Abstract
Immunoassay for detection of glucuronoxylomannan (GXM), the major capsular polysaccharide of Cryptococcus neoformans, is an important tool for diagnosis of cryptococcosis. However, immunoassays that are based solely or in part on detection with polyclonal antibodies may show serotype bias in detection of GXM, particularly limited sensitivity for serotype C. In this study, we describe detection of GXM in an antigen capture sandwich enzyme-linked immunosorbent assay (ELISA) that used a cocktail of two monoclonal antibodies (MAbs). MAb F12D2 was previously produced by immunization with GXM that had been treated to remove O-acetyl groups, a major source of serotype specificity. MAb F12D2 has a high degree of reactivity with GXM of serotypes A, B, C, and D, but the reactivity with serotype D was less than was found with other MAbs. MAb 339 is highly reactive with GXM of serotypes A and D. Use of a combination of the two MAbs produced an immunoassay that had the best properties of both MAbs, including good reactivity with serotype C, which is an emerging threat in sub-Saharan Africa. These results suggest that next-generation immunoassays for diagnosis of cryptococcosis may be formulated by (i) use of immunization and hybridoma screening strategies that are designed to prospectively meet the needs of immunoassay performance and (ii) careful selection of MAbs that span the expected polysaccharide serotypes in the subject patient population.
INTRODUCTION
Immunoassay for the capsular polysaccharide antigen of Cryptococcus neoformans has played an important role in diagnosis of cryptococcosis since introduction of the latex agglutination (LA) assay in 1963 (5). Immunoassays currently approved for diagnosis of cryptococcosis include latex agglutination and antigen capture sandwich enzyme-linked immunosorbent assay (ELISA). The antigen target for immunoassays in diagnosis of cryptococcosis is glucuronoxylomannan (GXM), the primary constituent of the capsule. GXM is a polysaccharide that has a (1→3)-α-mannopyran backbone with single β-d-xylopyranosyl and β-d-glucopyranosyluronic acid substituents (4, 11). The mannose backbone is variably O acetylated at C-6 (28). GXM occurs in four major serotypes, A, B, C, and D (29), and an intermediate AD serotype (15). The degrees of xylose substitution and O acetylation are the primary determinants of the structure for GXM of each serotype. The O-acetyl substituent is immunodominant in induction of antibodies to GXM and in recognition of GXM by polyclonal antibodies (9, 18) and monoclonal antibodies (MAbs) (2, 7, 12).
The classical latex agglutination assay for detection of cryptococcal polysaccharide in serum and cerebrospinal fluid (CSF) was produced with antibodies from rabbits that were immunized with whole yeast cells (5). Given the presence of O-acetyl groups on GXM of all serotypes and the immunodominance of the O-acetyl-dependent epitopes, it is likely that the serotype specificity of immunoassays that rely on polyclonal antibodies will reflect the antibody response of the rabbits to immunodominant O-acetyl epitopes.
With the emergence of serotype C as a relatively common cause of cryptococcosis in sub-Saharan Africa (20, 27), we reevaluated the serotype specificity of several commercially available immunoassays for GXM. Notably, even the first report of a latex agglutination assay described reduced reactivity with serotype C (5). Our results found reduced sensitivity for detection of purified GXM of serotype C. We next evaluated the utility of individual GXM MAbs for construction of an immunoassay with limited serotype bias. The results show that a cocktail of MAbs can be used to produce an immunoassay in enzyme-linked immunosorbent assay (ELISA) format that is highly sensitive for detection of GXM of all four major serotypes.
MATERIALS AND METHODS
C. neoformans and GXM.
C. neoformans strains used in this study were originally provided by R. Cherniak (Georgia State University, Atlanta, GA) and are maintained as frozen stock cultures. The chemotypes and structural components of these strains, as defined by Cherniak et al. (11), are summarized in Table 1. There is considerable variability in the expression of various structure reporter groups among strains of different serotypes. For this study, we selected representative strains on the basis of the chemotype and structure reporter types typical of each of the four major serotypes (1). GXM was isolated from supernatant fluids from broth cultures of each strain as described previously (7). Briefly, yeast cells were grown for 4 days at 30°C on synthetic medium (10) and killed by overnight treatment with formaldehyde. GXM was isolated and purified by differential precipitation with two cycles of precipitation with hexadecyltrimethylammonium bromide (CTAB) and ethanol as described previously (8). Following differential precipitation, GXM was solubilized in acetate buffer (10% sodium acetate crystals and 1% acetic acid) and reprecipitated two times with ethanol to remove residual CTAB. The precipitate was dried by washing with absolute ethanol followed by acetone.
Table 1.
Serotype, chemotype, and GXM structure of C. neoformans strains a
| Strain | Serotype | Chemotype | % of repeating units with structure reporter group: |
|||||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | |||
| CN6 | A | 5 | 67 | 17 | 15 | |||
| MU-1 | A | 4 | 100 | |||||
| 184 | B | 5 | 10 | 86 | 4 | |||
| 409 | B | 7 | 100 | |||||
| 34 | C | 8 | 33 | 67 | ||||
| 298 | C | 8 | 48 | 52 | ||||
| 24066 | C | 8 | 100 | |||||
| 127 | D | 1 | 73 | 27 | ||||
| M0024 | D | 1 | 26 | 74 | ||||
Serotype, chemotype, and structure reporter group data are from reference 11 and R. Cherniak (personal communication).
MAbs.
The GXM MAbs used in this study were 3C2, 471, 339, 1255, and F12D2. The production and characteristics of these MAbs have been reported previously (3, 7, 12, 19, 25). Key features of the antibodies are summarized in Table 2.
Table 2.
Characteristics of GXM MAbs
| MAb | GXM used for immunization | Isotype | Serotype reactivity | Reference(s) |
|---|---|---|---|---|
| 3C2 | Serotype C | IgG1 | A, B, C, and D | 3, 19, 25 |
| 471 | Serotype A | IgG1 | A, B, C, and D | 3, 19, 25 |
| 339 | Serotype B | IgG1 | A, B, and D | 3, 19, 25 |
| 1255 | Serotype A | IgG1 | A, B, and D | 3, 12, 19 |
| F12D2 | De-O-Aa | IgG1b | A, B, C, and D | 7 |
De-O-A, de-O-acetylated GXM of serotype A.
The F12D2 MAb used in this study is a subclass switch variant (IgG3→IgG1) of MAb F12D2 described in reference 7.
Immunoassays for detection of GXM.
All immunoassays were done at room temperature (21°C). The following kits for detection of GXM were obtained from the indicated manufacturers: Latex-Cryptococcus antigen test (Immuno-Mycologics, Inc.; lot 159CU), cryptococcal antigen latex agglutination system (CALAS; Meridian Bioscience, Inc.; lot 140100.256), Crypto-La test (Inverness Medical; lot 0916289), and Premier cryptococcal antigen (Meridian Bioscience, Inc.; lot 602096.064). Purified GXM was dissolved in phosphate-buffered saline (PBS) to produce a 2-mg/ml stock solution. For latex agglutination assays, the GXM stock was diluted using buffer provided for the respective assay. An identical 2-fold dilution series starting with 8 μg GXM per ml was used for study of all three kits. Assays were performed as recommended in the package insert for each product. For all assays, mixing of the latex beads with dilutions of GXM was done within 30 min after dilution of the purified GXM. Endpoints for agglutination were reported by observers on a scale of 0 to 4+ using descriptions from each kit to assign an agglutination score. For the Meridian CALAS, observers also referred to a supplied reaction photograph. Results were recorded from the observations of four independent observers. The results from the four observers on a scale of 0 to 4+ were plotted against the log of the GXM concentration in ng/ml. A linear regression was plotted through the linear portion of this semilog plot; the concentration of GXM in ng/ml that corresponded to a 2+ agglutination was calculated from the regression and taken as the endpoint.
The Premier cryptococcal antigen assay is an antigen capture immunoassay. The test was performed as indicated in the assay instructions using the 2-mg/ml GXM stock for each strain of each serotype. The stock solution was diluted to 200 μg GXM/ml immediately before use. Serial dilutions were prepared and the assay was performed according to manufacturer directions. Optical density at 450 nm (OD450) was recorded for all wells. The GXM concentration that produced an OD of 0.5 in a log-log plot of OD450 versus ng GXM per ml was calculated as described below and was reported as the endpoint.
An ELISA was constructed from the GXM MAbs listed in Table 2 for detection of GXM. In this immunoassay, microtiter plates were coated overnight with the capture MAb or a combination of MAbs (1 μg MAb per ml PBS). The plates were washed 3 times and blocked for 90 min with PBS-Tween (PBS containing 0.05% Tween 20). Use of Tween as a blocking agent is based on preliminary experiments which found that background levels are not further reduced by use of additional blocking agents, e.g., serum or powdered milk, below levels found by blocking with Tween alone (data not shown). Purified GXM in PBS-Tween solution was added in serial 2-fold dilutions and incubated for 90 min. The plates were washed 3 times with PBS-Tween, horseradish peroxidase (HRPO)-conjugated GXM MAb was added (1 μg/ml PBS-Tween plus 0.5% nonfat dry milk), and the plates were incubated for 90 min. Finally, the plates were washed 3 times with PBS-Tween and incubated for 30 min with TMB (3,3′,5,5′-tetramethylbenzidine) substrate (KPL, Gaithersburg, MD). Stop solution (1 M H3PO4) was added, and the absorbance was read at 450 nm. The log OD was plotted against the log antigen concentration in ng/ml. Control wells containing all reactants but with sample-free reaction buffer in place of the sample dilution were used to calculate background. All OD readings were corrected for background (typically ≤0.06) by subtracting background values. After correction for background, the log-log plot (log OD versus log GXM concentration) produces a straight line with a slope of 1 in the region of receptor excess, receptor excess in this case being an excess of capture antibody relative to the GXM analyte (21, 22). The data in the log-log plot were fitted to a linear regression, and the concentration of GXM in ng/ml that produced an OD of 0.5 was calculated from the regression and taken as the endpoint.
RESULTS
An initial experiment assessed the sensitivity of several commercially available immunoassays for detection of purified GXM from the four major serotypes. GXM from two isolates each of serotypes A, B, and D and three isolates of serotype C was used for analysis. The results (Table 3) showed sensitivity for serotypes A, B, and D for all kits that was largely in the range of 20 to 70 ng GXM/ml buffer. In contrast, there was at least 10-fold-less sensitivity for GXM of serotype C (range, 260 to >2,000 ng/ml).
Table 3.
Sensitivities of four commercially available immunoassays for detection of GXM of different serotypes
| Immunoassay | Assay formatb | Minimum concn of GXM (ng/ml) producing a positive resulta |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
|||||||
| CN6 | MU-1 | 184 | 409 | 34 | 298 | 24066 | 127 | M0024 | ||
| Immuno-Mycologics | LA | 24 | 32 | 27 | 68 | 432 | 260 | 460 | 62 | 63 |
| Meridian CALAS | LA | 18 | 20 | 52 | 22 | >2,000 | 470 | 360 | 44 | 65 |
| Inverness | LA | 38 | NTc | 64 | NT | >2,000 | 940 | >2,000 | 50 | NT |
| Meridian Premier | ELISA | 35 | 22 | 25 | 21 | >2,000 | >2,000 | >2,000 | 900 | 630 |
Results are shown for GXM isolated from different strains of each serotype. >2,000, GXM of some strains of serotype C produced negative results at all concentrations up to 2,000 ng/ml.
LA, latex agglutination; ELISA, antigen capture sandwich enzyme-linked immunosorbent assay.
NT, not tested.
Immunoassays were constructed in ELISA format using each of five GXM MAbs in the capture phase and the same HRPO-labeled MAb in the indicator phase. The results (Table 4) showed that MAbs F12D2, 3C2, and 471 performed well with GXM of serotypes A and B. MAbs 339 and 1255 performed best with serotype D GXM. MAb F12D2 performed best with serotype C.
Table 4.
Sensitivity of antigen capture ELISA constructed from various GXM MAbs for detection of GXM of the four major serotypes
| MAb used for construction of ELISAa | Minimum concn of GXM (ng/ml) producing a positive resultb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
||||||
| CN6 | MU-1 | 184 | 409 | 34 | 298 | 24066 | 127 | M0024 | |
| F12D2 | 0.40 | 0.41 | 0.41 | 0.47 | 6.8 | 2.1 | 2.3 | 1.9 | 2.9 |
| 3C2 | 0.77 | 0.61 | 0.67 | 0.77 | 26 | 5.2 | 6.7 | 2.5 | 2.8 |
| 471 | 0.58 | 0.45 | 0.60 | 0.56 | 360 | 30 | 37 | 4.3 | 2.3 |
| 339 | 0.82 | 0.90 | 8.1 | 7.5 | >2,000 | 1,200 | >2,000 | 1.1 | 0.56 |
| 1255 | 1.9 | 1.3 | 6.7 | 6.2 | 1,100 | 40 | 110 | 1.4 | 0.80 |
The indicated MAb was used for the capture phase of the ELISA, and a HRPO conjugate of the same MAb was used in the detection phase.
Results are shown for GXM isolated from different strains of each serotype. >2,000, GXM of some strains of serotype C at concentrations of 2,000 ng/ml failed to produce an OD of 0.5.
The results in Table 4 suggested that a combination of MAbs F12D2 and 339 might be best suited for detection of GXM of all four serotypes. Consequently, an experiment was done to evaluate the performance of each MAb alone or in combination in either the capture or the detection phase. The results (Table 5) showed that use of the different HRPO-labeled MAbs in the detection phase reflected the differential sensitivity noted in Table 4. Specifically, HRPO-F12D2 was less sensitive for serotype D, regardless of the MAb used for antigen capture. Optimal immunoassay performance was achieved by use of a combination (50:50) of MAb F12D2 (best sensitivity for GXM of serotypes A, B and C) and MAb 339 (best sensitivity for serotype D).
Table 5.
Optimization of ELISA performance using combinations of MAbs F12D2 and 339
| MAbs used for ELISA constructiona |
Minimum concn of GXM (ng/ml) producing a positive resultb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
|||||||
| Capture MAb(s) | Indicator MAb(s) | CN6 | MU-1 | 184 | 409 | 34 | 298 | 24066 | 127 | M0024 |
| F12D2 | F12D2 | 0.47 | 0.29 | 0.78 | 0.80 | 6.8 | 2.1 | 2.3 | 2.8 | 3.2 |
| F12D2 | 339 | 1.1 | 0.70 | 3.7 | 5.0 | 1,700 | 220 | >2,000 | 0.93 | 1.1 |
| F12D2 | F12D2/339 | 0.64 | 0.43 | 1.1 | 1.2 | 9.5 | 2.8 | 2.8 | 0.91 | 1.0 |
| 339 | F12D2 | 0.82 | 0.51 | 0.94 | 1.1 | 2,000 | 98 | 1,500 | 2.7 | 2.4 |
| 339 | 339 | 0.83 | 1.0 | 9.7 | 11 | >2,000 | 1,100 | >2,000 | 1.0 | 0.65 |
| 339 | F12D2/339 | 1.0 | 0.62 | 2.0 | 2.0 | >2,000 | 155 | 1,900 | 0.97 | 0.61 |
| F12D2/339 | F12D2 | 0.46 | 0.27 | 0.74 | 0.75 | 6.6 | 1.9 | 2.0 | 2.4 | 2.8 |
| F12D2/339 | 339 | 1.2 | 0.71 | 3.6 | 4.4 | 2,000 | 420 | >2,000 | 0.67 | 0.51 |
| F12D2/339 | F12D2/339 | 0.76 | 0.44 | 0.85 | 0.86 | 10 | 2.7 | 2.8 | 0.63 | 0.51 |
The indicated MAb or a 50:50 mixture of the two MAbs was used in either the capture phase of the ELISA or as a HRPO conjugate (indicator MAb[s]).
Results are shown for GXM isolated from different strains of each serotype. >2,000, GXM of some strains of serotype C at concentrations of 2,000 ng/ml failed to produce an OD of 0.5.
DISCUSSION
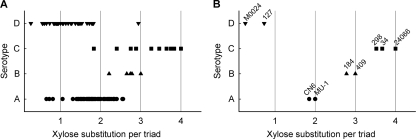
Glucuronoxylomannan occurs in four major serotypes (13, 14, 29). The primary structural differences between serotypes are the levels of O acetylation on the mannan backbone and the extent of xylose substitution (reviewed in reference 17). O acetylation is greatest and xylose substitution is least with serotype D; conversely, O acetylation is least and xylose substitution is greatest with serotype C. Serotypes A and B fall between these extremes. However, the structures of GXM from individual strains of each serotype are a continuum between the structures of serotypes D and C. This continuum is illustrated in Fig. 1A, which shows the levels of xylose substitution on 108 isolates of different serotypes (11). GXM preparations used for the present study were chosen to represent prototype GXM structures for each serotype, i.e., ratios of approximately 1, 2, 3, and 4 xylose residues per mannose triad for serotypes D, A, B, and C, respectively (Fig. 1B).
Fig. 1.
Serotype-dependent variability in extent of xylose substitution on the mannose backbone. (A) Data for 54 strains of serotype A, 7 strains of serotype B, 11 strains of serotype C, and 36 strains of serotype D. Values were calculated using information from reference 11 and R. Cherniak (personal communication). (B) Values for GXM from strains used in the present study.
The O-acetyl group is an immunodominant epitope on GXM (2, 9, 12, 18). As a consequence, antibodies that require O acetylation for recognition of GXM predominate in the polyclonal antibody response. This result predicts that polyclonal antibodies produced in response to polysaccharides with high levels of O acetylation, e.g., serotypes D and A, will have reduced reactivity with GXM from strains of serotype C, which has limited O acetylation. Conversely, antibodies produced in response to immunization with de-O-acetylated GXM are predicted to have more-limited reactivity with serotypes having high levels of O acetylation. Studies to date indicate that these predictions are, in fact, largely correct. For example, MAb F12D2 was produced in response to immunization with de-O-acetylated serotype A GXM. Results in Table 4 show that MAb F12D2 was more reactive with serotype C GXM than MAbs produced via immunization with native serotype A GXM (MAbs 471 and 1255). Indeed, the serotype C reactivity of MAb F12D2 exceeded that of MAb 3C2, which was produced by immunization with serotype C GXM. In another example, the original report of latex agglutination for detection of cryptococcal polysaccharide was based on the use of polyclonal antibody from rabbits immunized with whole cells (5). The latex beads had limited reactivity with serotype C polysaccharide.
In the present study, we relied on a MAb (F12D2) that was prepared from mice immunized with de-O-acetylated serotype A GXM as the foundation for immunoassay construction. Use of de-O-acetylated polysaccharide avoided the strong immunodominance of the O-acetyl group in the antibody response and allowed for isolation of MAbs specific for immunorecessive epitopes (7). MAb F12D2 proved to be highly reactive with GXM of serotypes A, B, C, and D but had less reactivity with serotype D than two other MAbs that were raised against fully acetylated GXM (MAbs 339 and 1255) (Table 4).
One means to compensate for deficiencies in any one MAb for detection of GXM of multiple serotypes is use of a cocktail of MAbs chosen to bridge such deficiencies. A combination of the panreactive MAb F12D2 with MAb 339, which was more reactive with serotype D, produced an immunoassay with the desirable traits of both antibodies. The cocktail produced an immunoassay with sensitivity for GXM of all four serotypes that exceeded the sensitivity found with any antibody alone (Table 5). The results further showed that deficiencies in reactivity across serotypes were most apparent when MAbs were used in the indicator phase. The increased avidity found with multivalent attachment during the capture phase likely compensates for lower binding activity.
Initial studies of cryptococcosis in patients with AIDS found a predominance of serotype A isolates, suggesting that serotype A selectively infected AIDS patients (6, 23, 24, 26). However, there are several recent reports of serotype C in AIDS patients in sub-Saharan Africa, where the frequency of serotype C has been reported to be as high as 14% (16, 20, 27). Given the more limited sensitivity of several available immunoassays for serotype C GXM, these reports of cryptococcosis produced by serotype C highlight the need for attention to serotype bias in assay construction.
Finally, several caveats should be noted. First, the comparative results shown for various immunoassays currently available for diagnosis of cryptococcosis are derived from use of purified GXM of different serotypes (Table 3). Classification of the strains is based on serotypic and structural analysis (11). The molecular types of these strains have not been determined. Second, reactivity of the MAbs with serotype AD GXM has not been evaluated. Given that the chemical and antigenic structure of serotype AD GXM falls between those of serotypes A and D, it is highly likely that reactivity of the cocktail of MAbs F12D2 and 339 will similarly fall between the results shown for serotypes A and D GXM. Third, these results are based on dilution of GXM in the buffer provided for each kit. In our experience, absolute sensitivity of an immunoassay for GXM (ng/ml) but not relative sensitivity for different serotypes can be influenced by buffer composition, time to assay, etc. (unpublished observations). Finally, our results do not reflect a clinical evaluation of patient serum or CSF, the relevant matrices for diagnosis of cryptococcosis. Moreover, we have made no assumptions regarding the assay sensitivity that is actually needed for diagnosis of cryptococcosis using clinical samples from a symptomatic patient. Our results, however, do suggest possible limitations of current immunoassays for diagnosis of infection by strains of serotype C. A clinical evaluation of immunoassays based on cocktails of GXM MAbs is in progress.
In summary, we found that several assays for detection of cryptococcal antigen have reduced sensitivity for serotype C GXM. Bias in sensitivity to different serotypes can be reduced by use of cocktails of MAbs chosen to be reactive with all four serotypes. Use of MAbs for detection of GXM of all four serotypes may be the basis for rational design of next-generation immunoassays for diagnosis of cryptococcosis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-014209 from the National Institute of Allergy and Infectious Diseases.
We thank Robert Cherniak for providing cultures and structural information on GXM from various isolates of C. neoformans.
The University of Nevada, Reno has licensed the use of MAbs F12D2 and 339 to Immuno-Mycologics, Inc., Norman, OK, for development of immunoassays for diagnosis of cryptococcosis.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Bacon B. E., Cherniak R., Kwon-Chung K. J., Jacobson E. S. 1996. Structure of the O-deacetylated glucuronoxylomannan from Cryptococcus neoformans Cap70 as determined by 2D NMR spectroscopy. Carbohydr. Res. 283:95–110 [DOI] [PubMed] [Google Scholar]
- 2. Belay T., Cherniak R. 1995. Determination of antigen binding specificities of Cryptococcus neoformans factor sera by enzyme-linked immunosorbent assay. Infect. Immun. 63:1810–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belay T., Cherniak R., Kozel T. R., Casadevall A. 1997. Reactivity patterns and epitope specificities of anti-Cryptococcus neoformans monoclonal antibodies by enzyme-linked immunosorbent assay and dot enzyme assay. Infect. Immun. 65:718–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharjee A. K., Bennett J. E., Glaudemans C. P. J. 1984. Capsular polysaccharides of Cryptococcus neoformans. Rev. Infect. Dis. 6:619–624 [DOI] [PubMed] [Google Scholar]
- 5. Bloomfield N., Gordon M. A., Elmendorf D. F., Jr. 1963. Detection of Cryptococcus neoformans antigen in body fluids by latex particle agglutination. Proc. Soc. Exp. Biol. Med. 114:64–67 [DOI] [PubMed] [Google Scholar]
- 6. Bottone E. J., Salkin I. F., Hurd N. J., Wormser G. P. 1987. Serogroup distribution of Cryptococcus neoformans in patients with AIDS. J. Infect. Dis. 156:242. [DOI] [PubMed] [Google Scholar]
- 7. Brandt S., Thorkildson P., Kozel T. R. 2003. Monoclonal antibodies reactive with immunorecessive epitopes of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans. Clin. Diagn. Lab Immunol. 10:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherniak R., Morris L. C., Anderson B. C., Meyer S. A. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherniak R., Reiss E., Slodki M. E., Plattner R. D., Blumer S. O. 1980. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol. Immunol. 17:1025–1032 [DOI] [PubMed] [Google Scholar]
- 10. Cherniak R., Reiss E., Turner S. H. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr. Res. 103:239–250 [Google Scholar]
- 11. Cherniak R., Valafar H., Morris L. C., Valafar F. 1998. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin. Diagn. Lab. Immunol. 5:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckert T. F., Kozel T. R. 1987. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect. Immun. 55:1895–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans E. E. 1950. The antigenic composition of Cryptococcus neoformans. I. A serologic classification by means of the capsular and agglutination reactions. J. Immunol. 64:423–430 [PubMed] [Google Scholar]
- 14. Evans E. E. 1949. An immunologic comparison of twelve strains of Cryptococcus neoformans (Torula histolytica). Proc. Soc. Exp. Biol. Med. 71:644–646 [DOI] [PubMed] [Google Scholar]
- 15. Ikeda R., Nishikawa A., Shinoda T., Fukazawa Y. 1985. Chemical characterization of capsular polysaccharide from Cryptococcus neoformans serotype A-D. Microbiol. Immunol. 29:981–991 [DOI] [PubMed] [Google Scholar]
- 16. Karstaedt A. S., Crewe-Brown H. H., Dromer F. 2002. Cryptococcal meningitis caused by Cryptococcus neoformans var. gattii, serotype C, in AIDS patients in Soweto, South Africa. Med. Mycol. 40:7–11 [DOI] [PubMed] [Google Scholar]
- 17. Kozel T. R. 1989. Antigenic structure of Cryptococcus neoformans capsular polysaccharides, p. 63–86 In Kurstak E., Marquis G. (ed.), Immunology of fungal diseases. Marcel Dekker, Inc., New York, NY: [PubMed] [Google Scholar]
- 18. Kozel T. R., Gotschlich E. C. 1982. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 129:1675–1680 [PubMed] [Google Scholar]
- 19. Kozel T. R., et al. 2003. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect. Immun. 71:2868–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Litvintseva A. P., Thakur R., Reller L. B., Mitchell T. G. 2005. Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in sub-Saharan Africa. J. Infect. Dis. 192:888–892 [DOI] [PubMed] [Google Scholar]
- 21. Peterman J. H. 1991. Immunochemical considerations in the analysis of data from non-competitive solid-phase immunoassays, p. 47–65 In Butler J. E. (ed.), Immunochemistry of solid-phase immunoassay. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 22. Peterman J. H., Butler J. E. 1989. Application of theoretical considerations to the analysis of ELISA data. Biotechniques 7:608–615 [PubMed] [Google Scholar]
- 23. Rinaldi M. G., et al. 1986. Serotypes of Cryptococcus neoformans in patients with AIDS. J. Infect. Dis. 153:642. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu R. Y., Howard D. H., Clancy M. N. 1986. The variety of Cryptococcus neoformans in patients with AIDS. J. Infect. Dis. 154:1042. [DOI] [PubMed] [Google Scholar]
- 25. Spiropulu C., Eppard R. A., Otteson E., Kozel T. R. 1989. Antigenic variation within serotypes of Cryptococcus neoformans detected by monoclonal antibodies specific for the capsular polysaccharide. Infect. Immun. 57:3240–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swinne D., Nkurikiyinfura J. B., Muyembe T. L. 1986. Clinical isolates of Cryptococcus neoformans from Zaire. Eur. J. Clin. Microbiol. 5:50–51 [DOI] [PubMed] [Google Scholar]
- 27. Thakur R., et al. 2009. Prevalence of C. neoformans and C. gattii among patients with cryptococcal meningitis at a tertiary hospital in Gaborone, Botswana: March 2005-February 2007, abstr. 1048. Abstr. 47th Annu. Meet. IDSA, Philadelphia, PA [Google Scholar]
- 28. Turner S. H., Cherniak R. 1991. Multiplicity in the structure of the glucuronoxylomannan of Cryptococcus neoformans, p. 123–142 In Latgé J. P., Boucias D. (ed.), Fungal cell wall and immune response. Springer-Verlag, Berlin, Germany [Google Scholar]
- 29. Wilson D. E., Bennett J. E., Bailey J. W. 1968. Serologic grouping of Cryptococcus neoformans. Proc. Soc. Exp. Biol. Med. 127:820–823 [DOI] [PubMed] [Google Scholar]