Abstract
Children bear a large component of the global burden of cholera. Despite this, little is known about immune responses to cholera in children, especially those under 5 years of age. Cholera vaccine studies have demonstrated lower long-term protective efficacy in young children than in older children and adults. Memory B cell (MBC) responses may correlate with duration of protection following infection and vaccination. Here we report a comparison of immune responses in young children (3 to 5 years of age; n = 17), older children (6 to 17 years of age; n = 17), and adults (18 to 60 years of age; n = 68) hospitalized with cholera in Dhaka, Bangladesh. We found that young children had lower baseline vibriocidal antibody titers and higher fold increases in titer between day 2 and day 7 than adults. Young children had higher baseline IgG plasma antibody levels to Vibrio cholerae antigens, although the magnitudes of responses at days 7 and 30 were similar across age groups. As a surrogate marker for mucosal immune responses, we assessed day 7 antibody-secreting cell (ASC) responses. These were comparable across age groups, although there was a trend for older age groups to have higher levels of lipopolysaccharide-specific IgA ASC responses. All age groups developed comparable MBC responses to V. cholerae lipopolysaccharide and cholera toxin B subunit at day 30. These findings suggest that young children are able to mount robust vibriocidal, plasma antibody, ASC, and MBC responses against V. cholerae O1, suggesting that under an optimal vaccination strategy, young children could achieve protective efficacy comparable to that induced in adults.
INTRODUCTION
Cholera is an acute dehydrating diarrheal disease caused predominantly by the Vibrio cholerae O1 or O139 serogroup. Globally, the vast majority of cholera is caused by V. cholerae O1. Cholera is endemic in over 50 countries and affects 3 to 5 million people each year, causing more than 100,000 deaths (2, 41). In areas of the world in which cholera is endemic, children under 5 years of age have the highest burden of disease (12, 31), and during cholera epidemics, children and adults are both at risk of dehydrating illness (21, 24, 34). In a rural area of Bangladesh in which cholera is endemic, 80% of the population had detectable vibriocidal antibodies by the age of 15 years (26). Similarly, in a recent observational study in urban Bangladesh, household contacts of patients with V. cholerae O1 infection who were ≤5 years of age had a significantly higher risk of developing infection than older family members in a 21-day observational period following identification of the index household case (15).
Despite the high global burden of cholera among children, vaccination of young children has shown lower protective efficacy than that achieved in adults, and the duration of protection has been shorter in children (3). In a field trial in Bangladesh of an oral killed cholera vaccine that contained whole-cell organisms together with cholera toxin B (CtxB) subunit (WC-BS), protective efficacy in children aged 2 to 5 years was 38% for the first 6 to 12 months, compared to 78% for those aged >5 years; furthermore, at a 3-year follow-up, protective efficacy had returned to baseline in young children, compared with a 40% efficacy for adults (8). However, in a recent trial of a different formulation of an oral killed whole-cell vaccine lacking CtxB (Shanchol; Shantha Biotechnics Ltd., India), in Kolkata, India, children aged 1.0 to 4.9 years and adults had comparable levels of protective efficacy during the 2-year period of observation (37). Currently, it is recommended that young children receive 3 doses of Dukoral, a WHO-prequalified cholera vaccine containing killed whole cells plus recombinant CtxB (Crucell, Sweden), with boosters every 6 months if there is an ongoing risk of cholera, while adults receive two initial doses, followed by a booster immunization every 2 years (3).
The reasons behind the lower immunogenicity and shorter duration of protection following use of some cholera vaccines in young children need to be better understood. As vaccine studies have been performed in areas in which cholera is endemic, older children and adults may have been primed by prior exposure to V. cholerae O1, resulting in more-robust immune responses. Memory B cells (MBCs) are generated after natural infection and vaccination and are thought to play a critical role in eliciting a rapid anamnestic antibody response upon antigenic reexposure (17). We have previously demonstrated the presence of memory B cell responses in adults with cholera in Bangladesh (14). We have also previously demonstrated that children are able to mount significant antibody responses to V. cholerae antigens following both natural infection and vaccination (7); however, currently there are no data on memory B cell responses in young children following cholera. Determining such responses in children with natural infection is pivotal for understanding the mechanism of lowered responses to vaccination in young children. The aim of this study was, therefore, to characterize the immune responses to wild-type V. cholerae O1 infection in young children compared to those in older children and adults, with a particular focus on differences in memory B cell responses between age groups.
MATERIALS AND METHODS
Study design and subject enrollment.
We enrolled patients presenting with cholera to the International Centre for Diarrheal Disease Research in Dhaka, Bangladesh (ICDDR,B). All patients were infected with V. cholerae O1 serotype Ogawa. After obtaining informed consent from patients or guardians, we drew venous blood samples on days 2, 7, and 30 following presentation. For each time point, we measured vibriocidal antibodies and lipopolysaccharide (LPS)- and CtxB-specific IgG and IgA antibody responses. We also studied the mucosal immune responses by determining the LPS- and CtxB-specific antibody-secreting cell (ASC) responses. We assessed LPS and CtxB-specific MBC responses on days 2 and 30 after onset of illness. This study was approved by the Research and Ethical Review Committees of the ICDDR,B, as well as the Institutional Review Board of the Massachusetts General Hospital.
Isolation of PBMCs.
We recovered peripheral blood mononuclear cells (PBMCs) by differential centrifugation on Ficoll-Isopaque (Pharmacia, Piscataway, NJ) and stored plasma at −70°C for use for immunological assays. PBMCs were washed and suspended at a concentration of 1 × 107 cells/ml in RPMI complete medium (Gibco, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT). We then immediately used fresh cells in the ASC assay and the MBC assay, both as described below.
Vibriocidal antibody assay.
We measured vibriocidal antibody levels in plasma as previously described (30), using guinea pig complement (ICDDR,B Animal Resources Branch, Dhaka, Bangladesh) and the homologous serotype of V. cholerae O1 Ogawa (strain X-25049) as the target organism. We defined the vibriocidal titer as the reciprocal of the highest dilution resulting in >50% reduction of the optical density compared to that of control wells without plasma.
Antigen-specific antibody assays.
We assessed CtxB-specific IgG and IgA and LPS-specific IgG, IgA, and IgM responses in plasma by using enzyme-linked immunosorbent assay (ELISA) techniques as previously described (30). Briefly, we coated 96-well polystyrene plates (Nunc A/S, Denmark) with GM1 ganglioside (0.3 nM; gift from A. M. Svennerholm, University of Gothenburg, Sweden) overnight followed by recombinant CtxB (2.5 μg/ml; gift from A. M. Svennerholm) or V. cholerae O1 LPS (2.5 μg/ml). We added 100 μl/well of plasma (diluted in 0.1% bovine serum albumin [BSA] in PBS-Tween, 1:100 for CtxB and 1:25 for LPS) and detected the presence of antigen-specific antibody using horseradish peroxidase (HRP)-conjugated anti-human IgG, IgA, or IgM (Jackson ImmunoResearch, West Grove, PA) and ortho-phenylenediamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.1% hydrogen peroxide. We read plates kinetically at 450 nm for 5 min, assessed the maximal rate of optical density change (mOD/min), and, to compare across plates, calculated ELISA units that represented the ratio of the test sample to a standard of pooled convalescent-phase sera present on each plate (16).
ASC assay.
We assessed peripheral blood IgG and IgA ASC responses using an enzyme-linked immunosorbent spot (ELISPOT) procedure (14, 30). Briefly, we coated nitrocellulose-bottomed plates (Mahan-4550; Millipore, Bedford, MA) with LPS (2.5 μg/ml) and GM1 ganglioside (3 nM), followed by recombinant CtxB (2.5 μg/ml) or affinity-purified goat anti-human immunoglobulin (5 μg/ml; Jackson Immunology Research, West Grove, PA), to assess total IgG and IgA cells. Wells were coated with keyhole limpet hemocyanin (KLH, 2.5 μg/ml; Pierce Biotechnology, Rockford, IL) as a negative control. After wells were blocked with RPMI containing 10% FBS, we added freshly isolated PBMCs (5 × 105 PBMCs/well to antigen-specific wells and 1 × 105 PBMCs/well to immunoglobulin-coated wells, which were then serially 10-fold diluted). After the plates were incubated for 3 h at 37°C in an incubator with 5% CO2, the plates were washed and alkaline phosphatase-conjugated goat anti-human IgG and HRP-conjugated IgA (Southern Biotech, Birmingham, AL) were added. We incubated plates overnight and developed them with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP-NBT; Sigma, St. Louis, MO) for IgG spots and 3-amino-9-ethyl carbazole (AEC) for IgA spots. We averaged independent counts visualized by two individuals using a stereomicroscope (blue spots indicating IgG and red spots indicating IgA on the same wells).
MBC assay.
We assessed MBC responses as previously described (10, 14). Briefly, we plated freshly harvested cells in 24-well cell culture plates (BD Biosciences, San Jose, CA) at 5 × 105 PBMCs/well in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, 200 units/ml penicillin, 200 μg/ml streptomycin, and 50 μM beta-mercaptoethanol, with or without a stimulatory cocktail optimized to stimulate antigen-independent proliferation and differentiation of memory B cells into ASCs (10). This cocktail consists of the mitogens CpG oligonucleotide (6 μg/ml; Operon, Huntsville, AL), a 1/100,000 dilution of crude pokeweed mitogen extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma, St. Louis, MO). We also tested a novel stimulatory cocktail recently published in the literature (38), consisting of 3 μg/ml CpG oligonucleotide, 20 ng/ml human recombinant B cell-activating factor (rBAFF; Peprotech), and 5 ng/ml recombinant interleukin-15 (rIL-15; Peprotech), on the PMBCs of 7 healthy controls and 8 cholera patients. We incubated plates at 37°C in an incubator with 5% CO2 for 6 days, after which we washed and evaluated MBC responses by using an ELISPOT format as described above. We placed 80% of cells from each well into CtxB- or LPS-coated ELISPOT plates for detection of antigen-specific IgG and IgA MBC responses and 20% of cells from each well in anti-human Ig-coated ELISPOT plates to assess total IgG and IgA MBC responses. We incubated these plates for 5 h at 37°C, washed them, and then incubated them overnight with horseradish peroxidase-conjugated mouse anti-human IgG and IgA (Hybridoma Reagent Laboratory, Baltimore, MD). We developed plates using AEC and averaged counts read by two independent readers. We expressed antigen-specific memory B cell responses as the percentage of antigen-specific ELISPOT counts per total stimulated isotype-specific Ig-reactive cells. Wells coated with KLH were used as negative controls. For inclusion in analysis, we required sufficient stimulation of total antibody isotype-specific memory B cells (defined as ≥3-fold increase for stimulated versus nonstimulated wells), fewer than three antigen-specific cells in the corresponding sample's unstimulated wells, and fewer than three antigen-specific cells in the corresponding sample's KLH MBC assay. Application of these criteria resulted in the inclusion of approximately 90% of all data.
Statistical analysis.
We compared baseline characteristics by using the chi-square test for categorical variables and Kruskal-Wallis one-way analysis of variance for continuous variables. We compared immunologic responses between two age groups by using the Mann-Whitney U test. For comparison of trends of responses across the three groups of increasing age, we used the Jonckheere-Terpstra test. Statistical significance is defined as a P value of ≤0.05. A trend was defined as a P value between 0.05 and 0.10. We performed statistical analyses using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Study population.
We enrolled a total of 17 young children (ages 3 to 5 years), 17 older children (ages 6 to 17 years), and 68 adults (ages 18 to 60 years). Baseline demographic, microbiological, and clinical data of each group are displayed in Table 1. There were no significant differences between age groups for any of the potential confounding demographic variables evaluated, including use of soap, place of defecation, source of drinking water, and blood group.
Table 1.
Demographic and clinical characteristics of study subjects
| Characteristic | Value for group of age: |
P value | ||
|---|---|---|---|---|
| 3–5 yr (n = 17) | 6–17 yr (n = 17) | 18–60 yr (n = 68) | ||
| No. of patients who completed 30-day follow-up | 15 | 16 | 62 | |
| Mean age, yr (range) | 4.4 (3–5) | 9.5 (6–14) | 33.0 (18–59) | |
| No. (%) of patients of female gender | 9 (53) | 7 (41) | 27 (40) | 0.61 |
| Mean duration of diarrhea prior to hospitalization, h (SD) | 14 (15) | 22 (18) | 19 (16) | 0.40 |
| Mean total intravenous fluid received at hospital, ml/kg body wt (SD) | 231 (166) | 184 (135) | 159 (128) | 0.15 |
| Mean duration of hospital stay, h (SD) | 48 (28) | 38 (18) | 40 (22) | 0.38 |
| No. (%) of subjects who used antibiotics in week prior to hospitalization | 5 (29) | 4 (24) | 16 (24) | 0.88 |
Vibriocidal response.
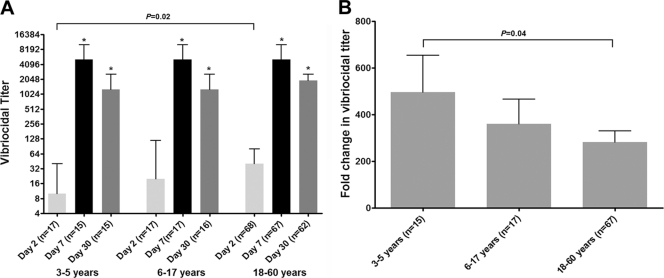
We measured vibriocidal antibody responses to the homologous O1 Ogawa serotype on study days 2, 7, and 30. Almost all (99%) patients mounted a ≥4-fold increase in vibriocidal titer by day 7. Young children had lower baseline (day 2) titers than adults (P = 0.02) (Fig. 1A) and had a higher fold increase in titer by day 7 than adults (P = 0.04) (Fig. 1B). There were no significant differences in the vibriocidal antibody titers at convalescence among the various age groups (days 7 and 30).
Fig. 1.
(A) Median vibriocidal antibody titers by age group, with error bars representing interquartile ranges. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 2) titer. (B) Mean fold changes in vibriocidal titer between days 2 and 7. Error bars represent standard errors.
Antigen-specific antibody responses in plasma.
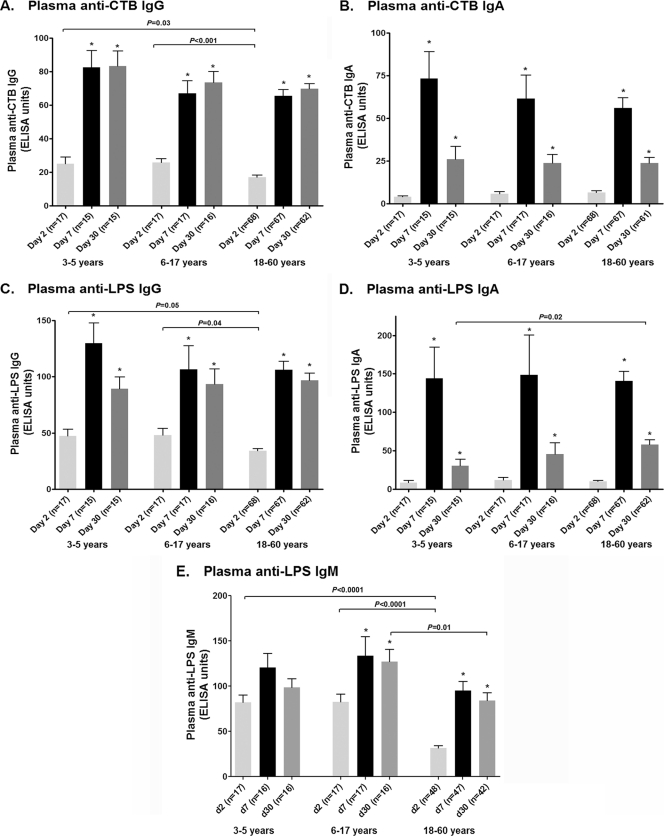
All age groups had significantly higher IgG and IgA antibody levels to CtxB and LPS at days 7 and 30 than at day 2 (Fig. 2). Younger children had higher baseline (day 2) plasma LPS (P = 0.05) and CtxB (P = 0.03) IgG levels than adults, although no significant differences between age groups were seen at days 7 and 30. The day 2 and day 7 antigen-specific IgA levels in plasma were comparable in all groups, although adults had higher LPS IgA responses on day 30 than young children (P = 0.02). Both younger and older children had higher day 2 IgM levels to LPS than adults (P < 0.001), and older children had higher levels at day 30 than adults (P = 0.01).
Fig. 2.
Mean plasma antibody responses by age group as measured by ELISA, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 2).
Antigen-specific ASC responses.
We assessed CtxB- and LPS-specific ASC responses on days 2, 7, and 30 (Fig. 3A to D). Baseline (day 2) ASC levels were comparable across all age groups. As expected, maximal ASC responses occurred on day 7 in all age groups, and levels returned to baseline by day 30. There was a trend for the older age groups to have higher LPS IgA responses at day 7 than the youngest group (P = 0.07). Younger children had a significantly higher number of total circulating IgA ASCs than adults for all days examined (Fig. 3E).
Fig. 3.
(A to D) Mean absolute numbers of antigen-specific ASC responses by age group, as measured by ELISPOT assay, per 5 × 106 PBMCs, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 2). (E) Mean numbers of total ASC IgA responses by age group. Error bars represent standard errors.
Antigen-specific MBC responses.
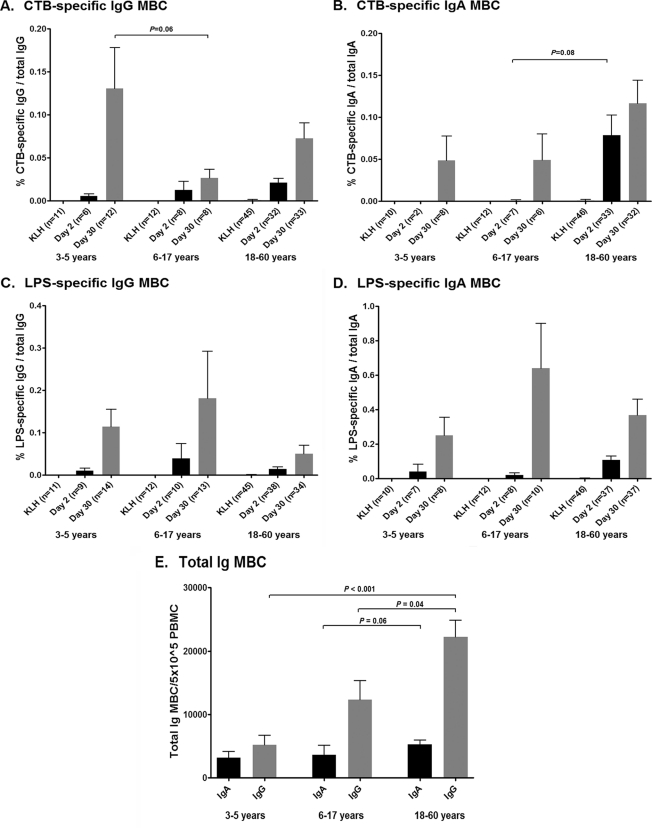
We compared two published methods to stimulate and assess MBC responses (10, 38). We found no differences in either the total or the antigen-specific MBC IgA and IgG responses between the two stimulation methods in 8 patients and 7 healthy controls. We here report the results obtained using the method of Crotty et al. (10). We assessed CtxB- and LPS-specific IgG and IgA memory B cell responses on days 2 and 30 (Fig. 4A to D). Baseline (day 2) MBC levels were comparable across all age groups, antigens, and isotypes, with the exception that there was a trend for baseline IgA anti-CtxB MBC levels to be higher in adults than in children (P = 0.08 for adults versus older children). Importantly, we detected MBC responses on day 30 in all age groups, for both antigens and for both antibody isotypes. IgA MBC responses were comparable across all age groups. The CtxB IgG MBC response at day 30 tended to be higher in young children than in older children (P = 0.06). The LPS IgG MBC responses at day 30 were similar across all age groups. Following stimulation, infected adults had a larger total MBC IgG response than infected younger (P < 0.001) and older (P = 0.04) children on day 30 (Fig. 4E). This pattern was also observed with the total MBC IgA response but to a lesser degree that was not significant (P = 0.06 for older children versus adults).
Fig. 4.
(A to D) Mean antigen-specific memory B cell responses by age group, as measured by ELISPOT assay after 6-day polyclonal stimulation, as percentage of total memory B cells, with error bars representing standard errors of the mean. (E) Mean numbers of total stimulated MBCs on day 30 by age group. Error bars represent standard errors.
DISCUSSION
Although children bear a significant component of the global burden of cholera, relatively little is known about immune responses in children with cholera (7), especially in children less than 5 years of age. In this study, we compared immune responses in young children, older children, and adults with cholera in an area of cholera endemicity. The vibriocidal antibody response is one of the most commonly used markers for assessing protection against cholera. It is thought to be a surrogate marker of protection since it detects the presence of complement binding anti-V. cholerae antibodies in the peripheral blood. In areas of the world in which cholera is endemic, the mean vibriocidal antibody titer increases with increasing age, presumably reflecting previous exposure to V. cholerae (13). However, there is no level of vibriocidal antibody titer absolutely associated with protection against cholera (32), and we and others have previously shown that vibriocidal responses decline to baseline within 6 to 12 months after an episode of severe cholera in Bangladesh (14). This is despite the fact that protection against cholera exists for at least 3 years (the last period examined) following experimental exposure and rechallenge with the organism (22), and mathematical modeling suggests that previous infection with V. cholerae affords significant protection that lasts up to 10 years (20). In the present study, we found that young children had the lowest baseline vibriocidal antibody levels, presumably a reflection of the fact that older children and adults may have already been exposed to V. cholerae infections. Despite this, the magnitudes of the vibriocidal antibody response at convalescence were equivalent in all age groups, including younger children, showing that young children are capable of mounting robust vibriocidal antibodies after exposure.
The vibriocidal response is largely directed against LPS (27), and we have recently demonstrated that LPS IgA responses, as well as CtxB IgA responses, also correlate with protection against cholera (15). In our present study, we showed that children had higher CtxB IgG levels in peripheral blood at baseline than adults, possibly reflecting the presence of cross-reactive antibodies that recognize both cholera toxin (CT) and the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) (13, 36), the latter a common cause of acute watery diarrhea in young children in resource-limited areas of the world (29). Despite these different baseline levels, all age groups had comparable CtxB IgA and IgG responses at convalescence. Baseline LPS IgG levels in blood were also slightly higher in children than adults, but the cause of this small difference is uncertain; however, the magnitudes of LPS-specific IgG responses in plasma at convalescence were comparable across all age groups. Baseline IgM responses to LPS in children were significantly higher than those in adults, which is opposite what was observed for vibriocidal antibody. Despite the fact that the vibriocidal antibody is mostly IgM directed to LPS (23, 25), we have previously observed that no correlation exists between plasma IgM and vibriocidal antibodies in adults with cholera (18). The LPS-specific IgA response in adults was higher than that in young children on day 30; this observation may reflect the ability of adults to mount more-prominent immune responses to T cell-independent antigens than young children (19). In summary, despite some differences in immune responses, young children were able to mount prominent antitoxin and antibacterial B cell responses in blood, and the magnitudes were comparable to those observed in older children and adults.
V. cholerae is a noninvasive mucosal pathogen. To assess mucosal immune responses, we measured ASC responses in peripheral blood. Following mucosal stimulation, intestinal lymphocytes transiently migrate in the peripheral circulation before rehoming to intestinal tissue (11). This migration peaks 7 to 10 days after mucosal challenge. We found that young children were able to mount prominent anti-CtxB ASC responses that peaked on day 7 and that these responses were comparable to those observed in older children and adults. There was a trend toward older age groups having a higher day 7 LPS-specific IgA ASC response than the youngest group, which corresponded with the higher plasma LPS IgA response on day 30 in adults that we observed. These observations may reflect a lower mucosal response to T cell-independent antigens, such as LPS, in young children (9), and these responses may increase with age due to immune maturation and continued pathogen exposure. Interestingly, younger children had a higher total pool of IgA-producing ASCs in peripheral blood than adults, which corresponds with observations that the number of plasma cells is also inversely correlated with age (5).
We have previously shown that memory B cell responses to V. cholerae O1 are detectable for at least 1 year after infection in adults and persist even after plasma antibodies and ASCs have returned to baseline levels (14). We were thus particularly interested in evaluating MBC responses in children compared to those in adults. In our current study, we found that both younger and older children were able to mount CtxB- and LPS-specific IgG and IgA MBC responses at day 30 that were comparable to those observed in adults. There was a trend for younger children to mount higher levels of CtxB IgG MBC responses than older children, perhaps reflecting more-recent exposure to the LT antigen of ETEC in young children. In comparison, there was a nonsignificant trend for adults to have higher baseline CtxB IgA MBCs. The cause and significance of this observation are uncertain, although the trend could reflect more-recent exposure to V. cholerae.
We also found that the total stimulated IgG MBCs increased with age, a phenomenon previously reported in a population from a region of malaria endemicity (39). A similar trend was noted for the IgA MBC compartment. These findings may be expected, as circulating MBCs are likely a reflection of a cumulative lifetime of antigenic exposures. When the absolute numbers of antigen-specific MBC spots per million PBMCs were measured, adults had a higher baseline LPS IgA MBC level than young children, which may reflect previous exposure; however, when this measurement was calculated as a percentage of total MBCs, this trend disappeared, given the overall higher number of MBCs among PBMCs in adults.
A limitation to our study is that due to difficulties inherent in enrollment of young children, the sample size of the groups of children in our study is relatively small compared to that of our adult cohort. In addition, in this study we followed children only up to 30 days postinfection. The persistence of the MBC response in young children, compared to that seen in adults (14), may further reveal the mechanism behind differences in vaccine efficacy, and a study addressing this issue is ongoing.
Current cholera immunization strategies focus on oral, whole-cell killed and live attenuated vaccines. These vaccines are protective, but their duration of protection is less than that afforded by wild-type infection, even in adults (20, 35). The immunogenicity of protection appears to be of a shorter duration in young children than in adult vaccine recipients (35, 37), despite the fact that children under 5 years of age also bear a large burden of cholera in areas of the world in which cholera is endemic (12). Natural cholera infection includes exposure to cholera toxin, which is not only an enterotoxin but also a potent immunoadjuvant (33). We have recently analyzed memory B cell responses in adult recipients of an oral cholera vaccine in Bangladesh and noted that responses were both less prominent and of shorter duration than those induced by wild-type disease (1). We hypothesize that these reduced responses following vaccination compared to infection may reflect the absence of the immunoadjuvantative properties of CT in current oral cholera vaccines. Experimental studies in animal models evaluating oral adjuvanticity of the toxin have demonstrated that CT stimulates a greater mucosal immune response than CtxB alone (4, 6); unfortunately, the use of CT as a vaccine component is limited due to its toxicity, although mutant toxoids are being explored for their use in vaccines (28, 40). The precise differences in immune stimulation between wild-type disease and current vaccines remain to be elucidated, and further studies are warranted to account for the age-specific differences in long-term vaccine efficacy seen with current vaccine formulations. However, our current observation that young children with cholera are able to mount immune responses, including memory B cell responses, comparable to those induced in older children and adults suggests that an optimal vaccination strategy for young children should be possible and could achieve protective efficacy comparable to that induced in adults.
ACKNOWLEDGMENTS
This work was supported by the ICDDR,B and grants from the National Institutes of Health, including the National Institute of Allergy & Infectious Diseases (U01 AI058935 [S.B.C. and E.T.R.], R03 AI063079 [F.Q.[, and U01 AI077883 [E.T.R.]), a Training Grant in Vaccine Development from the Fogarty International Center (TW05572 [M.M.A., M.S.B., and F.Q.]), an American Recovery and Reinvestment Act (ARRA) FIC Postdoctoral Fellowship in Global Infectious Diseases (TW05572 [D.T.L.]), Career Development awards (K01) from the Fogarty International Center (TW007409 [J.B.H.] and TW07144 [R.C.L.]), the Infectious Diseases Society of America Medical Scholars Program (E.J.K.), the American Society of Tropical Medicine and Hygiene Benjamin H. Kean Traveling Fellowship in Tropical Medicine (E.J.K.), a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (R.C.L.), and the Harvard Initiative for Global Health Postdoctoral Fellowship in Global Infectious Diseases (D.T.L.).
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Alam M. M., et al. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin. Vaccine Immunol. 18:844–850 doi:10.1128/CVI.00562-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous. 2009. Cholera: global surveillance summary, 2008. Wkly. Epidemiol. Rec. 84:309–324 [PubMed] [Google Scholar]
- 3. Anonymous. 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:117–128 [PubMed] [Google Scholar]
- 4. Blanchard T. G., Lycke N., Czinn S. J., Nedrud J. G. 1998. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against Helicobacter felis. Immunology 94:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caraux A., et al. 2010. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologicaae 95:1016–1020 doi:10.3324/haematol.2009.018689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen K. S., Strober W. 1990. Cholera holotoxin and its B subunit enhance Peyer's patch B cell responses induced by orally administered influenza virus: disproportionate cholera toxin enhancement of the IgA B cell response. Eur. J. Immunol. 20:433–436 doi:10.1002/eji.1830200230 [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury F., et al. 2008. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr. Infect. Dis. J. 27:986–992 doi:10.1097/INF.0b013e3181783adf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clemens J. D., et al. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273 [DOI] [PubMed] [Google Scholar]
- 9. Clutterbuck E. A., et al. 2008. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin. Vaccine Immunol. 15:182–193 doi:10.1128/CVI.00336-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crotty S., Aubert R. D., Glidewell J., Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111–122 doi:10.1016/j.jim.2003.12.015 [DOI] [PubMed] [Google Scholar]
- 11. Czerkinsky C., et al. 1987. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc. Natl. Acad. Sci. U. S. A. 84:2449–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deen J. L., et al. 2008. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Negl. Trop. Dis. 2:e173 doi:10.1371/journal.pntd.0000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glass R. I., et al. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236–242 [DOI] [PubMed] [Google Scholar]
- 14. Harris A. M., et al. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850–3856 doi:10.1128/IAI.00369-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris J. B., et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221 doi:10.1371/journal.pntd.0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. John M., Bridges E. A., Miller A. O., Calderwood S. B., Ryan E. T. 2002. Comparison of mucosal and systemic humoral immune responses after transcutaneous and oral immunization strategies. Vaccine 20:2720–2726 [DOI] [PubMed] [Google Scholar]
- 17. Kelly D. F., Pollard A. J., Moxon E. R. 2005. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA 294:3019–3023 doi:10.1001/jama.294.23.3019 [DOI] [PubMed] [Google Scholar]
- 18. Kendall E. A., et al. 2010. Development of immunoglobulin M memory to both a T-cell-independent and a T-cell-dependent antigen following infection with Vibrio cholerae O1 in Bangladesh. Infect. Immun. 78:253–259 doi:10.1128/IAI.00868-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein Klouwenberg P., Bont L. 2008. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin. Dev. Immunol. 2008:628963 doi:10.1155/2008/628963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koelle K., Rodo X., Pascual M., Yunus M., Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 doi:10.1038/nature03820 [DOI] [PubMed] [Google Scholar]
- 21. Legros D., et al. 2000. Epidemiology of cholera outbreak in Kampala, Uganda. East Afr. Med. J. 77:347–349 [DOI] [PubMed] [Google Scholar]
- 22. Levine M. M., et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 23. Losonsky G. A., et al. 1996. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect. Immun. 64:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luque Fernandez M. A., et al. 2011. Descriptive spatial analysis of the cholera epidemic 2008-2009 in Harare, Zimbabwe: a secondary data analysis. Trans. R. Soc. Trop. Med. Hyg. 105:38–45 doi:10.1016/j.trstmh.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 25. Majumdar A. S., Ghose A. C. 1981. Evaluation of the biological properties of different classes of human antibodies in relation to cholera. Infect. Immun. 32:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mosley W. H., Benenson A. S., Barui R. 1968. A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull. World Health Organ. 38:327–334 [PMC free article] [PubMed] [Google Scholar]
- 27. Neoh S. H., Rowley D. 1970. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J. Infect. Dis. 121:505–513 [DOI] [PubMed] [Google Scholar]
- 28. Norton E. B., Lawson L. B., Freytag L. C., Clements J. D. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 18:546–551 doi:10.1128/CVI.00538-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qadri F., Svennerholm A. M., Faruque A. S., Sack R. B. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483 doi:10.1128/CMR.18.3.465-483.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qadri F., et al. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sack R. B., et al. 2003. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J. Infect. Dis. 187:96–101 doi:10.1086/345865 [DOI] [PubMed] [Google Scholar]
- 32. Saha D., et al. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318–2322 doi:10.1086/421275 [DOI] [PubMed] [Google Scholar]
- 33. Sanchez J., Holmgren J. 2011. Cholera toxin—a foe & a friend. Indian J. Med. Res. 133:153–163 [PMC free article] [PubMed] [Google Scholar]
- 34. Sasaki S., Suzuki H., Igarashi K., Tambatamba B., Mulenga P. 2008. Spatial analysis of risk factor of cholera outbreak for 2003-2004 in a peri-urban area of Lusaka, Zambia. Am. J. Trop. Med. Hyg. 79:414–421 [PubMed] [Google Scholar]
- 35. Sinclair D., Abba K., Zaman K., Qadri F., Graves P. M. 2011. Oral vaccines for preventing cholera. Cochrane Database Syst. Rev. 3:CD008603 doi:10.1002/14651858.CD008603.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith N. W., Sack R. B. 1973. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J. Infect. Dis. 127:164–170 [DOI] [PubMed] [Google Scholar]
- 37. Sur D., et al. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702 doi:10.1016/S0140-6736(09)61297-6 [DOI] [PubMed] [Google Scholar]
- 38. Tengvall S., Lundgren A., Quiding-Jarbrink M., Svennerholm A. M. 2010. BAFF, stimulatory DNA and IL-15 stimulates IgA(+) memory B cells and provides a novel approach for analysis of memory responses to mucosal vaccines. Vaccine 28:5445–5450 doi:10.1016/j.vaccine.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 39. Weiss G. E., et al. 2010. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 6:e1000912 doi:10.1371/journal.ppat.1000912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf A. A., et al. 2008. Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster ganglioside GM1 molecules. Infect. Immun. 76:1476–1484 doi:10.1128/IAI.01286-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zuckerman J. N., Rombo L., Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 doi:10.1016/S1473-3099(07)70138-X [DOI] [PubMed] [Google Scholar]