Abstract
Complement-mediated bactericidal activity has long been regarded as the serological correlate of protective immunity against Neisseria meningitidis. This was affirmed in 2005 at a WHO-sponsored meningococcal serology standardization workshop. The assay currently employed by most laboratories involves determining surviving bacterial colony counts on agar as a readout which is labor-intensive, time-consuming, and not amendable to rapid data analysis for clinical trials. Consequently, there is an acute need to develop a sensitive, high-throughput bactericidal assay to enable a rapid and robust assessment of the effectiveness of vaccine candidates. To this end, we have developed an automated, kinetic assay based on the fluorescent respiration product of resazurin which reduces assay volume, shortens assay time, and facilitates automation of data analysis. We demonstrate proof of concept for applicability of this high-throughput system with multiple meningococcal strains and utilizing different lots of human complement. The assay is robust and highly reproducible. Titers obtained by the fluorescence readout method are strongly correlated with the data obtained using the conventional, agar plate-based assay. These results demonstrate that the detection of bacteria that have survived the bactericidal reaction by measuring metabolic activity using a fluorescent dye as an alternative readout is a promising approach for the development of a high-throughput bactericidal assay.
INTRODUCTION
Goldschneider et al. documented that serum bactericidal activity is inversely correlated with age-related incidence of invasive meningococcal disease for serogroups A, B, and C and demonstrated that the presence of bactericidal antibodies is predictive of protection from the disease (7). Serum bactericidal titer was used as the primary endpoint measurement for licensure of serogroup C polysaccharide conjugate vaccines (3) and additionally for groups A, W135, and Y for licensure of tetravalent polysaccharide conjugate vaccines (4). In clinical trials for group B outer membrane vesicle vaccines, serum bactericidal titers correlated with clinical efficacy and were used as the primary measure of protection against disease in several countries, including New Zealand, Norway, and Brazil (9, 10, 12, 16). The importance of serum bactericidal activity as a primary endpoint for evaluation of group B vaccines was reaffirmed in a report issued by a WHO meeting on correlates of protection and standardization for Neisseria meningitidis group B in March of 2005 (2).
The assay format of the serum bactericidal assay (SBA) has remained largely unchanged since Goldschneider et al. described the in vitro test in 1969. In the conventional SBA, test serum or plasma is serially diluted 2-fold usually from a starting dilution of 1:2 or 1:4. Each dilution of the test sample is then incubated with bacteria in the presence of an exogenous source of active complement. Bactericidal antibodies in test samples bind to the surface of the bacteria, resulting in the activation of complement proteins and the formation of the membrane attack complex, which results in bacteriolysis. Bacteria that survive complement-mediated lysis are enumerated by plating on solid growth medium. Bactericidal antibody titers are calculated as the reciprocal dilution of test sample that kills 50% or more of the bacteria inoculum in the assay. The enumeration of CFU in the conventional methodology restricts several assay parameters of the SBA, including assay volume, input number of bacteria, number of replicates tested, assay duration, and the requirement of a human operator for colony plating. The process is mostly performed manually, and interoperator variability has to be established as a source of error when an assay is being qualified for clinical trials.
The burdens of the conventional assay are particularly apparent during clinical trials when volumes of test samples are limiting, particularly in infants, but test population and breadth of bacterial strains to be tested are large. For meningococci, the preferred SBA method involves use of human complement obtained from normal volunteer donors. This is important because meningococci have known surface proteins that bind complement components in a species-specific manner in order to mask the bacteria and protect them from lysis (8). Many normal human donors have intrinsic bactericidal activity against meningococci; hence, a specific complement source must be obtained for each meningococcal isolate. Miniaturizing the test is important not only from the point of view of economy of specimen but also for conservation of the valuable resources of human donor complement.
In this study, we describe a modified version of the SBA that is termed the high-throughput SBA (HT-SBA). It adheres to the same assay principles as the conventional SBA but differs in that the bactericidal reaction is not plated on agar but instead receives a mixture of liquid growth medium and the cell-permeable redox substrate resazurin dye (alamarBlue). The assay is miniaturized to a 384-well format with reduced volume and can be easily implemented with laboratory robotic systems, thereby significantly increasing assay throughput. Resazurin is reduced to the fluorescent product resorufin in viable cells. Both resazurin and resorufin are nontoxic to cells allowing a homogenous assay format and kinetic measurement of fluorescent signal over time. The signal is proportional to the number of metabolically active cells and can be used to calculate a bactericidal titer directly from the microplate using a fluorescence plate reader. The HT-SBA differs from previous attempts to develop an alternative non-agar-based readout for the bactericidal reaction (13–15) in that it utilizes a kinetic measurement to optimize the time point selected for titer determination for each assay run. Our kinetic approach allows for the optimization of the signal differential between wells with high versus low bacterial counts following the bactericidal reaction such that the most sensitive measurements are achieved. Any changes in assay parameter values such as different growth characteristics of various bacterial strains are captured in the kinetic growth data, allowing for better accuracy in titer calculations. We demonstrate a strong correlation of titers between the conventional and HT-SBA using different MenB strains, lots of human complement, and test samples.
MATERIALS AND METHODS
Strains and reagents.
Meningococcal group B clinical isolates NZ98-254, H44/76, and 5/99, with serotype and antigenic compositions previously described (5), were stored frozen as stock cultures at −80°C in 10% skim milk (232100; Becton Dickinson). Strains were thawed and grown overnight on chocolate agar at 37°C in 5% CO2 for use the following day in the bactericidal assay.
Serum/plasma samples.
The serum samples used for this study were from two randomized, single-blind phase I studies conducted to assess the safety, tolerability, and immunogenicity of a meningococcal serogroup B recombinant vaccine administered alone or in combination with outer membrane vesicles from the Norwegian serogroup B strain H44/76 (17). The serum panel was selected to give a broad range of bactericidal titers. Authors were blinded as to which vaccine group the individual samples were derived. When plasma was used as the source of human complement, heparin (H3149; Sigma) was added to both the conventional and the high-throughput assays at a 1.0-U/ml final concentration.
Conventional SBA.
The standardized serum bactericidal assay (SBA) was performed using human complement as described previously (1). Frozen stock meningococcal strains were thawed and grown overnight on chocolate agar at 37°C in 5% CO2 for use in the bactericidal assay. The following day, isolated colonies were pooled and then subcultured in Mueller-Hinton broth (MHB) (275730; Becton Dickinson) for approximately 2 h to the mid-log phase of growth. Bacteria were then diluted in assay diluent, consisting of Dulbecco's phosphate-buffered saline (DPBS) (21-030-CV; Mediatech) with 1% bovine serum albumin (BSA) (A7888; Sigma) supplemented with 0.1% glucose (G5767; Sigma) to a concentration of approximately 2.0 × 104 bacteria/ml for use in the assay. Test sera were serially diluted 2-fold in assay diluent in 96-well plates starting from a 1:2 dilution and then incubated for 60 min with bacteria and 25% human serum complement lacking intrinsic bactericidal activity in a total reaction volume of 80 μl. Aliquots were spread onto chocolate agar plates and grown overnight. Colonies were counted after 16 to 20 h of growth, and the 50% titer of each test specimen was determined relative to the time zero (t0) inoculum. Bacterial cultures were grown in a humidified incubator at 37°C in 5% CO2 at each step.
HT-SBA.
The high-throughput serum bactericidal assay (HT-SBA) adheres to the same assay principles as the conventional SBA but diverged from the standard assay as follows. The bactericidal reaction was performed in a 384-well microtiter black flat-bottom plate (781209; Greiner) in 20 μl of a reaction volume. Unless otherwise stated, all experiments used a 5-μl starting inoculum of 1.5 × 105 CFU/ml. Following completion of the bactericidal reaction, each well received 20 μl of a mixture of liquid growth medium consisting of MHB or DPBS, supplemented with glucose and alamarBlue (DAL1100; Invitrogen); the plate was sealed with a gas-permeable optically clear film (2978-2100; USA Scientific) and incubated at 37°C in 5% CO2 in 98% humidity. alamarBlue was prepared fresh the day of use. A serum normalization control series that lacks bactericidal activity against the strain of interest was included in each experiment to monitor the effect of serum quenching of resorufin. At hourly intervals, the plate was removed from the incubator and read to detect the fluorescence signal generated when resazurin is reduced to resorufin by bacterial respiration using a fully automated robotic platform built by GNF Systems (11). The data were collected for 16 h and then analyzed for titer determination in a three-step process. First, the bacterial growth curve derived from changes in fluorescence was plotted using controls that monitor growth in human plasma or serum complement lacking bactericidal activity. Second, a time point used for titer determination where the bacteria were in the exponential growth phase and the signal was at least 50% of the plateau was selected. Third, after the time point was selected, the data were normalized to the above-mentioned serum normalization control series and analyzed by a four-parameter logistic curve fitting as described in detail in Results. The reciprocal serum dilution that resulted in a 50% reduction of fluorescence (IC50) relative to the normalized maximum signal was reported as the titer of the sample.
RESULTS
Determination and optimization of assay parameter values. (i) Identification of grow-out media.
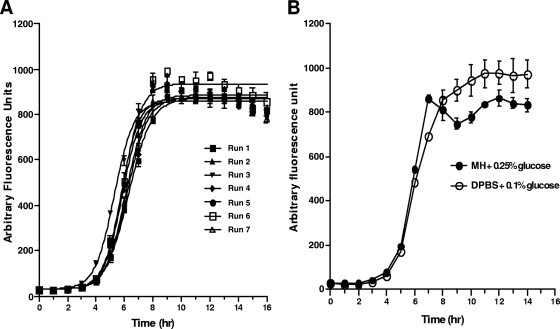
Successful miniaturization of the SBA was dependent upon the identification of a readout method that will allow the quantification directly in the well of bacteria that survive challenge with antibody and active complement. This can be accomplished only with suitable grow-out media that allow surviving bacteria to recover and proliferate. In Fig. 1A, growth curves are shown from 7 successive runs for the group B strain, NZ 98/254, using Mueller-Hinton broth (MHB) supplemented with 0.25% glucose and alamarBlue as the grow-out medium. The curves are plotted following treatment for 1 h with serum complement which has no intrinsic bactericidal activity. This postreaction growth phase is analogous to plating a sample on chocolate agar, which also allows growth of surviving bacteria that will go on to form colonies. The growth curves of NZ 98/254 following incubation with complement are highly reproducible, with the exponential phase of growth typically between the 4th and 9th hours following addition of the grow-out medium. As the scope of the study was expanded to include different bacterial strains, complement sources, and test sera, it was determined that MHB supplemented with 0.25% glucose was not universally applicable for use as a grow-out medium. In order to circumvent this problem, growth in alternative medium was examined. DPBS supplemented with 0.1% glucose was subsequently found to support NZ98/254 growth similar to MHB (Fig. 1B) and have the best compatibility profile for all strains tested.
Fig. 1.
(A) Growth curve of NZ98-254 in MHB-0.25% glucose measured by fluorescence signal generated when resazurin is reduced into resorufin by bacterial respiration. (B) Comparable growth is observed when NZ98-254 was grown in MHB-0.25% glucose or DPBS-0.1% glucose, both containing 25% human serum. Data points represent the averages and standard deviations for six separate fluorescence measurements.
(ii) Time point selection for titer determination.
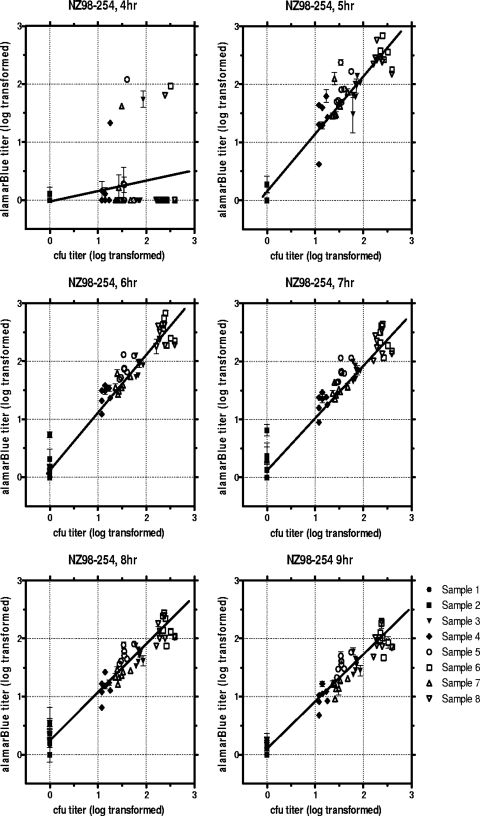
In each of the seven individual experiments represented in Fig. 1, eight human serum samples were tested in parallel by both the conventional and the HT-SBA methods and the resulting titers were compared. In the HT-SBA, titers were calculated for all eight samples at each time point from 4 to 9 h after the addition of alamarBlue. These results are plotted for each time point in Fig. 2. As demonstrated by the linear regression of titers (Table 1), in this series of assays the HT-SBA data obtained at the 5-, 6-, and 7-h time points showed the best agreement with the titers obtained in the conventional SBA. In order to automate time point selection and reduce operator bias, a performance-based selection process was assessed. In this case, the time point just before the fluorescence signal reached 90% of the signal plateau (AFU90) was used. The AFU90 can differ from experiment to experiment, as observed in the seven runs shown in Fig. 1, presumably due to slight variation in bacterial inoculum at the start of each assay. In this set of runs, the actual time points corresponding to the AFU90 ranged between 7 and 8 h. When the AFU90 was used as the selection criterion, the linear regression of titers remained high (R2 = 0.907).
Fig. 2.
Correlation of serum titers from nonimmunized human adults (samples 1 and 2) and immunized human adults (samples 3, 4, 5, 6, 7, and 8) determined by the HT-SBA compared to those determined by the conventional assay at six different time points.
Table 1.
Linear regression values for data shown in Fig. 2
| Time point |
ya |
||
|---|---|---|---|
| a | b | R2 | |
| 4 | 0.039 | 0.133 | 0.045 |
| 5 | 0.117 | 1.024 | 0.932 |
| 6 | 0.160 | 0.992 | 0.950 |
| 7 | 0.198 | 0.909 | 0.934 |
| 8 | 0.240 | 0.813 | 0.927 |
| 9 | 0.103 | 0.793 | 0.945 |
| AFU90b | 0.187 | 0.907 | 0.935 |
y = a + b × x, where a is the y intercept and b is the slope.
The time point at which the fluorescence signal reaches 90% of the signal plateau (AFU90) ranged between 7 and 8 h.
(iii) Serum normalization.
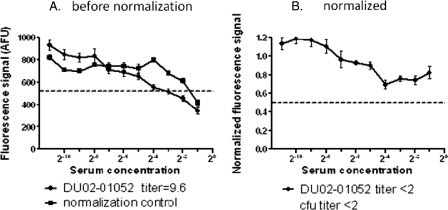
In the SBA, test samples were serially diluted and then combined with exogenous complement and bacteria. Consequently, each individual sample in a dilution series has a different final concentration of serum. It is a documented feature of alamarBlue that the fluorescence signal can be quenched by the presence of serum (6). This quenching phenomenon was also observed in the HT-SBA and can result in an overestimation of titer. In order to correct for this effect, we introduced an experimental control, the serum normalization series. This series involves the use of a negative-control serum, which lacks intrinsic bactericidal activity, serially diluted in the same manner as the test sera. This control series lacks bactericidal activity; hence, any loss in the fluorescence signal, as the serum concentration increases, is due to serum absorption of resorufin (Fig. 3A, closed squares). In order to normalize data obtained for each serum dilution series, the median fluorescence intensity of the triplicate values of the serum normalization control is computed for each combination of time point and dilution point. Each test serum value is then divided by the normalization factor for its corresponding time and dilution. In the example shown in Fig. 3A, test sample 2 had a titer of <2 by the conventional SBA method; however, in the HT-SBA the titer for sample 2 would have been 9.6 without normalization (closed diamonds). After normalization using corresponding dilution points from the normalization control serum, when recalculated the titer was determined to be <2, in agreement with the titer obtained from the conventional SBA (Fig. 3B).
Fig. 3.
Panel A shows the fluorescence signal generated by test serum (closed diamonds) and the normalization control (closed squares). Panel B demonstrates that after normalization, the test serum has little bactericidal activity, and the titer is determined to be <2. The dashed line represents approximately 50% killing signal.
(iv) Curve fitting.
After the appropriate time point was chosen from the postreaction grow-out phase of the assay for interpretation of data and the raw values were normalized to correct for the serum quenching effect, the antibody titer for each test sample was determined as the IC50 value of the reciprocal of the dilution by fitting sample fluorescence data with a 4-parameter logistic equation. The curve-fitting constraints of the normalized data were set to have an upper limit bound between 0.8 and 1.5, a lower limit between 0 and 0.15, and a Hill slope between −3.0 and +3.0. One outlier is allowed for each curve fitted. The equation used for this analysis is as follows: response = bottom + {[top − bottom]/[1 + (concentration/IC50)−Hill]}.
Comparison of assays.
The relative performance of the HT-SBA was assessed, including assay reproducibility, robustness, and linearity, to determine the comparability of results in parallel with the conventional SBA. For the conventional SBA, each sample run was assayed once in a 96-well microtiter plate per test and the bactericidal reaction was plated on agar in duplicate for titer determination. The HT-SBA was carried out in higher-density 384-microtiter-plate format, with the reaction volume reduced by 75%. Test serum was assayed in triplicate, and the fluorescence signal for each replicate was analyzed to determine the corresponding reciprocal serum dilution to the IC50 of the normalized fluorescence signal. A sample with a negative titer of <2 was assigned a value “1” for statistical analysis purposes. For all data discussed below, the time point just before the growth curve reached 90% of the plateau value point (AFU90) was used for titer calculation, unless otherwise stated. For the purpose of comparing the two methods, titer values obtained by the HT-SBA and the standardized SBA were both log transformed.
(i) Reproducibility.
To demonstrate that the HT-SBA is robust and can generate reproducible data, a panel of 8 human sera covering a range of titers were assayed seven times in parallel with the conventional SBA using strain NZ98/254, as shown in Fig. 2. Good agreement was seen between the two assays, with a Pearson product-moment correlation coefficient (R2) of 0.935 when the AFU90 was used as the basis for time point selection.
(ii) Robustness with different inoculum concentrations.
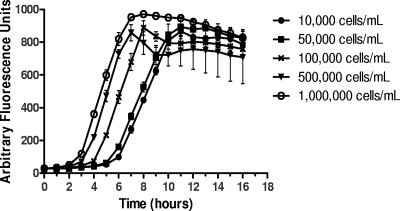
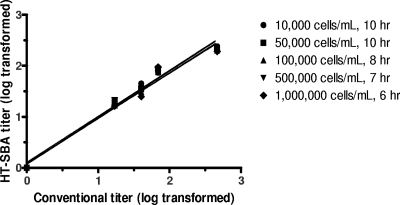
Given that the survival of bacteria after the bactericidal assay is assayed by fluorescence signal rather than enumeration of CFU on an agar plate, it is important to know the limitation and robustness of the HT-SBA when variable bacterial inoculum is used in each assay. To assess this, a panel of five test sera were tested against strain NZ98/254 with inoculum concentrations 10-fold higher and lower than the 105 CFU/ml typically used in the HT-SBA. In examining the growth curves, different inoculum concentrations generated fluorescence signals at different rates and reached the plateau at different time points (Fig. 4). Given the difference in growth kinetic, the data set from each inoculum was calculated based on the AFU90 from each run. The data from these runs are summarized in Fig. 5 and Table 2, which demonstrates that varying the concentration of bacterial inoculum added to each well in the HT-SBA from 104 to 106 per ml had little effect on the correlation of bactericidal titers compared to the conventional SBA, where the inoculum added is approximately 104.
Fig. 4.
Growth curves of NZ98/254 at different starting inoculum concentrations. Shown are average fluorescence signals and standard deviations for 6 wells.
Fig. 5.
Comparison of titers from serum samples (n = 5) obtained by the conventional assay and the alamarBlue assay over a range of starting inoculum concentrations.
Table 2.
Linear regression values shown in Fig. 5
| No. of cells/ml (time point) | Slope | y intercept when x = 0.0 | r2 |
|---|---|---|---|
| 10,000 (10 h) | 0.9107 ± 0.03386 | 0.07361 ± 0.05833 | 0.985 |
| 50,000 (10 h) | 0.9040 ± 0.04631 | 0.1041 ± 0.07977 | 0.972 |
| 100,000 (8 h) | 0.8862 ± 0.03774 | 0.08079 ± 0.06501 | 0.980 |
| 500,000 (7 h) | 0.8984 ± 0.03348 | 0.06638 ± 0.05767 | 0.985 |
| 1,000,000 (6 h) | 0.8893 ± 0.05166 | 0.08170 ± 0.08897 | 0.964 |
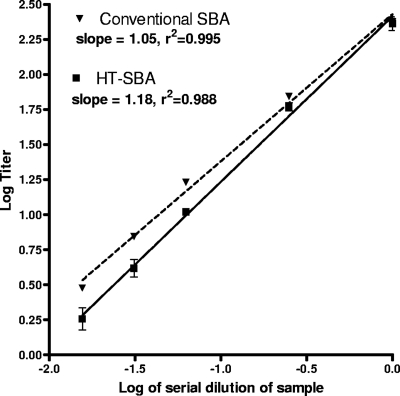
(iii) Linearity.
In order to assess linearity of the HT-SBA, a series of samples were made from a high-titer serum which was diluted by factors of 4, 16, 32 and 64 and then tested in concurrent assays with the conventional or HT-SBA using strain NZ98/254. Samples were diluted in assay diluent consisting of DPBS and 1% bovine serum albumin. As shown in Fig. 6, using simple linear regression we observed linearity between the titers obtained and the predilution factor in both assay methods, with a slope of 1.18 and R2 values of 0.988 for the HT-SBA and a slope of 1.05 and an r2 of 0.995 for the conventional SBA.
Fig. 6.
Demonstration of the linearity of the alamarBlue assay by serial dilution of the test sample. A test sample is prediluted at different ratios (x axis) and assayed in parallel for CFU or alamarBlue readouts. In both cases, the titer calculated is log transformed and plotted on the y axis. Simple linear regression between titer determined and dilution factor were determined, and the slope and correlation coefficients are listed in the legend.
(iv) Additional meningococcal strains.
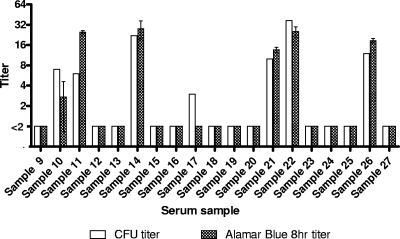
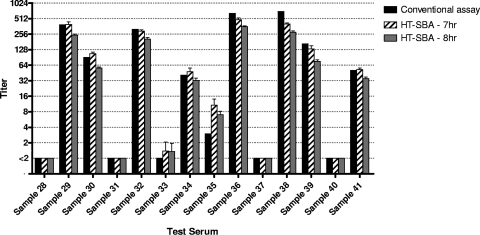
Strain H44/76 was tested concurrently in the conventional and HT-SBA in two independent experiments. Serum samples with low titers (<64) were assayed (Fig. 7), and good agreement in titers was observed between the conventional and the HT-SBA methods (R2 = 0.8659). No false positives for the HT-SBA were detected. In a similar comparison using strain 5/99 and a sample set with a broader range of titers, between <2 and 1,024 (Fig. 8), good agreement in titers was also observed between the two methods at 7 h (R2 = 0.98) and at 8 h (R2 = 0.9865) of bacterial growth after the bactericidal reaction in the HT-SBA.
Fig. 7.
Comparison of titers from the conventional assay and the HT-SBA using a panel of low-titer serum samples from nonimmunized human adult donors against the 44/76-SL strain.
Fig. 8.
Comparison of titers from nonimmunized human adults (samples 28, 31, 33, 35, 37, and 40) and immunized human adults (samples 29, 30, 32, 34, 36, 38, 39, and 41) obtained for strain 5/99 in the conventional assay and the HT-SBA after 7 or 8 h of growth following the bactericidal reaction.
(v) Use of plasma complement.
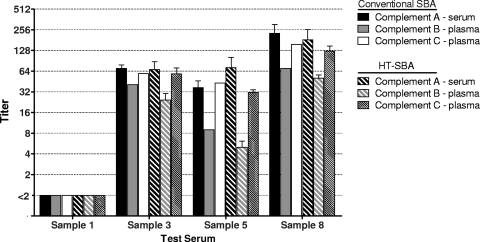
With the use of plasma as the source of exogenous complement in combination with low serum dilutions, clotting in assay wells has been observed in the conventional assay in some instances. This can be controlled with the addition of anticlotting agents, such as heparin, which was used in both the standard assay and the HT-SBA. Given the format changes in the HT-SBA, a subset of serum samples was tested using aphaeresis plasma as the complement source to determine the compatibly of plasma with the HT-SBA. Samples were assayed in both the conventional assay and the HT-SBA with either serum or plasma complement and the resulting titers compared. Results were consistent across both assay methods, largely independent of the complement source used. The only notable difference was associated with plasma complement B, which in both assays displayed titers slightly lower than those obtained with serum complement (Fig. 9).
Fig. 9.
Comparison of different human complement sources on titers from both the conventional assay and the HT-SBA.
DISCUSSION
One of the most critical stages in vaccine development is the testing of vaccine effectiveness in the target population. A clinical trial often involves thousands of subjects and multiple samples collected from each individual during various stages of the trial. It is important to have an efficient method for assaying the samples and determining the effectiveness of the vaccine. The SBA is the gold standard assay for the assessment of meningococcal vaccines. The manual format in the conventional assay poses significant demands on critical reagents due to high volume requirements, which places constraints on the number of assays that can be performed on any given specimen and limits the throughput of the assay.
The high-throughput bactericidal assay adheres to the same assay principle as the standard SBA. Meningococcal bacteria were incubated with immune serum and human complement, and the percentage of bacteria that survive was determined. The procedure diverges from that of the conventional SBA in that the surviving bacteria in each well were provided with a mixture of liquid growth medium and respiration dye resazurin, which is used to monitor bacterial growth following the reaction, instead of plating the bactericidal reaction on a solid growth medium to allow surviving bacteria to form colonies. The bacteria reduce the dye to resorufin by respiration, which generates a fluorescence signal that accumulates over time. The data were collected kinetically and analyzed to determine a titer based on survival curves generated by the 2-fold serial dilution of sera from immunized subjects. The growth curve of bacteria in normal human serum lacking intrinsic bactericidal activity is plotted from data collected each hour for 16 h. A time point wherein the increase in fluorescence signal is linear with respect to time and the signal is at least 50% of the plateau is selected for test sample titer determinations. Once the time point is chosen, all data were normalized to correct for nonspecific fluorescence quenching by serum. For each test sample, the curve is then fitted by the four-parameter logistic model and the bactericidal titer is calculated as the reciprocal dilution of the half-maximal peak of growth.
The HT-SBA differs from previous attempts to develop fluorescent or colorimetry-based readouts for the bactericidal reaction (13–15) in that it utilizes a kinetic measurement to optimize the time point selected for titer determinations. This is one of the key features of the HT-SBA. This kinetic approach is utilized to ensure the most robust correlation between the fluorescence signal and the number of surviving bacteria present in the test well. For this reason, care was taken that the surviving bacteria in the wells were in the log phase of growth while the alamarBlue was present, thereby optimizing the signal differential between wells with high bacterial counts from wells where few bacteria survived the bactericidal reaction. From these studies, we have concluded that the optimal time frame for titer calculation by fluorescence data analysis corresponds to those time points where the accumulation of fluorescence signal is at its steepest and the fluorescent signal is at least 50% of the signal plateau. This kinetic approach allows the assay to be easily adapted to changes in assay parameter values resulting from different growth characteristics of various bacterial strains and sources of reagents (e.g., different complement sources).
A second important feature which is needed for accurate data acquisition is the inclusion of the serum normalization control. In the HT-SBA, the overall concentration of test serum or plasma can range from 75% to 25% of the reaction volume, depending on the starting dilution of the test sample in combination with the complement used in the test. The fluorescence signal of alamarBlue can be quenched at high serum concentrations; therefore, correcting for the effect can greatly reduce the occurrence of false positives in the assay.
A third feature of importance to the HT-SBA is the use of an appropriate growth medium to promote robust recovery of bacteria that have survived the bactericidal reaction which, in our experience, needs to be empirically determined. In pilot studies, Muller-Hinton broth was a reasonable choice for this purpose, as it performed well and was used to prepare bacteria for inclusion in the bactericidal reaction itself. As the strain panel was extended, we found that the growth of bacteria for some strains was suboptimal, which might have resulted from unexpected incompatibility between components in the postreaction grow-out phase of the HT-SBA. Several different types of media were examined, including yeast extract and medium supplemented with Isovitalex, all with various results (data not shown). We found that DPBS supplemented with glucose appeared to be a generally compatible medium; however, there is no guarantee that this will be true for any new strain tested.
An important difference between the conventional SBA and the HT-SBA is the baseline that is used to define the bactericidal effect of antibodies and complement. Many laboratories that perform the conventional SBA, including our own, use the number of input CFU, also termed the t0 bacterial count, as the basis for determining the 50% kill endpoint. Thus, the number of surviving bacteria must be less than half the number of input bacteria for a sample to be scored as having a positive titer. A widely used alternative to this approach employs CFU enumerated at t60 to determine the 50% kill endpoint of a test sample. With this approach, titer determination is based not on the number of input bacteria but rather on the number that have survived during the 60-min incubation time comparable to all sample tests. It should be noted that titer determination in the HT-SBA is based on the fluorescent signal associated with the serial dilution of each sample. It is not possible to get a t0-equivalent fluorescence reading in the HT-SBA, because the combination of bacterial inoculum and respiration rate will not produce sufficient fluorescent product (resorufin) within the first few measurements. Only after 3 to 4 h of bacterial growth is the fluorescent signal generated by resorufin detectible above the background. In the HT-SBA, all bacteria were plated simultaneously and the fluorescence signals from the surviving bacteria incubated with test sera were compared with the fluorescence signal from a negative-control sample incubated with bacteria and complement but without antibody. Thus, the 50% threshold used for titer determination in the HT-SBA represents inhibition of bacterial growth in comparison to the appropriate growth and negative controls rather than a reduction below half of the input CFU level. For the strains that were evaluated in this study, very good agreement was seen between the endpoint titer results from the conventional assay using t0 for titer calculations and the growth kinetics methodology used in the HT-SBA.
The goal of developing the HT-SBA was to improve the assay throughput while maintaining good agreement with the titers obtained using the conventional bactericidal assay. In this report, we described the development of a high-throughput assay and proof-of-principle studies which detail the reproducibility, linearity, robustness, and applicability of the assay with various meningococcal strains and complement sources. Even when the test conditions were varied, the HT-SBA result maintained a strong correlation with the result obtained with the standardized agar-based assay. The HT-SBA has two significant improvements upon previous work in the development of alternative readouts of SBA (13–15): (i) the collection of kinetic fluorescence readings during the bacteria recovery phase allows us to identify the best time point for titer determination, optimizing assay sensitivity, and (ii) the introduction of a serum normalization control is used to correct for signal quenching, which improves the accuracy of titer determination.
The advantages of the HT-SBA format for clinical studies are numerous: (i) the process can now be fully automated, thereby limiting operator's exposure to biohazardous materials; (ii) it will be easier to standardize across different laboratories and operators; (iii) lower sample volume usage, which is particularly useful for the measurement of vaccine effectiveness in infant populations; (iv) reagent costs and manual assay time will be significantly reduced; and (v) the higher-density format also allows each test sample to be tested in a wider dilution range, reducing the need for repeat testing. In each case, the HT-SBA is a clear improvement over the conventional, agar-based bactericidal assay, which enables the HT-SBA to be applied in large-scale studies with greater efficiency without compromising data quality.
ACKNOWLEDGMENT
This work was supported in part under Defense Threat Reduction Agency contract no. HDTRA1-07-9-0001, awarded by the U.S. Government.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Borrow R., et al. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab Immunol. 12:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borrow R., et al. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 24:5093–5107 [DOI] [PubMed] [Google Scholar]
- 3. Campbell H., Borrow R., Salisbury D., Miller E. 2009. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine 27:B20–B29 [DOI] [PubMed] [Google Scholar]
- 4. Gill C. J., Baxter R., Anemona A., Ciavarro G., Dull P. 2010. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo(R)) or Menactra(R) among healthy adolescents. Hum. Vaccin. 6:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giuliani M. M., et al. 2010. Measuring antigen-specific bactericidal responses to a multicomponent vaccine against serogroup B meningococcus. Vaccine 28:5023–5030 [DOI] [PubMed] [Google Scholar]
- 6. Goegan P., Johnson G., Vincent R. 1995. Effects of serum protein and colloid on the alamarBlue assay in cell cultures. Toxicol. In Vitro 9:257–266 [DOI] [PubMed] [Google Scholar]
- 7. Goldschneider I., Gotschlich E. C., Artenstein M. S. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granoff D. M., Welsch J. A., Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holst J., et al. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734–737 [DOI] [PubMed] [Google Scholar]
- 10. Lennon D., et al. 2009. Fast tracking the vaccine licensure process to control an epidemic of serogroup B meningococcal disease in New Zealand. Clin. Infect. Dis. 49:597–605 [DOI] [PubMed] [Google Scholar]
- 11. Michael S., et al. 2008. A robotic platform for quantitative high-throughput screening. Assay Drug Dev. Technol. 6:637–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milagres L. G., et al. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mountzouros K. T., Howell A. P. 2000. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group B Neisseria meningitidis. J. Clin. Microbiol. 38:2878–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodríguez T., et al. 2003. Validation of colorimetric assay to detect complement-mediated antibody-dependent bactericidal activity against serogroups B and C Neisseria meningitidis. Biologicals 31:209–212 [DOI] [PubMed] [Google Scholar]
- 15. Romero-Steiner S., et al. 2004. Measurement of serum bactericidal activity specific for Haemophilus influenzae type b by using a chromogenic and fluorescent metabolic indicator. Clin. Diagn. Lab. Immunol. 11:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sierra G. V., et al. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195–210 [PubMed] [Google Scholar]
- 17. Toneatto D., et al. Early clinical experience with a candidate meningococcal B recombinant vaccine (rMenB) in healthy adults. Hum. Vaccin., in press [DOI] [PubMed] [Google Scholar]