wide. A critical limitation of the assay is the lack of standardization between laboratories with respect to the antigens used. The IHA relies upon the agglutination of sheep red cells in the presence of serum antibodies to polysaccharide and lipopolysaccharide antigens derived from defined strains of B. pseudomallei. However, these antigens remain poorly characterized and are likely to be variable between isolates.
In the Townsville Hospital (Queensland, Australia) cohort of patients with culture-proven melioidosis, approximately 50% were seronegative by IHA at presentation (11). Furthermore, a significant proportion (approximately 10%) of these failed to seroconvert on serial testing over time, with no specific clinical features clearly associated with this phenomenon (11). We postulated that the limited number of B. pseudomallei strains used to provide sensitizing antigens for the IHA may account for this failure to detect the presence of antibody. Thus, in this study we aimed to employ antigens derived from B. pseudomallei isolates cultured from individual IHA-nonreactive patient serum samples to be tested in an IHA format against convalescent-phase sera from the same patients. IHA-nonreactive sera were also tested by enzyme immunoassay (EIA) to detect B. pseudomallei-specific IgG. We also performed lymphocyte proliferation assays to determine whether patients who had IHA-nonreactive sera had developed a B. pseudomallei-specific cell-mediated immune (CMI) response (13). Our hypothesis was that patients with persistently IHA-nonreactive sera may demonstrate seroreactivity if antigens derived from their own cultured isolates were used in the assay preparation. Since antibody detection may not necessarily reflect the totality of the patient's immune response or protective immunity, we aimed to additionally demonstrate evidence of specific CMI to B. pseudomallei in these individuals.
MATERIALS AND METHODS
Study setting and patient selection.
Townsville Hospital, a tertiary referral center for tropical north Queensland, services a population of approximately 250,000. Melioidosis is a relatively common condition in this region, especially during the rainy season (approximately from December to February). We also receive isolates from patients admitted and treated in district hospitals. We retrospectively examined the records of all patients with culture-confirmed melioidosis from clinical specimens in our laboratory between January 1996 and January 2010. All patients showing a persistently nonreactive IHA (tested at baseline and at least 1 month after presentation) and for whom sera and bacterial isolates had been stored were included. All available patients with persistently IHA-nonreactive sera who had culture-proven melioidosis were requested to participate in further studies of cell-mediated immunity. Those enrolled were paired with an age- and sex-matched control group of healthy individuals from the same geographic region with no history of melioidosis and IHA-negative serology. Ethical approval for this study was obtained from the Townsville Health Service District Ethics Committee.
Indirect hemagglutination assay.
The standard IHA was performed as previously described using sheep erythrocytes sensitized with antigens from five selected strains of B. pseudomallei (1). Serum samples were heat inactivated and then incubated with nonsensitized sheep red cells to remove nonspecific agglutinins. Sample sera were then titrated in microwell plates, and antigen-sensitized red cells were added. The presence of antibody was confirmed by red cell agglutination. A titer of ≥1:40 was considered positive. Nonsensitized ovine red cells were used as controls. The modified IHA used different sensitizing antigens, prepared as follows. Isolates of B. pseudomallei cultured from each of the patients with persistently IHA-nonreactive sera were taken from frozen storage (−80°C), incubated on Columbia horse blood agar in ambient air at 37°C for 24 h, and checked for purity. Antigens were then prepared from these isolates using a crude heat-killed and filtered extract. Back titrations against known IHA-reactive serum (at a titer of 1:80) were performed on each antigen to determine optimal antigen concentrations. Some antigen preparations demonstrated gross red cell hemolysis unless serially diluted. As a result, optimal concentrations of these antigens could not be determined, and a presumptive 1:40 antigen dilution was used. An IHA with red cells sensitized with patient-derived antigen tested against the patient's own convalescent-phase serum along with low-positive (1:80), high-positive (1:320), and negative (<1:5) controls was then performed using the method previously described by Ashdown (1).
Enzyme immunoassay IgG.
The EIA to detect B. pseudomallei IgG was performed as previously described, with minor modifications (3). Briefly, antigens were prepared from eight defined strains of B. pseudomallei by heating, sonication, and filtration. Optimal antigen dilution was obtained by titration against known IgG-positive and -negative controls. EIA plates (Nunc, Copenhagen, Denmark) were coated with 100 μl of diluted antigen in borate-saline buffer and incubated overnight at 4°C. To eliminate nonspecific binding, the plates were then blocked using 5% bovine serum albumin by incubation at 35°C for 90 min and washed with phosphate-buffered saline (PBS) plus 0.05% Tween 20 solution (PBS-Tween). Samples were then tested in duplicate using 50 μl of patient serum diluted 1:100 with PBS-Tween and incubated at room temperature for 30 min. The plates were then washed, 50 μl of horseradish peroxidase-IgG conjugate (Panbio, Australia) was added, and then plates were incubated for a further 30 min. After another washing step, 100 μl of tetramethylbenzidine and hydrogen peroxide (TMB; Panbio, Australia) substrate was added. After 10 min the reaction was terminated using 1 M phosphoric acid, and the reactions were read by automated plate reader at 450/620 nm (Multiskan; Flow Laboratories, McLean, VA). All test sera were run with negative, low-positive, and high-positive controls. Results were expressed as enzyme immunoassay units (EIU) calculated from the following formula: EIU = (test absorbance − negative-control absorbance)/(low-positive-control absorbance − negative-control absorbance) × 100. EIU values of ≤25 were considered negative, values between 26 and 50 were considered equivocal, and values that were >50 were considered positive.
Lymphocyte proliferation assay.
A panel of seven B. pseudomallei crude antigens were prepared from seven clinical B. pseudomallei strains comprised of six strains isolated from each of the patients with IHA-nonreactive sera who were participating in this study and one previously characterized B. pseudomallei isolate of low virulence, NCTC 13179 (13). Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by density centrifugation over Ficoll-Paque Plus (GE Healthcare Biosciences, Australia) and cultured as previously described (5). Triplicate wells (1 × 105 PBMC/well) were stimulated with the seven B. pseudomallei antigens (1 μg/ml) and phytohemagglutinin ([PHA] positive control; 10 μg/ml). Cultures were then incubated at 37°C with 5% CO2. Proliferative responses were assessed at 24-h intervals between 96 and 168 h of culture by determining [3H]thymidine incorporation (1.25 μCi/ml for 4 h; GE Healthcare Biosciences, Australia) with outputs measured in counts per minute (cpm). Stimulation indexes (SI) were calculated (SI = cpmstimulated/cpmunstimulated). The highest SI recorded over the four time points was selected to compare the proliferative response of PBMC from IHA-nonreactive patient sera to samples from healthy controls.
RESULTS
During the study period from January 1996 to January 2010, 177 patients were identified with culture-confirmed melioidosis. Of those that had serial IHA testing performed, 14 had persistently nonreactive sera over time. Eleven of these patients had stored sera and bacterial isolates available and were included in the study. Six of the patients (two males and four females) with IHA-nonreactive sera were also available for CMI studies.
Indirect hemagglutination assay.
During IHA antigen titration, 4 of the 11 B. pseudomallei antigen preparations showed gross red cells hemolysis unless serially diluted, possibly suggesting the presence of a hemolysin, a phenomenon that has been previously observed with B. pseudomallei (4). Nine samples showed a negative (≤1:5) result in the modified IHA using the individual patient-derived antigen, one was borderline (1:20), and one was positive at the cutoff value (1:40) (Τable 1). All nonreactive test sera showed appropriate results in positive and negative controls.
Table 1.
Comparison of IHA results from culture-positive persistently nonreactive patient sera by a standard IHA with an IHA using patient isolate-derived antigens and EIA IgG
| Isolate no. | Standard IHA titer | Modified IHA titer (dilution)a | Control IHA titer (dilution) |
EIA IgG | ||
|---|---|---|---|---|---|---|
| Low positive (1:80) | High positive (1:320) | Nonsensitized red cells | ||||
| 1 | <1:5 | <1:5 (1:10) | 1:160 | 1:640 | <1:5 | Pos |
| 2 | <1:5 | <1:5 (1:10) | 1:80 | 1:320 | <1:5 | Equiv |
| 3 | <1:5 | <1:5 (1:40) | 1:80 | 1:80 | <1:5 | Neg |
| 4 | <1:5 | <1:5 (1:40) | 1:80 | 1:320 | <1:5 | Pos |
| 5 | 1:5 | <1:5 (1:40) | 1:160 | 1:320 | <1:5 | Pos |
| 6 | <1:5 | <1:5 (1:10) | 1:80 | 1:1,280 | <1:5 | Pos |
| 7 | <1:5 | <1:5 (1:40) | 1:80 | 1:320 | <1:5 | Pos |
| 8 | 1:5 | 1:40 (1:40) | 1:320 | 1:1,280 | <1:5 | Pos |
| 9 | 1:10 | <1:5 (1:40) | 1:40 | 1:160 | <1:5 | Pos |
| 10 | <1:5 | 1:5 (1:40) | 1:160 | 1:320 | <1:5 | Neg |
| 11 | 1:20 | 1:20 (1:10) | 1:160 | 1:1,280 | <1:5 | Pos |
Patient isolate-derived antigen.
Enzyme immunoassay IgG.
All 11 patients with a nonreactive IHA were tested by EIA to detect B. pseudomallei-specific IgG. Of these, eight (73%) were positive on at least one convalescent-phase serum sample, two were negative, and one was equivocal (Table 1).
Lymphocyte proliferation.
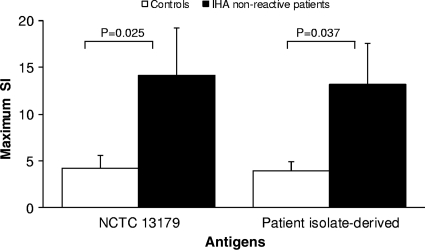
When stimulated with B. pseudomallei antigens, lymphocytes from IHA-nonreactive patient sera demonstrated significantly higher proliferation than lymphocytes from controls (Fig. 1). In addition, the B. pseudomallei-specific lymphocyte proliferative response of IHA-nonreactive patient sera was elicited in response to both NCTC 13179 and patient isolate-derived antigen.
Fig. 1.
Lymphocyte proliferation in IHA-nonreactive patient sera in response to B. pseudomallei antigens. PBMC isolated from the peripheral blood of IHA-nonreactive patient sera and controls were stimulated with B. pseudomallei antigens generated from either NCTC 13179 or individual isolates derived from the IHA-nonreactive patient sera. Proliferative responses were assessed at 24-h intervals between 96 and 168 h of culture by determining [3H]thymidine incorporation. Results are expressed as mean maximum SIs ± standard error of the mean. Maximum SIs were significantly higher in PBMC from patients with IHA-nonreactive sera than in samples from healthy controls following stimulation with antigens from either the patients' own bacterial isolates or isolate NCTC 13179. No significant differences were observed between the maximum SIs of patient PBMC stimulated with either isolate.
DISCUSSION
Antigens derived from isolates of B. pseudomallei cultured from IHA-nonreactive patient sera did not improve assay sensitivity in a modified IHA. The initial hypothesis that the poor test characteristics of the IHA reflect the use of a limited array of sensitizing antigens was not supported by the findings. Although the use of specific antigens derived from individual patients could not demonstrate the presence of antibody in corresponding sera from the same patients, these antigens were recognized appropriately by sera from known seropositive controls. Thus, it seems unlikely that the apparent failure to seroconvert is a product of the isolate itself or the antigens that are presented within the IHA format. A possible explanation might be that these patients had not developed antibodies to the particular antigens that are presented in the IHA format. To our knowledge, characterization of the B. pseudomallei antigens displayed by erythrocytes in the IHA has not been investigated. Sera from patients in the current study (4 out of 5) demonstrated seroconversion for hepatitis B surface antibody after vaccination, making anergy an unlikely explanation for the lack of antibodies reactive toward B. pseudomallei antigens in the IHA.
Importantly, the majority (73%) of IHA-nonreactive samples were found to be IgG positive by EIA. This suggests that specific antibodies were produced in response to B. pseudomallei infection in this subset of individuals; however, these were not directed against or were unable to bind the specific epitopes presented on the surface of erythrocytes in the IHA. These findings provide further evidence to support previous studies demonstrating the poor sensitivity of the IHA and the superior sensitivity of the EIA in the diagnosis of melioidosis (3, 11). However, while the EIA may be more sensitive than the IHA in confirming the diagnosis of melioidosis, the tendency of sera from patients with melioidosis to remain reactive by EIA for long periods after acute infection means that this assay may not be an effective tool for monitoring disease progression. Its specificity for diagnosing acute infection may also be affected by high background seroreactivity in the population (20). The specificity of the EIA, using the same method and in the same population as in this study, has previously been shown to be 95% (3). However, a fundamental problem of most B. pseudomallei EIAs is that they are rarely validated outside the laboratory that originally developed the assay or tested in a wide variety of populations. A recent study from Thailand using Bayesian latent-class models to generate new cutoff values for EIAs that employed five different antigens has suggested that the sensitivity and specificity of these assays may be much improved when one considers that the gold standard of microbiological culture is in itself imperfect (14).
We have previously used lymphocyte proliferation assays to provide evidence for the development of B. pseudomallei-specific CMI responses in patients who have recovered from melioidosis (5, 13). Using the same technique, this study has demonstrated that patients with IHA-nonreactive sera develop a strong B. pseudomallei-specific CMI response. In accordance with the modified IHA used in this study, no additional benefit was provided by using patient isolate-derived antigen to stimulate lymphocytes isolated from IHA-nonreactive patient sera. Demonstration of specific CMI responses in patients with melioidosis continues to be an underutilized indicator of exposure to B. pseudomallei. Findings of Barnes et al. (5) suggest a tentative link between the development of a B. pseudomallei-specific CMI response and protection against disease progression (5). Due to the intracellular nature of B. pseudomallei, CMI responses may play a more important role than antibodies in recovery from infection and in the development of immunity to this pathogen. Therefore, the incorporation of antigens triggering strong CMI responses in diagnostic tests and assays for monitoring disease progression may prove to be more clinically relevant than the current assays based on antibody responses. Furthermore, the use of molecular diagnostic techniques, such as nucleic acid detection, holds promise for the rapid recognition of melioidosis (10, 17) although some assays have lacked sufficient sensitivity (6, 7).
Limitations of this study are acknowledged. First, the number of participants in this study was limited by the relatively small proportion of patients with confirmed melioidosis demonstrating persistently IHA-nonreactive sera. Furthermore, the surprisingly high rate of erythrocyte hemolysis seen in some IHA antigen preparations made determination of the optimal antigen concentration uninterpretable for these isolates. However, the high- and low-positive controls were reasonably concordant in almost all cases, making major dilution errors less likely.
To date, our understanding of the immunopathogenesis of B. pseudomallei is limited. Antigens involved in the development of protective specific immunity have not been identified. Additional investigations are warranted to better characterize the immunological responses in patients with melioidosis, particularly those that remain persistently IHA negative, and to understand the reasons for the observed limitations of serological testing. Such an understanding is critical to the development of new immunoassays and effective vaccines for melioidosis. Identification of immunodominant antigens will be essential to this process, and recent work has begun to elucidate this area (9, 18).
In summary, the use of extracts of B. pseudomallei isolated from patients with IHA-nonreactive sera and culture-confirmed melioidosis as sensitizing antigens in a modified IHA tested against the patients' own convalescent-phase sera did not improve assay sensitivity. Our data suggest that the poor sensitivity of the IHA does not reflect the limited choice of strains used in the antigen preparation for the assay. Furthermore, the majority of patients with IHA-nonreactive sera were seroreactive for IgG as measured by EIA. We have also demonstrated that patients with IHA-nonreactive sera developed a strong B. pseudomallei-specific adaptive CMI response. These findings identify additional shortcomings in the current standard serological assay used in the diagnosis of melioidosis. The immunodominant antigens of B. pseudomallei have not yet been identified. However, the procedures for the preparation of antigens used in the IHA, EIA, and lymphocyte proliferation assay are different. As such, variations in the immunogenicity and concentration of bacterial antigens present in each preparation may alter the immune response being measured. The results suggest that the B. pseudomallei antigens used in the IHA format may not be sufficiently immunogenic for a subset of patients with melioidosis. Further work to identify immunodominant antigens of B. pseudomallei is warranted to facilitate the development of more reliable and sensitive diagnostic assays.
ACKNOWLEDGMENTS
We thank the Townsville Hospital Foundation and James Cook University for funding this project.
We thank the laboratory staff of the Townsville Hospital serology department for providing technical assistance with the assays.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 15 June 2011.
REFERENCES
- 1. Ashdown L. R. 1987. Indirect haemagglutination test for melioidosis. Med. J. Aust. 147:364–365 [DOI] [PubMed] [Google Scholar]
- 2. Ashdown L. R., Guard R. W. 1984. The prevalence of human melioidosis in Northern Queensland. Am. J. Trop. Med. Hyg. 33:474–478 [DOI] [PubMed] [Google Scholar]
- 3. Ashdown L. R., Johnson R. W., Koehler J. M., Cooney C. A. 1989. Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J. Infect. Dis. 160:253–260 [DOI] [PubMed] [Google Scholar]
- 4. Ashdown L. R., Koehler J. M. 1990. Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J. Clin. Microbiol. 28:2331–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes J. L., et al. 2004. Adaptive immunity in melioidosis: a possible role for T cells in determining outcome of infection with Burkholderia pseudomallei. Clin. Immunol. 113:22–28 [DOI] [PubMed] [Google Scholar]
- 6. Chantratita N., et al. 2008. Loop-mediated isothermal amplification method targeting the TTS1 gene cluster for detection of Burkholderia pseudomallei and diagnosis of melioidosis. J. Clin. Microbiol. 46:568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chantratita N., et al. 2007. Prospective clinical evaluation of the accuracy of 16S rRNA real-time PCR assay for the diagnosis of melioidosis. Am. J. Trop. Med. Hyg. 77:814–817 [PubMed] [Google Scholar]
- 8. Cheng A. C., O'Brien M., Freeman K., Lum G., Currie B. J. 2006. Indirect hemagglutination assay in patients with melioidosis in northern Australia. Am. J. Trop. Med. Hyg. 74:330–334 [PubMed] [Google Scholar]
- 9. Felgner P. L., et al. 2009. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc. Natl. Acad. Sci. U. S. A. 106:13499–13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gal D., Mayo M., Spencer E., Cheng A. C., Currie B. J. 2005. Short report: application of a PCR to detect Burkholderia pseudomallei in clinical specimens from patients with suspected melioidosis. Am. J. Trop. Med. Hyg. 73:1162–1164 [PubMed] [Google Scholar]
- 11. Harris P. N., Ketheesan N., Owens L., Norton R. E. 2009. Clinical features that affect indirect-hemagglutination-assay responses to Burkholderia pseudomallei. Clin. Vaccine Immunol. 16:924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ileri S. Z. 1965. The indirect haemagglutination test in the diagnosis of melioidosis in goats. Br. Vet. J. 121:164–170 [DOI] [PubMed] [Google Scholar]
- 13. Ketheesan N., et al. 2002. Demonstration of a cell-mediated immune response in melioidosis. J. Infect. Dis. 186:286–289 [DOI] [PubMed] [Google Scholar]
- 14. Limmathurotsakul D., et al. 2011. Enzyme-linked immunosorbent assay for the diagnosis of melioidosis: better than we thought. Clin. Infect. Dis. 52:1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malczewski A. B., Oman K. M., Norton R. E., Ketheesan N. 2005. Clinical presentation of melioidosis in Queensland, Australia. Trans. R. Soc. Trop. Med. Hyg. 99:856–860 [DOI] [PubMed] [Google Scholar]
- 16. Sirisinha S. 1991. Diagnostic value of serological tests for melioidosis in an endemic area. Asian Pac. J. Allergy Immunol. 9:1–3 [PubMed] [Google Scholar]
- 17. Supaprom C., et al. 2007. Development of real-time PCR assays and evaluation of their potential use for rapid detection of Burkholderia pseudomallei in clinical blood specimens. J. Clin. Microbiol. 45:2894–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson D. B., et al. 2008. In silico analysis of potential diagnostic targets from Burkholderia pseudomallei. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl. 1):S61–S65 [DOI] [PubMed] [Google Scholar]
- 19. White N. J. 2003. Melioidosis. Lancet 361:1715–1722 [DOI] [PubMed] [Google Scholar]
- 20. Wuthiekanun V., et al. 2004. Evaluation of immunoglobulin M (IgM) and IgG rapid cassette test kits for diagnosis of melioidosis in an area of endemicity. J. Clin. Microbiol. 42:3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]