Abstract
Waning vaccine-induced immunity against Bordetella pertussis is observed among adolescents and adults. A high incidence of pertussis has been reported in this population, which serves as a reservoir for B. pertussis. A fifth dose of reduced antigen of diphtheria-tetanus-acellular-pertussis and inactivated polio vaccine was given as a booster dose to healthy teenagers. The antibody activity against B. pertussis antigens was measured prior to and 4 to 8 weeks after the booster by different assays: enzyme-linked immunosorbent assays (ELISAs) of IgG and IgA against pertussis toxin (PT) and filamentous hemagglutinin (FHA), IgG against pertactin (PRN), opsonophagocytic activity (OPA), and IgG binding to live B. pertussis. There was a significant increase in the IgG activity against PT, FHA, and PRN following the booster immunization (P < 0.001). The prebooster sera showed a geometric mean OPA titer of 65.1 and IgG binding to live bacteria at a geometric mean concentration of 164.9 arbitrary units (AU)/ml. Following the fifth dose, the OPA increased to a titer of 360.4, and the IgG concentration against live bacteria increased to 833.4 AU/ml (P < 0.001 for both). The correlation analyses between the different assays suggest that antibodies against FHA and PRN contribute the most to the OPA and IgG binding.
INTRODUCTION
Bordetella pertussis is the causative agent of pertussis, or whooping cough. The vaccination of infants and children has proven to be an efficient strategy to reduce whooping cough in the general population (27). However, in spite of high vaccination coverage, B. pertussis still circulates, and the adult population with waning immunity against this organism is thought to be the main reservoir (5, 35). Many countries with highly immunized populations experience a resurgence of pertussis, particularly among adolescents and adults (4, 17). This has been true in particular for Norway, which has had the highest recorded incidence of pertussis for all European countries the past few years (http://www.euvac.net/graphics/euvac/pdf/pertussis_2009.pdf). The reported incidence is probably underestimated, and serological surveys indicate a much higher incidence rate (9). The vaccine-induced immunity is reported to last for 4 to 12 years, almost similar to infection-acquired immunity, which can last for 4 to 20 years (16, 32, 42). As an attempt to prolong the immunity against pertussis, in 2006 Norway introduced a fourth diphtheria-tetanus-acellular-pertussis and inactivated polio vaccine (DTaP-IPV) dose to children at the age of 7. However, to improve disease control and limit the risk of transmitting B. pertussis to susceptible infants, the immunization strategies may be further reinforced. There is an ongoing debate whether other age groups also should be vaccinated, e.g., adolescents and new parents (12).
High levels of antibodies against B. pertussis indicate previous infection or recent vaccination and are associated with protection against pertussis. However, the antibody specificity and antibody effector mechanisms for protection remain to be established. Phagocytosis may be an important mechanism to clear the bacteria, and we have shown previously that there is a high correlation between opsonophagocytic activity (OPA) and IgG antibodies against FHA and PT in sera from military recruits (1). Conflicting results have been found regarding the OPA and immunity to pertussis, and only a few studies have investigated the OPA in sera after immunization with acellular pertussis vaccine (19, 36, 40). In this study, we analyzed the immune response against B. pertussis in teenagers aged 15 to 16 years before and after a fifth dose of DTaP-IPV vaccine was given. They had followed the three-dose immunization regimen during their first year of life and received a fourth dose at the age of 7. The immune response was analyzed using enzyme-linked immunosorbent assays (ELISAs) against selected pertussis antigens, by OPA, and by IgG binding to live B. pertussis. The vaccine response to diphtheria, tetanus, and polio virus and safety data are to be published separately.
MATERIALS AND METHODS
Study population.
Eighty teenagers aged 15 to 16 years were given a fifth dose of DTaP-IPV (Boostrix Polio; GlaxoSmithKline) (containing 8 μg PT, 8 μg FHA, and 2.5 μg pertactin [PRN] per dose). They had followed the regular three-dose immunization regimen within their first year of life (DTwP-IPV), and they also received a fourth dose (Infanrix-Polio; GlaxoSmithKline) when they were 7 years old, participating in a vaccine study as described earlier (34). Sera from 80 pupils were collected at the time when the fifth dose was administered, and 75 participants (94%) completed the study and gave a blood sample 4 to 8 weeks later. The present study was conducted in compliance with the principles of the Declaration of Helsinki and the Norwegian Regional Committee for Medical Research Ethics, and the Norwegian Medicines Agency approved the study. Written informed consent was obtained from all participants and parents.
Bacterial strain.
A nasopharyngeal clinical isolate of B. pertussis (NIPH reference number 105/06) from a 30-year-old woman with clinical pertussis was used as the target in the opsonophagocytic assay and in the IgG binding assay. It was typed as serotype 1,3 using in-house typing sera that have been evaluated against international typing reagents. This serotype is representative of the ongoing epidemic strains prevailing the last 10 years. The bacteria were grown on charcoal agar plates for 2 days prior to use.
ELISA measurements against vaccine antigens.
The specific immune response against selected vaccine antigens were analyzed by two different commercial enzyme-linked immunosorbent assays (ELISAs): PT-IgG antibodies were analyzed using Bordetella pertussis toxin IgG by Serion ELISA classic (Institute Virion\Serion GmbH, Würzburg, Germany) according to the manufacturer's instructions. The specification of this PT-IgG activity in FDA-U/ml refers to the U.S. Reference Pertussis Antiserum (Human) Lot 3 (FDA), and the lower limit of detection was 5 FDA-U/ml. Sera with activity less than this were assigned a value of 2.5 FDA-U/ml for statistical analysis. In addition, IgG and IgA antibodies against PT and FHA were analyzed using Pertusscan 2 + 2 (Euro-Diagnostica AB, Malmö, Sweden), and the results were reported as a percentage of the negative cutoff (i.e., an optical density of 0.3 equals 100%). These kits are widely used in pertussis serology in Norway, and both were included in this study for comparison. In these commercial assays, only one dilution of test sera is analyzed, i.e., a 1:101 dilution in the Serion kit and a 1:500 dilution in the Pertusscan kit. Antibodies against PRN were measured by an in-house ELISA slightly modified from that of Mead et al. (28). Briefly, PRN (a gift from G. Berbers, RIVM, the Netherlands) was coated in 1 μg/ml in carbonate buffer. The sera were diluted in four 2-fold dilutions and were calculated against the WHO reference serum 06/140, containing 65 IU/ml PRN-IgG, using four-parameter curve analyses. An in-house reference serum included in each experiment revealed the high reproducibility of the tests, with a coefficient of variation of <10%. Pre- and postimmunization samples were always analyzed on the same plate.
Opsonophagocytic activity.
OPA was measured as respiratory burst, as previously described (1). Briefly, live B. pertussis cells were used as target cells, and dihydrorhodamine 123 (Molecular Probes, Invitrogen)-primed polymorphonuclear neutrophils (PMNs) were used as effector cells. All sera were heated to 56°C to inactivate complement. As a complement source, we added external human serum (10%) that had been passed through a protein G column at 4°C to remove IgG antibodies. Two-fold dilutions of sera were tested. The percent respiratory burst of the PMNs was examined by flow cytometry (CyFlowML; Partec, GmbH, Münster, Germany), and the highest reciprocal serum dilution giving ≥50% respiratory burst of the PMNs was recorded as the serum titer. Pre- and postvaccine sera were always analyzed on the same plate. A late-convalescent-phase serum from a confirmed pertussis case collected 18 months after the onset of disease was included on every plate and showed a reproducibility of ±1 titer of the assay.
Quantification of IgG binding to live B. pertussis by flow cytometry.
Specific IgG antibodies binding to live B. pertussis were measured by a flow cytometry method, as previously described (1). We used the same preparation of live bacteria as that used in the OPA assay. The sera were diluted 2-fold, and pre- and postvaccination sera were always included on the same plate. The samples were analyzed with the Partec CyFlowML flow cytometer, and the geometric mean fluorescence intensity was used to calculate the actual serum antibody concentration using GraphPad Prism software, version 1.02 (GraphPad Software Inc., San Diego, CA). The B. pertussis-specific serum IgG concentrations are reported in arbitrary units (AU) against an in-house reference serum defined as 1,000 AU/ml.
IgG subclass quantification.
IgG subclasses were quantified against whole-cell B. pertussis ELISA. The bacteria were grown for 2 to 3 days, harvested, and washed in phosphate-buffered saline (PBS) with 0.02% Na-azide.
ELISA microplates were coated with 100 μl B. pertussis (optical density at 650 nm, 0.02) overnight at 37°C or for 2 to 3 days at 4°C. Paired sera were tested at 1:20 and 1:80 dilutions on the same plate, and all four subclasses were measured on the same day using the same coating batch. The following subclass-specific mouse anti-human IgG were utilized: anti-IgG1 (HP6091), 1:8,000; anti-IgG2 (HP6002), 1:16,000; anti-IgG3 (HP6050), 1:16,000; anti-IgG4 (HP6011), 1:16,000; all were obtained from WHO. The mouse antibodies were detected by a goat anti-mouse IgG-alkaline phosphatase (ALP) conjugate (Sigma-Aldrich) and was developed by the substrate nitrophenyl phosphate (NPP). The results were read in arbitrary optical density units related to an in-house pertussis reference serum.
Statistics.
Statistical analyses were done using Sigma Plot 3.1 (Systat Software, Chicago, IL). Geometric mean concentrations (GMCs) and GM titers (GMTs) with corresponding 95% confidence intervals were calculated on sera collected prior to and 4 to 8 weeks after the fifth dose. Mann-Whitney rank-sum tests were run to test for differences between groups, and P < 0.05 was considered significant. Linear regression analyses on log-transformed data were performed to look for relationships between the assays, and Pearson's correlation coefficients were calculated.
RESULTS
Antibody levels against PT, FHA, and PRN.
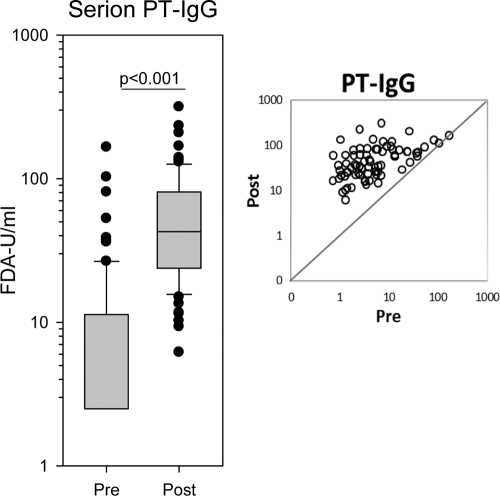
With the Serion IgG-PT ELISA, the IgG GMC against PT was 5.7 FDA-U/ml (range, 2.5 to 166 FDA-U/ml) prior to the fifth dose (Fig. 1). Four to 8 weeks after the booster, the IgG-PT GMC response increased to 43.7 FDA-U/ml (range, 6 to 316 FDA-U/ml; P < 0.001). However, 26 of 75 (35%) participants who gave the second serum samples did not gain more than 30 FDA-U/ml against PT, whereas 20 (27%) reached values of ≥80 FDA-U/ml. More than half (53%) of the participants demonstrated <5 FDA-U/ml of IgG antibodies in their prebooster samples. After immunization, this group obtained an IgG-PT GMC of 31.0 FDA-U/ml.
Fig. 1.
Serum IgG response against pertussis toxin (PT) at the time of the fifth dose of DTaP and 4 to 8 weeks later as measured by the Serion ELISA classic. The boxes span the 25th to 75th percentiles, the whiskers indicate the 10th and 90th percentiles, and the bullets represent the individual outliers. n=80 and 75 in pre- and postvaccination groups, respectively. The scatter plot shows the PT-IgG distribution of the 75 paired pre- and postvaccination sera. The diagonal represents line of identity. Results are from one experiment.
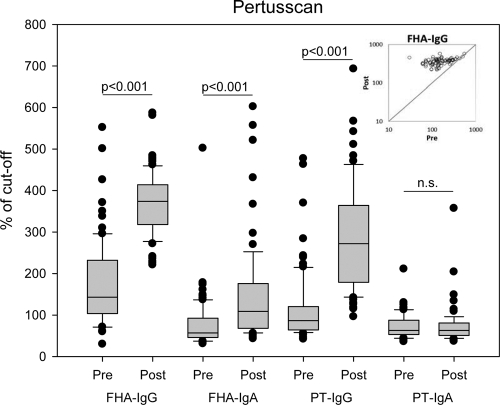
Using the Pertusscan assay, we observed significant responses of IgG against both PT and FHA and of IgA against FHA from before and after the fifth dose (P < 0.001) (Fig. 2). Only two persons had an IgA response against PT. Before the booster dose, 80% were positive for IgG against FHA (i.e., above the cutoff), whereas 33% were positive for IgG antibodies against PT.
Fig. 2.
Box plot showing the serum IgG and IgA responses against PT and FHA as measured by the Pertusscan ELISA of the sera before and after the administration of the fifth dose; n=80 and 75 in pre- and postvaccination groups, respectively. The scatter plot in the inset shows the FHA-IgG distribution of the 75 paired pre- and postvaccination sera. Results are from one experiment.
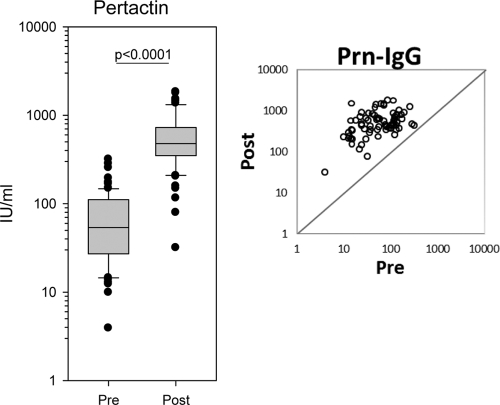
Against PRN, the GMC was 51.8 IU/ml (range, 3.9 to 318.0 IU/ml) in the prebooster sera; this increased to a GMC of 484.6 IU/ml (range, 31.8 to 1,837.0 IU/ml) 4 to 8 weeks postbooster (P < 0.001) (Fig. 3).
Fig. 3.
Box plot showing the serum PRN-IgG measured by the in-house ELISA of the sera before and after the administration of the fifth dose. A single measurement using serial 2-fold dilutions of serum was examined; n=80 and 75 in pre- and postvaccination groups, respectively. The scatter plot shows the PRN-IgG distribution of the 75 paired pre- and postvaccination sera.
Opsonophagocytosis.
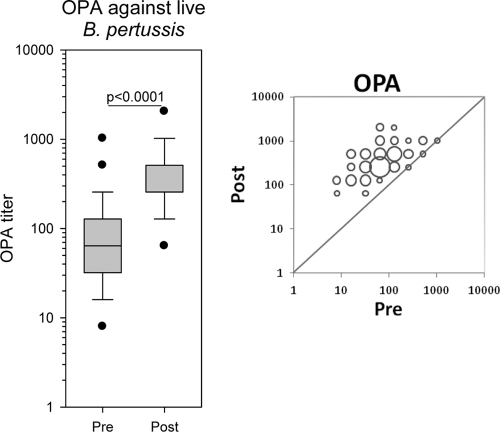
The OPA results demonstrated that antibodies raised against acellular pertussis vaccine containing PT, FHA, and PRN clearly promote OPA in neutrophils. Before the booster dose, the OPA GMT was 65.1 (range, 8 to 1,024), and 4 to 8 weeks after the booster dose it had increased to a GMT of 360.4 (range, 64 to 2,048) (P < 0.001) (Fig. 4). We observed the highest increases in OPA titers among those who had the lowest prebooster OPA titers. The subjects with prebooster titers of ≤64 (n=51) achieved a 7.7-fold increase in OPA titer after immunization, and the subjects with prebooster OPA titers of >64 (n=24) obtained a 3.4-fold increase in titers. Three participants revealed no increase in OPA titer; however, they all had elevated titers in their prebooster samples (titers of 256, 512, and 1,024). No participants showed any decline in OPA after the fifth dose.
Fig. 4.
Box plot showing the opsonophagocytic activity (OPA) of sera before and after the fifth dose. OPA is measured as the titer of respiratory burst as described in Materials and Methods. A single measurement using serial 2-fold dilutions of serum was examined; n=80 and 75 in pre- and postvaccination groups, respectively. The bubble plot shows the OPA distribution of the 75 paired pre- and postvaccination sera. The bubble size correlates to the number of paired sera with identical titers before and after vaccination.
IgG binding to live B. pertussis.
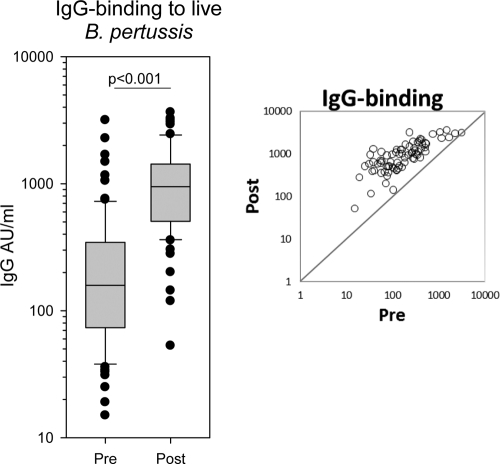
The IgG GMC measured against live B. pertussis was 164.9 AU/ml (range, 15 to 3,166 AU/ml) before the booster, increasing to 833.4 AU/ml (range, 53 to 3,652 AU/ml) after the booster (P < 0.001) (Fig. 5). As for the OPA, the IgG responses were most pronounced in individuals with lower levels in the prebooster sera.
Fig. 5.
Box plot showing IgG binding against live B. pertussis in sera before and after the fifth dose. The activities were quantified in arbitrary units using an in-house reference serum. A single measurement using serial 2-fold dilutions of serum was examined; n=80 and 75 in pre- and postvaccination groups, respectively. The scatter plot shows the distribution of IgG binding of the 75 paired pre- and postvaccination sera.
One participant did not respond to the booster dose in any of the pertussis tests used and displayed only negative to borderline activities in all assays.
Three participants had Serion PT-IgG levels of >80 FDA-U/ml in their prebooster serum samples, which according to the kit instruction manual indicates acute or recent infection by B. pertussis (8, 14) (Table 1). They also revealed elevated PT-IgG with the Pertusscan ELISA. One serum (no. 83) had increased FHA-IgG and PRN-IgG levels before the booster. This serum showed a raised OPA (titer, 512) and also IgG binding (3,166 AU/ml) to live bacteria, whereas the two former sera (no. 3 and 5) demonstrated rather low OPA and IgG binding activities against live B. pertussis. Interestingly, after the booster immunization the FHA-IgG levels, and in particular the PRN-IgG levels, OPA, and IgG binding activity, of these two participants had increased severalfold, whereas the IgG-PT level remained unchanged (Table 1).
Table 1.
Serological results from three participants with suspected recent pertussis prior to the booster immunization
| Subject no. | Assay result before and after immunization |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serion PT-IgG (FDA-U/ml) |
PRN-IgG (IU/ml) |
Pertusscan FHA-IgG (% cutoff) |
OPA (titer) |
IgG binding (AU/ml) |
||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| 3 | 81 | 131 | 70.2 | 1,496.5 | 165 | 462 | 64 | 2,048 | 231 | 3,237 |
| 5 | 166 | 168 | 54.2 | 237.4 | 162 | 282 | 64 | 256 | 133 | 465 |
| 83 | 103 | 113 | 318.0 | 450.3 | 552 | 581 | 512 | 512 | 3,166 | 3,141 |
IgG subclasses.
The IgG subclass response was measured against whole bacteria by ELISA. The IgG response was dominated by the IgG1 subclass, which increased from a GMC of 0.23 AU/ml before the booster to 0.59 AU/ml after the booster (P < 0.001). About 30% of the subjects demonstrated an IgG4 response following the booster (GMC from 0.15 to 0.21 AU/ml; P < 0.001). Most of the sera had detectable IgG2 antibodies in the prebooster samples, but there was no significant change of this subclass after the fifth dose. The IgG3 subclass level was low compared to those of the other subclasses (GMC of 0.11 to 0.12 from before and after the fifth dose).
Correlation between assays.
We demonstrated significant correlation between the various assays applied in this study (P < 0.001 for all), except for the PT-IgA, which did not change following the booster immunization and thus revealed low/no correlation with the other assays (Table 2). Although the Pertusscan ELISA is a semiquantitative assay (particularly at the higher antibody levels), we still got very good correlations between PT-IgG levels measured by the Pertusscan and the Serion kits (r2=0.92). For correlations to OPA, PRN showed the highest correlation (r2=0.81) followed by IgG binding to live B. pertussis (r2=0.79), FHA-IgG (r2=0.69), and PT-IgG (r2=0.61). The OPA revealed higher correlation to the IgG than to the IgA antibodies measured, indicating that specific IgG antibodies are the main inducers of OPA in these sera.
Table 2.
Pearson's correlations (r2) between different assays (n=155)
| Assay | OPA | IgG binding | PT-IgG (Serion) | FHA-IgG (Pertusscan) | FHA-IgA (Pertusscan) | PT-IgG (Pertusscan) | PT-IgA (Pertusscan) |
|---|---|---|---|---|---|---|---|
| IgG binding | 0.79 | ||||||
| PT-IgG (Serion) | 0.61 | 0.55 | |||||
| FHA-IgG (Pertusscan) | 0.69 | 0.67 | 0.64 | ||||
| FHA-IgA (Pertusscan) | 0.44 | 0.36 | 0.44 | 0.41 | |||
| PT-IgG (Pertusscan) | 0.61 | 0.55 | 0.92 | 0.66 | 0.45 | ||
| PT-IgA (Pertusscan) | 0.01 | 0.00 | 0.01 | 0.00 | 0.14 | 0.03 | |
| PRN-IgG | 0.81 | 0.76 | 0.58 | 0.66 | 0.31 | 0.59 | 0.00 |
DISCUSSION
In this study, we have analyzed the antibody levels against B. pertussis before and after a fifth dose of pertussis vaccine was given to healthy teenagers aged 15 to 16 years. The vaccine was well tolerated with no serious adverse events. They had been vaccinated against pertussis with three doses during their first year of life and with a fourth dose when they were 7 years old. The prebooster results demonstrated that the immunity against B. pertussis was low 9 years after the fourth dose. This is in accordance with other studies confirming waning vaccine-induced immunity over time, reaching undetectable ELISA antibody levels in a large proportion of the population (10, 16, 42). Three of the participants demonstrated high levels of PT-IgG in their prebooster sera, possibly caused by recent infection. This indicates immunity below the level of protection of this cohort and argues for a booster dose at this age.
Prior to the fifth dose, more subjects had detectable antibodies to PRN and FHA than to PT, as has been shown previously (10, 13, 24). This may reflect immune stimulation by other Bordetella species or other bacteria known to have cross-reactive epitopes to PRN or FHA (3, 20, 37), but also that PRN and FHA are stronger antigens and give rise to longer-lasting antibodies (18, 24, 26).
A significant increase in the antibody response to all vaccine antigens was observed following the fifth dose. This is in accordance with previous studies using a reduced-antigen-content vaccine, even though the vaccine schedules and age groups were somewhat different (38, 39, 43). There was no significant IgA response to PT; however, two participants had a PT-IgA response (more than 2-fold increase) (30). The immunization of adolescents and adults seems to induce more PT-IgA than that in younger individuals, possibly as a result of a previous encounter with live B. pertussis (22, 24).
Although low antibody levels were detected against PT, FHA, and PRN in the prebooster sera, they still demonstrated OPA and IgG binding activities (Fig. 3 and 4), which may be relevant for protection. These antibodies probably bind to other surface-exposed epitopes and are induced from a previous encounter with B. pertussis or other cross-reactive antigens.
This is one of a few studies that document increased vaccine-induced OPA against pertussis in human sera after revaccination with an acellular pertussis vaccine (19, 36, 40). In an earlier study, we found increased OPA in sera from military recruits who also had corresponding high levels of antibodies against pertussis (1). Those antibodies likely were reminiscent of previous infections rather than induced by vaccination, and the specificities of these antibodies probably were directed against a whole range of B. pertussis epitopes. In the present study, the increased OPA activity observed following the fifth vaccine dose must have been induced by antibodies directed against the pertussis antigens in the vaccine, i.e., PT, FHA, and PRN. It has been demonstrated previously that neutralizing antibodies against adenylate cyclase toxin facilitate phagocytosis (41); however, this antigen is not in the vaccine.
The B. pertussis strain (105/06) used is from a clinical isolate assumed to be representative of the ongoing Norwegian epidemic. In addition, a subset of sera (14%) was analyzed for OPA against two other clinical B. pertussis strains, one from 2003 (strain 1809/03) and one from 2011 (strain 12130), both of serotype 1,3. The OPA responses obtained with these two strains were almost identical to the responses observed with the 105/06 strain.
The correlation analysis suggests that antibodies against PRN and FHA are most important for the OPA after immunization with PT-, FHA-, and PRN-containing pertussis vaccine. As the FHA-IgG response is measured using a semiquantitative assay (Pertusscan), the correlation coefficients calculated are likely to be underestimated. We generally observed slightly better correlations between the assays in this study than in a previous study among healthy, nonvaccinated military recruits (1). This is most likely explained by the higher dispersion of antibody activities between pre- and postvaccination sera than in sera from normal adults. The importance of PRN in OPA also has been demonstrated by Hellwig et al. using purified PRN and fimbriae to deplete specific antibodies (19).
Three participants had high IgG anti-PT responses prior to the booster (indicating previous infection), and two of these had low FHA-IgG and low PRN-IgG responses. These two also had lower OPA and IgG binding to live bacteria. However, after the fifth dose they demonstrated an increased FHA-IgG response and a particularly strong IgG-PRN response, and interestingly a corresponding increase in OPA and IgG binding (Table 1). This may indicate that PT-IgG is not particularly important for the OPA, in contrast to PRN-IgG and FHA-IgG, and it suggests that most of the PT epitopes are more or less concealed for antibody binding when the molecule is associated with the bacterial outer membrane, as illustrated by Burns (6).
Live B. pertussis is used as the target in both the OPA and IgG binding assays. By using live bacteria, we exclude antibody activities against other immunogenic determinants that are not expressed on live bacteria and therefore possibly of less importance for eliminating the pathogen. Antibodies binding to antigens on live B. pertussis, e.g., adhesion molecules like PRN and FHA, may have a protective function by reducing the interaction between bacteria and respiratory epithelium. Mucosal secretion contains significant amounts of IgG transudate from blood plasma; these antibodies may contribute to reducing colonization similarly to what is observed after Haemophilus influenzae type B vaccination (11). In addition, both FHA and, in particular, PT exist as secreted molecules, and antibodies directed against many epitopes on these antigens may aid in neutralizing the biological activities of these molecules (7, 27).
It still is uncertain which antigens are most important in these functional assays. Absorption experiments with different purified antigens may shed light on this issue. By using sera from both vaccinees and convalescents, we will examine the importance of different antibody specificities regarding OPA and IgG binding.
The OPA protocol used in this study measures respiratory bursts of the PMNs and is not a bacterial killing assay. However, results from previous studies using similar OPA protocols and PMNs as effector cells indicate that the internalized B. pertussis bacteria are readily killed in this process (23, 25, 33). In addition to OPA, the direct complement killing of the bacteria also may play a role. We have preliminary data showing that prebooster serum with low antibody levels lacks bactericidal activity, whereas after the booster a severalfold increase in bactericidal titer is obtained.
The IgG subclass response was determined using an ELISA coated with whole-cell B. pertussis. The abundance of IgG1 also is reported by Giammanco et al. (15). This subclass is highly efficient in promoting phagocytosis and in activating the classical complement pathway (2, 29). The IgG2 subclass did not respond significantly following the booster, and the binding observed possibly reflects IgG2 produced against other cross-reacting determinants, e.g., polysaccharide antigens. The low IgG3 response also was demonstrated by Giammanco et al. after acellular immunization of 1-year-old children. IgG3 often is produced against protein determinants; however, it peaks promptly after a booster and declines rapidly within 4 to 6 weeks, as shown for IgG3 against a meningococcal outer membrane vesicle vaccine (31).
It has been demonstrated recently that pertussis toxin inhibits neutrophil recruitment in a mouse model (21). Neutralizing antibodies against PT, combined with antibodies that confer opsonophagocytic activity, might prove to be favorable for protecting immunity against pertussis and also to minimize the time of carriage.
ACKNOWLEDGMENTS
We thank Per Wium and Aud Befring, Lørenskog municipality, for their collaboration, and we thank the participating teenagers for making the trial possible. Special thanks to Guy Berbers, RIVM, Netherlands, for providing the pertactin used in this study.
Footnotes
Published ahead of print on 15 June 2011.
REFERENCES
- 1. Aase A., et al. 2007. Opsonophagocytic activity and other serological indications of Bordetella pertussis infection in military recruits in Norway. Clin. Vaccine Immunol. 14:855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aase A., Michaelsen T. E. 1994. Opsonophagocytic activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand. J. Immunol. 39:581–587 [DOI] [PubMed] [Google Scholar]
- 3. Barenkamp S. J., Leininger E. 1992. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect. Immun. 60:1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berbers G. A., de Greeff S. C., Mooi F. R. 2009. Improving pertussis vaccination. Hum. Vaccin. 5:497–503 [DOI] [PubMed] [Google Scholar]
- 5. Bisgard K. M., et al. 2004. Infant pertussis: who was the source? Pediatr. Infect. Dis. J. 23:985–989 [DOI] [PubMed] [Google Scholar]
- 6. Burns D. L. 2003. Type IV transporters of pathogenic bacteria. Curr. Opin. Microbiol. 6:29–34 [DOI] [PubMed] [Google Scholar]
- 7. Coutte L., et al. 2003. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 197:735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Melker H. E., et al. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Melker H. E., Versteegh F. G. A., Schellekens J. F. P., Teunis P. F. M., Kretzschmar M. 2006. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J. Infect. 53:106–113 [DOI] [PubMed] [Google Scholar]
- 10. Edelman K., et al. 2007. Immunity to pertussis 5 years after booster immunization during adolescence. Clin. Infect. Dis. 44:1271–1277 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez J., et al. 2000. Prevention of Haemophilus influenzae type B colonization by vaccination: correlation with serum anti-capsular IgG concentration. J. Infect. Dis. 182:1553–1556 [DOI] [PubMed] [Google Scholar]
- 12. Forsyth K. D., von Konig C.-H. Wirsing, Tan T., Caro J., Plotkin S. 2007. Prevention of pertussis: recommendations derived from the second Global Pertussis Initiative roundtable meeting. Vaccine 25:2634–2642 [DOI] [PubMed] [Google Scholar]
- 13. García-Corbeira P., Dal-Ré R., Aguilar L., García-de-Lomas J. 2000. Seroepidemiology of Bordetella pertussis infections in the Spanish population: a cross-sectional study. Vaccine 18:2173–2176 [DOI] [PubMed] [Google Scholar]
- 14. Giammanco A., et al. 2003. European Sero-Epidemiology Network: standardisation of the assay results for pertussis. Vaccine 22:112–120 [DOI] [PubMed] [Google Scholar]
- 15. Giammanco A., et al. 2003. Analogous IgG subclass response to pertussis toxin in vaccinated children, healthy or affected by whooping cough. Vaccine 21:1924–1931 [DOI] [PubMed] [Google Scholar]
- 16. Gustafsson L., Hessel L., Storsaeter J., Olin P. 2006. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics 118:978–984 [DOI] [PubMed] [Google Scholar]
- 17. He Q., Mertsola J. 2008. Factors contributing to pertussis resurgence. Future Microbiol. 3:329–339 [DOI] [PubMed] [Google Scholar]
- 18. Heininger U., Cherry J. D., Stehr K. 2004. Serologic response and antibody-titer decay in adults with pertussis. Clin. Infect. Dis. 38:591–594 [DOI] [PubMed] [Google Scholar]
- 19. Hellwig S. M. M., Rodriguez M. E., Berbers G. A. M., de Winkel J. G. J. V., Mooi F. R. 2003. Crucial role of antibodies to pertactin in Bordetella pertussis immunity. J. Infect. Dis. 188:738–742 [DOI] [PubMed] [Google Scholar]
- 20. Hodder S. L., et al. 2000. Antibody responses to Bordetella pertussis antigens and clinical correlations in elderly community residents. Clin. Infect. Dis. 31:7–14 [DOI] [PubMed] [Google Scholar]
- 21. Kirimanjeswara G. S., Agosto L. M., Kennett M. J., Bjornstad O. N., Harvill E. T. 2005. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J. Clin. Investig. 115:3594–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knuf M., et al. 2006. Immunogenicity of a single dose of reduced-antigen acellular pertussis vaccine in a non-vaccinated adolescent population. Vaccine 24:2043–2048 [DOI] [PubMed] [Google Scholar]
- 23. Lamberti Y., Perez Vidakovics M. L., van der Pol L. W., Rodriguez M. E. 2008. Cholesterol-rich domains are involved in Bordetella pertussis phagocytosis and intracellular survival in neutrophils. Microb. Pathog. 44:501–511 [DOI] [PubMed] [Google Scholar]
- 24. Le T., et al. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J. Infect. Dis. 190:535–544 [DOI] [PubMed] [Google Scholar]
- 25. Lenz D. H., Weingart C. L., Weiss A. A. 2000. Phagocytosed Bordetella pertussis fails to survive in human neutrophils. Infect. Immun. 68:956–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Littmann M., Hulsse C., Riffelmann M., von Konig C. H. Wirsing. 2008. Long-term immunogenicity of a single dose of acellular pertussis vaccine in paediatric health-care workers. Vaccine 26:2344–2349 [DOI] [PubMed] [Google Scholar]
- 27. Mattoo S., Cherry J. D. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meade B. D., et al. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96:570–575 [PubMed] [Google Scholar]
- 29. Michaelsen T. E., Garred P., Aase A. 1991. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 21:11–16 [DOI] [PubMed] [Google Scholar]
- 30. Müller F. M., Hoppe J. E., von Konig C. H. Wirsing. 1997. Laboratory diagnosis of pertussis: state of the art in 1997. J. Clin. Microbiol. 35:2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naess L. M., et al. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754–764 [DOI] [PubMed] [Google Scholar]
- 32. Riffelmann M., Littmann M., Hulsse C., von Konig C. H. 2009. Antibody decay after immunisation of health-care workers with an acellular pertussis vaccine. Eur. J. Clin. Microbiol. Infect. Dis. 28:275–279 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez M. E., et al. 2001. Fc receptor-mediated immunity against Bordetella pertussis. J. Immunol. 167:6545–6551 [DOI] [PubMed] [Google Scholar]
- 34. Sandbu S., et al. 2001. Should school children receive pertussis vaccine? Tidsskr. Nor. Laegeforen. 121:1464–1468 [PubMed] [Google Scholar]
- 35. Schellekens J., von Konig C. H., Gardner P. 2005. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr. Infect. Dis. J. 24:S19–S24 [DOI] [PubMed] [Google Scholar]
- 36. Stefanelli P., Ippoliti R., Fazio C., Mastrantonio P. 2002. Role of immune sera in the in-vitro phagocytosis of Bordetella pertussis strains. Microb. Pathog. 32:135–141 [DOI] [PubMed] [Google Scholar]
- 37. Stehr K., et al. 1998. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP Vaccine, or DT vaccine. Pediatrics 101:1–11 [DOI] [PubMed] [Google Scholar]
- 38. Tran Minh N. N., et al. 1999. Acellular vaccines containing reduced quantities of pertussis antigens as a booster in adolescents. Pediatrics 104:e70. [DOI] [PubMed] [Google Scholar]
- 39. Vergara R., et al. 2005. Reduced-antigen-content-diphtheria-tetanus-acellular-pertussis and inactivated polio vaccine as a booster for adolescents 10 to 14 years of age. Eur. J. Pediatr. 164:377–382 [DOI] [PubMed] [Google Scholar]
- 40. Weingart C. L., Keitel W. A., Edwards K. M., Weiss A. A. 2000. Characterization of bactericidal immune responses following vaccination with acellular pertussis vaccines in adults. Infect. Immun. 68:7175–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weingart C. L., Mobberley-Schuman P. S., Hewlett E. L., Gray M. C., Weiss A. A. 2000. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 68:7152–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wendelboe A. M., Van R. A., Salmaso S., Englund J. A. 2005. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24:S58–S61 [DOI] [PubMed] [Google Scholar]
- 43. Zepp F., et al. 2007. Immunogenicity of reduced antigen content tetanus-diphtheria-acellular pertussis vaccine in adolescents as a sixth consecutive dose of acellular pertussis-containing vaccine. Vaccine 25:5248–5252 [DOI] [PubMed] [Google Scholar]