Abstract
Investigation of antigenic determinants of the microaerophilic obligate intracellular bacterium Lawsonia intracellularis using a mass spectrometry approach identified a novel bacterial protein present in an extract of cell culture medium aspirated from heavily infected in vitro cell cultures. Western immunoblotting analysis of SDS-PAGE-resolved proteins using immune sera pooled from L. intracellularis-infected pigs revealed the presence of a strongly immunoreactive band of ∼72 kDa. Liquid chromatography-electrospray ionization-tandem mass spectrometry analysis of this component and database mining using a fully annotated L. intracellularis genome sequence and the comprehensive GenBank prokaryotic genomic database highlighted the presence of a protein that shares little sequence similarity with other prokaryotic proteins and appears to be highly species specific. Detailed bioinformatic analyses identified the protein as member of the autotransporter protein family of surface-exposed proteins, and the designation LatA (Lawsonia autotransporter protein A) is suggested. Recognition of recombinant LatA on Western blots by a panel of sera from infected and control pigs corresponded 100% with a commercial serodiagnostic that relies on in vitro culture of this fastidious organism. LatA therefore represents a potential candidate for the development of a rapid and species-specific serodiagnostic reagent.
INTRODUCTION
Lawsonia intracellularis is the etiological agent of proliferative enteropathy, or ileitis, a commercially significant disease of pigs with a worldwide distribution (17, 24, 30). As well as impacting the health and welfare of pigs, proliferative enteropathy has been reported in a wide variety of other animals, including the horse, hamster, rabbit, rat, guinea pig, ferret, deer, dog, wolf, fox, ostrich, emu, and rhesus macaque (30, 48).
This Gram-negative, microaerophilic obligate intracellular bacterium replicates in the cytoplasm of infected cells, with a tropism for immature enterocytes in the intestinal crypts. Here, it induces proliferation and, in turn, hyperplasia (24, 45) which results in various clinical manifestations. Proliferative hemorrhagic enteropathy is an acute form of the disease associated with bloody diarrhea and sudden death, affecting finishing pigs and replacement gilts, whereas a chronic condition, more common in younger pigs and known as “porcine intestinal adenomatosis,” is typified by wasting and loss of condition and may be accompanied by mild diarrhea. Herd infection results in considerable financial losses due to poor feed conversion and the costs of diagnosis and treatment.
Despite the impact of L. intracellularis on the farming industry worldwide, this bacterium is poorly characterized, and pathogenicity determinants remain unclear. This is due largely to the obligate intracellular lifestyle, fastidious in vitro growth requirements, and limited genetic pliability which mean that study of the organism using conventional laboratory techniques is challenging. The current lack of information regarding virulence factors and pathophysiological mechanisms has consequently limited the development of novel therapies, vaccines, and diagnostic tools.
Current diagnostic tools are not without their drawbacks: PCR amplification of bacterial DNA from the feces of infected animals is routinely employed, but detection is limited to when bacteria are excreted (19, 39), and in situ detection of the bacterium within the intestines of infected animals can only be achieved postmortem (28). Serodiagnosis is considered to be a reliable and convenient indicator of exposure to the bacterium, particularly when evaluating the immune status of herds, (7, 21). Existing tools, however, rely on in vitro culture of L. intracellularis as the antigen source, which is highly demanding and subject to variation between culture batches.
Difficulties associated with propagating the organism in vitro and the efficient removal of extraneous host cell proteins during bacterial cell purification have to date precluded proteomic analyses of L. intracellularis. However, recent advances in proteomics technologies have facilitated the rapid and accurate detection of proteins within complex biological mixtures (3). In addition, the availability of a fully sequenced and annotated L. intracellularis genome has provided a valuable resource that enables mass spectrometry (MS) data to be mined against a corresponding genomic database. This provides a rapid, sensitive, and cost-effective means of detecting and identifying L. intracellularis proteins.
In the present study, liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) was applied to the identification of an immunogen present in cell cultures heavily infected with L. intracellularis. Bioinformatic analysis of the amino acid sequence identified the protein as a putative autotransporter, and it was subsequently designated LatA (Lawsonia autotransporter A). Further immunological investigation, facilitated by recombinant LatA (rLatA) and a panel of sera from naturally infected and uninfected pigs, has established the potential of this protein as a candidate for future applications in detection and control of infection.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The L. intracellularis isolate LR189/5/83 was obtained from the University of Edinburgh (35) and was cocultured in an adherent, nonpolarized, rat ileal epithelial cell line (IEC-18; ATCC-1589) as previously described (31) at 37°C under microaerophilic conditions (8.8% CO2, 8.0% O2). Recombinant plasmids were maintained in the Escherichia coli TOP10 strain (Invitrogen, Paisley, United Kingdom), which was routinely cultured under aerobic conditions on LB medium containing 50 μg/ml ampicillin. The E. coli BL21(DE3)/pLysS strain (Invitrogen, Paisley, United Kingdom) was used for expression of the recombinant LatA fusion protein and was grown on LB medium containing ampicillin (50 μg/ml) and chloramphenicol (35 μg/ml).
Sample preparation.
For preparation of L. intracellularis samples, 5 ml of cell culture medium from heavily infected L. intracellularis cell cultures was centrifuged at 200 × g for 5 min to remove mammalian cell debris. The supernatant was then centrifuged at 5,500 × g for 10 min to pellet the bacteria, which were washed three times in phosphate-buffered saline (PBS) before being resuspended in a final volume of 500 μl PBS.
SDS-PAGE and Western blotting.
Proteins were resolved on discontinuous Tris-glycine SDS-PAGE gels (4% stacking gel, 10% resolving gel) under reducing conditions (29). Approximately 50 μl L. intracellularis sample material prepared as described above was loaded into each of two sample wells of a Hoefer SE-600 vertical slab gel and separated at 200 V (constant voltage) over 4.5 h. Approximately 20 μg recombinant LatA fusion protein was resolved on an SDS-PAGE minigel over the entire gel width (8 cm) using the Mini-Protean III cell (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom) at 135 V (constant voltage) over 1 h. Resolved proteins were visualized using SimplyBlue SafeStain (Invitrogen, Paisley, United Kingom) or colloidal Coomassie blue stain (Genomic Solutions). Molecular size standards were included routinely on gels. For Western blotting, pig sera were kindly donated by Boehringer Ingelheim Vetmedica (1). Prior to receipt, serological status was confirmed at the source using the Enterisol ileitis enzyme-linked immunosorbent assay (ELISA) (bioScreen, Münster, Germany): pigs found to be seropositive were designated naturally infected, and seronegative animals were designated uninfected. Sera were centrifuged at 13,000 × g to remove debris prior to use. Proteins resolved on SDS-PAGE gels were transferred onto Hybond-C nitrocellulose membranes (GE Healthcare, Buckinghamshire, United Kingdom) using a semidry blotter (Fisher Scientific, Loughborough, United Kingdom). Membranes were blocked for 1 h in 2% bovine serum albumin (BSA) and then incubated with sera at a 1:5,000 dilution. Membranes were washed thoroughly in PBS-0.3% Tween 20, incubated with horseradish peroxidase (HRP)-conjugated anti-pig IgG (Sigma, Poole, United Kingdom) at a 1:10,000 dilution, and then washed again. Bound antibodies were detected using the Pierce enhanced chemiluminescence system (Thermo Fisher Scientific, Rockford, IL).
LC-ESI-MS/MS.
A protein band of 72 kDa was excised manually before performing standard in-gel destaining, reduction, alkylation, and trypsinolysis procedures (43). The sample was transferred to a high-performance liquid chromatography (HPLC) sample vial and stored at +4°C until required for LC-ESI-MS/MS analysis. Liquid chromatography was performed using an Ultimate 3000 nano-HPLC system (Dionex UK, Camberley, United Kingdom) comprising a WPS-3000 well-plate microautosampler, an FLM-3000 flow manager and column compartment, a UVD-3000 UV detector, an LPG-3600 dual-gradient micropump, and an SRD-3600 solvent rack controlled by Chromeleon chromatography software (Dionex). A micropump flow rate of 246 μl/min−1 was used in combination with a cap-flow splitter cartridge, affording a 1/82 flow split and a final flow rate of 3 μl/min−1 through a 5-cm by 200-μm inside diameter monolithic reversed phase column (Dionex-LC Packings) maintained at 50°C. Samples of 4 μl were applied to the column by direct injection. Peptides were eluted by the application of a 15-min linear gradient from 8 to 45% solvent B (80% acetonitrile, 0.1% [vol/vol] formic acid) and directed through a 3-nl UV detector flow cell. LC was interfaced directly with a three-dimensional high-capacity ion trap mass spectrometer (Esquire HCTplus; Bruker Daltonics, Bremen, Germany) via a low-volume (50 μl/min−1 maximum) stainless steel nebulizer (Agilent, catalog no. G1946-20260) and ESI. Parameters for tandem MS analysis were set as previously described (3).
Database mining.
Deconvoluted MS/MS data were submitted to an in-house MASCOT server and searched against a fully annotated L. intracellularis genomic database using the MASCOT search algorithm (40). The presentation and interpretation of MS/MS data were performed in accordance with published guidelines (47). To this end, the fixed and variable modifications selected were carbamidomethyl (C) and oxidation (M), respectively, and mass tolerance values for MS and MS/MS were set at 1.5 Da and 0.5 Da, respectively. Molecular weight search (MOWSE) scores (38) attained for individual protein identifications were inspected manually. Peptide hits containing an unbroken “b” or “y” ion series of a minimum of four amino acid residues were considered to be significant. BLASTP searches of the NCBI database were performed on amino acid sequences corresponding to contiguous ions to confirm assignment of peptide identification. Protein identifications were assigned when the protein was represented by at least two peptides which meet the criteria for peptide identification or by one peptide when the unbroken “b” or “y” ion series is double or more than the minimum number set for peptide identification and the corresponding amino acid string is exclusive to that protein. L. intracellularis genomic databases are available at GenBank, National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) under accession no. NC_008011, NC_008012, NC_008013, and NC_008014.
Bioinformatic analysis of LatA.
Similarity between the identified L. intracellularis protein and previously described proteins was ascertained using the BLAST algorithms (2); searches were initially conducted using BLASTP, and more distant similarities were identified using PSI-BLAST. Records describing conserved domains were retrieved from the Uniprot Knowledgebase (http://www.uniprot.org/help/uniprotkb) and Superfamily (http://supfam.mrc-lmb.cam.ac.uk/SUPERFAMILY/) databases (18, 49). The primary amino acid sequence was submitted to Interproscan (www.ebi.ac.uk/interpro/) (50) in order to predict further functional domains or motifs. To predict an N-terminal signal peptide, which is common to many proteins that are secreted, membrane-bound, or periplasmic, SignalP v3.0 was utilized (http://www.cbs.dtu.dk/services/SignalP/) (4, 14). Secondary structure prediction was carried out using the Phyre program (Protein Homology/analogY Recognition Engine) (http://www.sbg.bio.ic.ac.uk/phyre/index.cgi), which generates a consensus secondary structure based on predictions from J-Pred, PSI-Pred, and SS-PRO (8, 10, 12, 26).
Molecular biology techniques.
PCR amplification, restriction digests, DNA ligations, plasmid isolation, and transformations were carried out according to standard methods (42). DNA was visualized using GelRed (Cambridge Biosciences, Cambridge, United Kingdom) on agarose gels under UV. Taq polymerase, T4 DNA ligase and restriction endonucleases were purchased from Promega (Southampton, United Kingdom) and used in accordance with the manufacturer's recommendations.
Construction of recombinant plasmids containing LatA.
A region of the open reading frame corresponding to nucleotides 58 to 1452 of the LatA gene was amplified by PCR from L. intracellularis LR189/5/83 DNA using the primers 5′-CGGGTACCGCACTATATTTATAATGACATC-3′ (forward) and 5′-GCGAATTCTGGTCCATATATTGTTAACTGT-3′ (reverse), which were designed to introduce the restriction sites KpnI and EcoRI (underlined) into the amplified product (MWG-Biotech Ltd., Milton Keynes, United Kingdom). PCR products and pRSETA expression vector (Invitrogen, Paisley, United Kingdom) were cut with KpnI and EcoRI restriction endonucleases and ligated to generate the recombinant plasmid pEWX2. TOP10 competent cells were transformed with pEWX2 according to the manufacturer's instructions. Plasmid DNA from transformants was isolated using a QIAPrep spin minikit (Qiagen, Crawley, United Kingdom) and confirmed by restriction analysis.
Expression and purification of recombinant LatA protein.
pEWX2 was used to transform BL21(DE3)/pLysS cells according to the manufacturer's instructions. Freshly transformed isolates were used to inoculate 2 ml SOB medium (20 g tryptone, 5 g yeast extract, 0.186 g KCl, 0.5 g NaCl, and 1 M MgSO4 in 1 liter distilled water [dH2O]) containing ampicillin (50 μg/ml) and chloramphenicol (35 μg/ml) and were incubated overnight at 37°C shaking at 200 rpm. Transformants were subcultured by adding 2 ml overnight culture to 38 ml SOB medium and grown at 37°C with shaking at 200 rpm for 2 h. Expression of LatA was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma, Poole, United Kingdom), at a final concentration of 1 mM, for 4 h. The bacteria were harvested by centrifugation at 5,000 × g for 10 min, and the resulting pellets were stored at −20°C until further use. One-milliliter samples of culture prior to and 4 h after induction were taken to confirm the expression and solubility of the recombinant fusion protein carrying the N-terminal six-histidine tag. The 1-ml bacterial samples were lysed by freeze-thawing three times in PBS and pelleted at 13,000 × g for 10 min, and proteins in the soluble (supernatant) and insoluble (pellet) fractions were separated by SDS-PAGE and visualized with Coomassie SimplyBlue SafeStain (Invitrogen, Paisley, United Kingdom). A band of approximately 51 kDa, the predicted molecular mass of rLatA with combined polyhistidine tags (ExPASy Compute pI/Mw tool; http://www.expasy.org/tools/pi_tool.html) (6), was visible on SDS-PAGE gels in induced samples only and was recognized by anti-HisG-HRP monoclonal antibody (Invitrogen, Paisley, United Kingdom) on Western blots. This protein was present in the insoluble fraction (data not shown) and was subsequently enriched from the whole-cell lysate under denaturing conditions by immobilized metal affinity capture (IMAC) using ProBond nickel-chelating resin (Invitrogen, Paisley, United Kingdom) in accordance with the manufacturer's recommendations. Confirmatory identification of the affinity-purified protein as LatA was achieved by peptide mass fingerprinting and MS/MS sequencing using matrix-assisted laser desorption ionization-time of flight tandem mass spectrometry (MALDI-TOF MS/MS).
RESULTS
Identification and bioinformatic analysis of LatA.
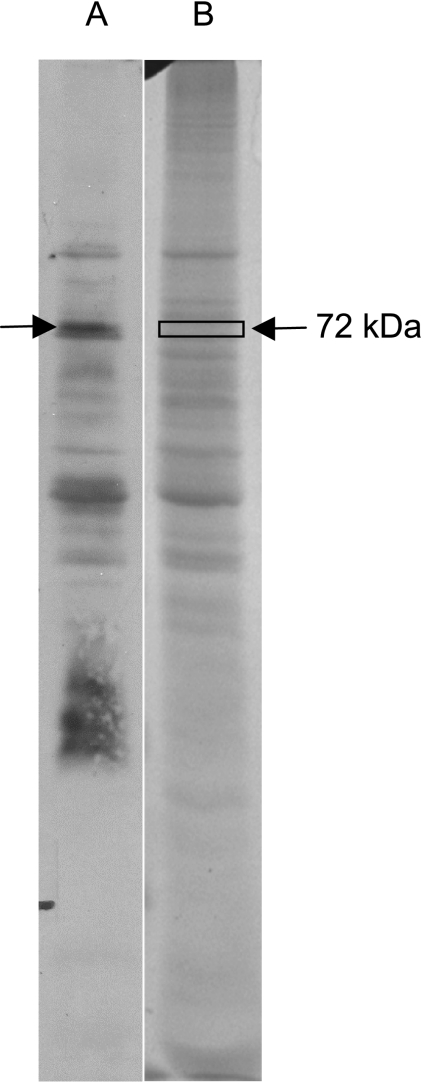
A 72-kDa protein in the L. intracellularis extract was strongly recognized by pooled sera from 10 pigs naturally infected with L. intracellularis on a Western blot (Fig. 1). A corresponding region of the SDS-PAGE gel was excised and submitted for analysis by LC-ESI-MS/MS. Within this excised region, one putative L. intracellularis protein, LI0649 (YP_595024), was detected with a MOWSE score of 56 and 4% coverage, represented by one peptide with an unbroken “y” ion series of eight amino acid residues. A BLASTP search of this amino acid sequence against the entire NCBI database revealed a 100% amino acid match with only LI0649.
Fig. 1.
Recognition of L. intracellularis proteins by sera from infected pigs. Shown is a Western blot of L. intracellularis protein extract probed with sera pooled from 10 pigs naturally infected with L. intracellularis (lane A) and the corresponding SDS-PAGE gel (lane B). A protein of approximately 72 kDa was strongly recognized by sera, and the corresponding region of the SDS-PAGE gel was excised and analyzed by LC-ESI-MS/MS.
BLAST algorithms were employed to identify proteins with sequence similarity to LI0649 in order to infer a putative biological function for this protein. BLASTP analysis revealed only two similar proteins, which are both encoded by L. intracellularis: the first was LIB004, a protein annotated as “Asn/Thr/Ser/Val rich protein,” encoded on L. intracellularis plasmid 2 (36% amino acid identity; E value, 3e−142), and the second was LI0045, a chromosomally encoded “hypothetical protein” (31% identity; 5e−104). Proteins encoded by species other than L. intracellularis were returned only by PSI-BLAST after the 2nd iteration and comprised outer membrane proteins, although these exhibited relatively low sequence similarity with high E values (>0.005). No new protein identifications were made on subsequent PSI-BLAST iterations up to the 5th iteration.
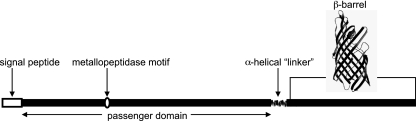
Given that initial bioinformatic analysis using BLASTP and PSI-BLAST searches showed only very low sequence similarity between LI0649 and proteins encoded by species other than L. intracellularis, additional in silico methodologies were adopted in order to predict the putative function(s) of LI0649. Structural domains predicted by in silico analysis are represented in Fig. 2. Data held in the Uniprot KB and Superfamily databases (accessed on 21 March 2011) suggest that the protein shares a feature with the autotransporter protein family, namely, the transmembrane β-barrel domain (IPR006315), which is conserved among autotransporter proteins. A second region corresponding to an ArgE/DapE/ACY1/CPG2/YscS-like metallopeptidase motif was also predicted (amino acids 227 to 236; IPR001261). These predictions were corroborated by a further search using Interproscan. The SignalP program was used to predict a signal peptide comprising the N-terminal 31 amino acids (probability, 0.944) with a potential cleavage site after Ala 31 (probability, 0.791). Using the Phyre program, similarities were also identified between the predicted secondary structure of LI0649 and previously determined autotransporter structures. In addition to the β-barrel (residues 587 to 851) and an α-helical linker domain (residues 556 to 583), short stretches of amino acids predicted to form β-sheets were identified in the N-terminal portion of the protein (residues 32 to 546). This suggests that LI0649 may have a β-helical passenger domain—a common structural motif shared by autotransporter proteins (15, 25, 37). In light of these results, which identify LI0649 as a putative autotransporter, this protein was designated LatA (Lawsonia autotransporter A).
Fig. 2.
Schematic diagram showing the predicted features of LatA, including the signal sequence (amino acids 1 to 31), the passenger domain (amino acids 32 to 546), the ArgE/DapE/ACY1/CPG2/YscS-like metallopeptidase motif (amino acids 227 to 236; IPR001261), the α-helical linker domain (amino acids 556 to 583), and the β-barrel domain (amino acids 587 to 851). A putative structural model of the LatA β-barrel domain was generated using the Phyre program.
Expression and purification of LatA.
Due to the difficulties of culturing L. intracellularis, it was not possible to prepare a purified extract of this protein from in vitro culture. Consequently, to facilitate further immunological investigations of the antigenicity of LatA, a recombinant protein was generated. Primers were designed to exclude both the hydrophobic N-terminal signal peptide and the conserved C-terminal regions of the natural protein. These exclusions facilitated the production and extraction of a recombinant protein with minimal antigenic cross-reactivity. Subsequently a sequence corresponding to amino acids 20 to 484 was cloned and expressed in BL21(DE3)/pLysS cells (rLatA).
Expression of rLatA was confirmed by SDS-PAGE and Western blotting. A large band of approximately 51 kDa corresponding to the predicted combined molecular mass of the expressed protein and polyhistidine tag was visible in induced samples only and was also recognized by the anti-HisG-HRP monoclonal antibody. Recombinant protein for further analysis was then extracted from cell extracts using an IMAC (immobilized metal affinity chromatography) nickel affinity column. The identity of the final affinity-purified protein was verified by peptide mass fingerprinting and MS/MS sequencing (data not shown).
Recognition of rLatA by a panel of pig sera.
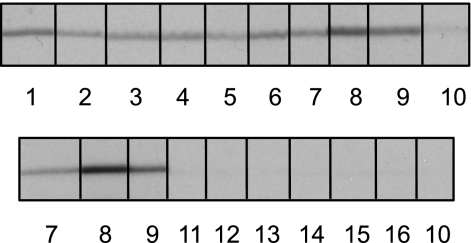
To determine the seroreactivity/specificity of LatA, purified recombinant protein was resolved over the entire width of an SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membrane was cut into a series of 6-mm-wide strips before being incubated with sera from nine pigs which were seropositive for L. intracellularis infection and seven seronegative pigs (Fig. 3). LatA was strongly recognized by all sera from seropositive animals, compared with seronegative animals, which showed negligible recognition, suggesting that LatA is a consistently antigenic protein.
Fig. 3.
Recognition of purified rLatA by pig sera. Western blots were probed with sera from infected animals (numbers 1 to 9) or uninfected animals (numbers 10 to 16). Sera from infected animals reacted specifically with rLatA, whereas reactivity of sera from uninfected animals was negligible. Induction of specific antibodies indicates that LatA is expressed during in vivo infection with L. intracellularis.
DISCUSSION
The inability to culture L. intracellularis independently of eukaryotic cells and the consequent limitations on genetic tractability have to date hindered investigative progress and prohibited identification of bacterial factors expressed during host-pathogen interactions. Mass spectrometric analysis of complex protein mixtures has proved to be a successful approach for investigating protein expression of obligate intracellular bacteria where technical difficulties in obtaining preparations of organisms free from extraneous host cell material previously hampered protein identification (9, 11, 22, 34, 36, 44). In this study, we used LC-ESI-MS/MS, which facilitates the detection and identification of proteins contained within a complex biological mixture, to identify a novel L. intracellularis antigen, LatA, expressed during in vitro infection.
Bioinformatic analysis of the LatA protein sequence identified conserved domains, including a β-barrel and an N-terminal signal peptide, and tentatively predicted the presence of a β-helical passenger domain, which suggests that LatA is a member of the monomeric (type Va) autotransporter protein family. These surface proteins generally contain an extreme N-terminal signal sequence, recognized by the Sec system, which facilitates export of the unfolded protein across the inner membrane to the periplasm, a C-terminal β-barrel domain, and a passenger domain between the signal peptide and the β-barrel, which includes the functional part of the protein (for a review, see reference 20). The passenger domain of the protein is proposed to translocate to the outer membrane through a pore formed by the β-barrel (41). The passenger domain may then be cleaved to release a functional molecule, or the whole protein may remain embedded in the outer membrane (16, 46). It is of note that the predicted molecular mass of LatA, calculated from the open reading frame, is 91.9 kDa, although the migration distance after SDS-PAGE indicated that the protein was approximately 72 kDa, which could suggest that cleavage of LatA may occur in some form. This may have occurred as a function of the identified metallopeptidase motif, which might have a role in autoproteolysis, although this is work in progress and remains to be confirmed.
Autotransporter proteins are widespread among Gram-negative bacteria, with several having been associated with functions related to virulence, including adhesins such as AIDA-I (E. coli) and pertactin (Bordetella pertussis) (5, 32) and proteolytic enzymes (13). Several autotransporters have been identified as immunodominant antigens, perhaps most notably, pertactin, which forms a component of the pertussis vaccine (23).
The identification of LatA as a putative surface protein and powerful inducer of humoral immunity led to a further investigation of antigenicity, which was enabled by the generation of a recombinant protein corresponding to a region of the passenger domain. The clear recognition of rLatA by sera from infected pigs indicates that this protein is antigenic and is consistently encountered by the immune system during infection. The antigenicity of this protein together with its apparent species specificity suggests that LatA may be exploited in the diagnosis of animals exposed to L. intracellularis. Current diagnostic tools for L. intracellularis infection are not ideal: diagnosis achieved by examination of infected guts based on gross morphology, histopathology, and immunohistopathology is only of use postmortem (28). PCR amplification of bacterial DNA from the feces of infected animals can be successfully employed but can only be achieved when L. intracellularis is excreted in feces (19, 27). Serodiagnosis can be attained through commercially available assays: an immunofluorescence assay, which is based upon infected cell lines fixed onto glass slides (Ileitest; Elanco Animal Health); and a blocking ELISA (Enterisol ileitis ELISA; bioScreen, Münster, Germany). These tools are sensitive, but disadvantaged by their reliance on in vitro-cultured L. intracellularis as the antigen for antibody capture. Production of whole-cell fractions is demanding and therefore costly, as well as being subject to batch variation. The use of an individual recombinant antigen offers a reliable alternative.
The data presented in this study indicate that LatA-specific antibody is present in sera from L. intracellularis-infected pigs and highlight its potential as an immunodiagnostic for the management and control of L. intracellularis-induced disease. LatA represents a potential candidate for a recombinant protein-based ELISA. This would provide a scaleable, reproducible serodiagnostic tool for L. intracellularis that also offers a convenient format for a multiplex test with other serodiagnostics. Serodiagnostic ELISAs have previously been developed based on outer membrane protein preparations (33). Work toward characterizing LatA as a functional autotransporter protein and its role in disease is under way.
ACKNOWLEDGMENTS
This work was supported by Biotechnology and Biological Sciences Research Council research grants (BB/C510532/1 and BB/E018939/1). The Moredun Research Institute receives funding from the Rural and Environment Research and Analysis Directorate of the Scottish government (Control of Bacterial Diseases).
We thank Knut Elbers and Michael Roof of Boehringer Ingelheim Vetmedica for arranging provision of sera from seropositive and seronegative animals. We also thank David Longbottom and Nick Wheelhouse for useful discussion, Douglas Park for formatting the figures, and Gina McAllister for assistance with electrophoresis.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Alberdi M. P., et al. 2009. Expression by Lawsonia intracellularis of type III secretion system components during infection. Vet. Microbiol. 139:298–303 [DOI] [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batycka M., et al. 2006. Ultra-fast tandem mass spectrometry scanning combined with monolithic column liquid chromatography increases throughput in proteomic analysis. Rapid Commun. Mass Spectrom. 20:2074–2080 [DOI] [PubMed] [Google Scholar]
- 4. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 5. Benz I., Schmidt M. A. 1992. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27). Infect. Immun. 60:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjellqvist B., et al. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14:1023–1031 [DOI] [PubMed] [Google Scholar]
- 7. Brandt D., Kaim U., Baumgartner W., Wendt M. 2010. Evaluation of Lawsonia intracellularis infection in a group of pigs in a subclinically affected herd from weaning to slaughter. Vet. Microbiol. 146:361–365 [DOI] [PubMed] [Google Scholar]
- 8. Bryson K., et al. 2005. Protein structure prediction servers at University College London. Nucleic Acids Res. 33:W36–W38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chao C. C., Chelius D., Zhang T., Mutumanje E., Ching W. M. 2005. Proteomic analysis of Rickettsia prowazekii. Ann. N. Y. Acad. Sci. 1063:87–89 [DOI] [PubMed] [Google Scholar]
- 10. Cheng J., Randall A. Z., Sweredoski M. J., Baldi P. 2005. SCRATCH: a protein structure and structural feature prediction server. Nucleic Acids Res. 33:W72–W76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coleman S. A., et al. 2007. Proteome and antigen profiling of Coxiella burnetii developmental forms. Infect. Immun. 75:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuff J. A., Barton G. J. 2000. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 40:502–511 [DOI] [PubMed] [Google Scholar]
- 13. Dutta P. R., Cappello R., Navarro-Garcia F., Nataro J. P. 2002. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 70:7105–7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emanuelsson O., Brunak S., von Heijne G., Nielsen H. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2:953–971 [DOI] [PubMed] [Google Scholar]
- 15. Emsley P., Charles I. G., Fairweather N. F., Isaacs N. W. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90–92 [DOI] [PubMed] [Google Scholar]
- 16. Eslava C., et al. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gebhart C. J., Barns S. M., McOrist S., Lin G. F., Lawson G. H. 1993. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int. J. Syst. Bacteriol. 43:533–538 [DOI] [PubMed] [Google Scholar]
- 18. Gough J., Karplus K., Hughey R., Chothia C. 2001. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 313:903–919 [DOI] [PubMed] [Google Scholar]
- 19. Guedes R. M., et al. 2002. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can. J. Vet. Res. 66:99–107 [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala'Aldeen D. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holyoake P. K., Emery D., Gonsalves J., Donahoo M., Collins A. 2010. Prevalence of antibodies to Lawsonia intracellularis in pig herds in Australia. Aust. Vet. J. 88:186–188 [DOI] [PubMed] [Google Scholar]
- 22. Huang H., et al. 2008. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect. Immun. 76:3405–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson G., et al. February 1993. Purification of a pertussis outer membrane protein. European patent EP0527753. [Google Scholar]
- 24. Jacobson M., Fellstrom C., Jensen-Waern M. 2010. Porcine proliferative enteropathy: an important disease with questions remaining to be solved. Vet. J. 184:264–268 [DOI] [PubMed] [Google Scholar]
- 25. Junker M., et al. 2006. Pertactin beta-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc. Natl. Acad. Sci. U. S. A. 103:4918–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelley L. A., Sternberg M. J. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 27. Knittel J. P., et al. 1998. Evaluation of antemortem PCR and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am. J. Vet. Res. 59:722–726 [PubMed] [Google Scholar]
- 28. Kroll J. J., Roof M. B., Hoffman L. J., Dickson J. S., Harris D. L. 2005. Proliferative enteropathy: a global enteric disease of pigs caused by Lawsonia intracellularis. Anim. Health Res. Rev. 6:173–197 [DOI] [PubMed] [Google Scholar]
- 29. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 30. Lawson G. H., Gebhart C. J. 2000. Proliferative enteropathy. J. Comp. Pathol. 122:77–100 [DOI] [PubMed] [Google Scholar]
- 31. Lawson G. H., McOrist S., Jasni S., Mackie R. A. 1993. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J. Clin. Microbiol. 31:1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leininger E., et al. 1991. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 88:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Longbottom D., et al. 2002. Serological diagnosis of ovine enzootic abortion by enzyme-linked immunosorbent assay with a recombinant protein fragment of the polymorphic outer membrane protein POMP90 of Chlamydophila abortus. J. Clin. Microbiol. 40:4235–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lopez J. E., et al. 2005. Identification of novel antigenic proteins in a complex Anaplasma marginale outer membrane immunogen by mass spectrometry and genomic mapping. Infect. Immun. 73:8109–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCluskey J., Hannigan J., Harris J. D., Wren B., Smith D. G. 2002. LsaA, an antigen involved in cell attachment and invasion, is expressed by Lawsonia intracellularis during infection in vitro and in vivo. Infect. Immun. 70:2899–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogawa M., et al. 2007. Proteome analysis of Rickettsia felis highlights the expression profile of intracellular bacteria. Proteomics 7:1232–1248 [DOI] [PubMed] [Google Scholar]
- 37. Otto B. R., et al. 2005. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J. Biol. Chem. 280:17339–17345 [DOI] [PubMed] [Google Scholar]
- 38. Pappin D. J., Hojrup P., Bleasby A. J. 1993. Rapid identification of proteins by peptide-mass fingerprinting. Curr. Biol. 3:327–332 [DOI] [PubMed] [Google Scholar]
- 39. Pedersen K. S., Holyoake P., Stege H., Nielsen J. P. 2010. Diagnostic performance of different fecal Lawsonia intracellularis-specific PCR assays as diagnostic tests for proliferative enteropathy in pigs: a review. J. Vet. Diagn. Invest. 22:487–494 [DOI] [PubMed] [Google Scholar]
- 40. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567 [DOI] [PubMed] [Google Scholar]
- 41. Pohlner J., Halter R., Beyreuther K., Meyer T. F. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458–462 [DOI] [PubMed] [Google Scholar]
- 42. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 43. Shevchenko A., Wilm M., Vorm O., Mann M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850–858 [DOI] [PubMed] [Google Scholar]
- 44. Skipp P., Robinson J., O'Connor C. D., Clarke I. N. 2005. Shotgun proteomic analysis of Chlamydia trachomatis. Proteomics 5:1558–1573 [DOI] [PubMed] [Google Scholar]
- 45. Smith D. G., Lawson G. H. 2001. Lawsonia intracellularis: getting inside the pathogenesis of proliferative enteropathy. Vet. Microbiol. 82:331–345 [DOI] [PubMed] [Google Scholar]
- 46. St Geme J. W., III, Cutter D. 2000. The Haemophilus influenzae Hia adhesin is an autotransporter protein that remains uncleaved at the C terminus and fully cell associated. J. Bacteriol. 182:6005–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor G. K., Goodlett D. R. 2005. Rules governing protein identification by mass spectrometry. Rapid Commun. Mass Spectrom. 19:3420. [DOI] [PubMed] [Google Scholar]
- 48. Tomanova K., et al. 2003. Lawsonia intracellularis in wild mammals in the Slovak Carpathians. J. Wildl. Dis. 39:407–411 [DOI] [PubMed] [Google Scholar]
- 49. Uniprot Consortium 2011. Ongoing and future developments at the Universal Protein Resource. Nucleic Acids Res. 39:D214–D219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zdobnov E. M., Apweiler R. 2001. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848 [DOI] [PubMed] [Google Scholar]