Abstract
The presence of hypergammaglobulinemia, autoantibodies, and circulating immune complexes suggests that humoral immunity may contribute to the pathogenesis of sarcoidosis. However, little is known about the role played by B cells in the development of this disease. Here we investigated the subpopulation distribution, response to stimulation, and levels of the nuclear transcription factor NF-κB/p65 in peripheral blood B cells from patients with severe chronic sarcoidosis. Patients with severe chronic sarcoidosis had absolute B-cell lymphopenia and exhibited significantly decreased frequencies and total numbers of memory (CD19+ CD27+) B cells. The reduced numbers of memory B cells in these patients reflected a decrease in the total numbers of class-switched (CD19+ CD27+ IgD−) and unswitched (CD19+ CD27+ IgD+) memory B cells and coincided with an increased frequency of circulating (CD19+/− CD20− CD27++) plasmablasts. Polyclonal stimulation of sarcoid B cells resulted in reduced expression of activation markers (i.e., CD25, CD69, and CD86), decreased proliferation, and impaired plasma cell differentiation. Baseline expression of p65 in B cells was reduced in 65% of the patients. These results suggest disturbed homeostasis, intrinsic signaling defects, and anergy within the peripheral B-cell compartments of patients with severe chronic sarcoidosis.
INTRODUCTION
Sarcoidosis is a cell-mediated immunological disorder characterized by granuloma development and the production of inflammatory cytokines by activated macrophages and T cells (29). In spite of the predominant involvement of cellular immunity in the pathogenesis of this disease, sarcoidosis is frequently associated with hypergammaglobulinemia (28), autoantibody production (66), and circulating immune complexes (18), humoral abnormalities typically found in patients with systemic autoimmunity (63). The clinical and pathological features of sarcoidosis (i.e., multisystemic involvement, arthritis, uveitis, myositis, conjunctivitis, neuritis, response to immunosuppressive therapy, and lymphocytic infiltration in affected tissues and organs) also mimic those of many systemic autoimmune diseases (50), and sarcoidosis has been reported to coexist with systemic lupus erythematosus (SLE), primary Sjögren's syndrome (pSS), and rheumatoid arthritis (RA) (62). Thus, a relationship between sarcoidosis and systemic autoimmune diseases has been proposed, and it is postulated that sarcoidosis and connective tissue diseases may share common immunopathogenic mechanisms (67). Sarcoidosis, however, does not meet Witebsky's criteria for autoimmune diseases, and therefore, the inclusion of sarcoidosis in the group of autoimmune disorders has not been generally accepted (57).
The peripheral B-cell compartments of patients with systemic autoimmunity are frequently altered (51). Different connective tissue disorders are correlated with distinct changes in the peripheral B-cell populations. In SLE, marked reductions in the levels of CD19+ CD27− naïve B cells, enhanced frequencies of CD19+ CD27+memory B cells, and increased numbers of CD19+/− CD27++ plasma cells were found (46), whereas a predominance of naïve B cells (with diminished frequencies and absolute numbers of memory B cells) and increased frequencies of IgD-expressing memory B cells (with similar distributions of peripheral naïve and memory B cells) were identified in patients with pSS and RA, respectively (8, 9, 26). It is thought that these homeostatic changes profoundly influence a variety of B-cell functions, such as antigen presentation, cytokine synthesis, and Ig production, and that these alterations in immune factors are essential to the pathogenesis of systemic autoimmune diseases (58). However, in spite of the striking clinical, pathological, and immunological similarities between sarcoidosis and systemic autoimmune diseases, the peripheral B-cell compartment of sarcoidosis patients has not been characterized.
Analysis of B-cell populations in patients with systemic autoimmunity and healthy individuals generally relies on the expression of four surface markers: CD19, IgD, CD38, and CD27 (51, 58). With this approach, two major classifications can be produced depending on the relative expression of either IgD and CD38 or IgD and CD27 on B cells. Thus, IgD CD38 staining can be used to identify naïve cells (CD19+ IgD+ CD38−), activated naïve cells (CD19+ IgD+ CD38+), pre-germinal-center cells (CD19+ IgD+ CD38++), centroblasts-centrocytes (CD19+ IgD− CD38++), plasma cells (CD19+ IgD− CD38+++), and memory cells (CD19+ IgD− CD38−). IgD CD27 staining builds on the notion of CD27 as a marker of memory B cells to distinguish between memory cells (CD27+) and naïve cells (CD27−). CD27+ memory cells can be divided into unswitched (IgD+) and class-switched (IgD−) memory cells. The various B-cell subpopulations exist in relatively similar ratios in healthy individuals (51).
Although many studies have shown multiple B-cell homeostatic abnormalities, very little is known regarding B-cell receptor (BCR) signaling in systemic autoimmunity (53). Yet the importance of understanding the regulation of BCR signaling pathways in human autoimmune diseases is underscored by multiple demonstrations in animal models that abnormalities in these pathways may result in systemic autoimmunity (27). Transgenic mice deficient in the src family protein tyrosine kinase Lyn develop an SLE-like syndrome with autoantibodies and nephritis (45). Consistent with this observation, a subset of SLE patients have reduced levels of Lyn and a significant increase in levels of the protein tyrosine phosphatase CD45 in lipid raft microdomains (24). Impaired expression and activity of the immunoglobulin-binding receptor CD32 is another factor that might account for intrinsic changes in B-cell signaling in SLE (30). CD32 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) that, upon phosphorylation, recruits protein tyrosine phosphatases that destabilize and downregulate the BCR signaling complex (59). The importance of CD32 in providing negative signals is demonstrated by the generation of enhanced IgG autoantibody responses and spontaneous autoimmunity in susceptible strains of CD32-deficient mice (10). In accordance with this, various studies have shown decreased CD32 expression in CD27+ B cells from patients with SLE (39). Although a role for B-cell intrinsic signaling defects in the pathogenesis of autoimmune diseases is increasingly apparent, no studies targeted to defining possible signaling abnormalities in sarcoid B cells have been performed.
We hypothesize that the peripheral B-cell compartment and B-cell signaling may be altered in sarcoidosis patients and that these disturbances may be essential to the pathogenesis of the disease. As a first step in studying this idea, we investigated the total numbers and frequencies of peripheral B-cell populations, the response of B cells to stimulation through CD40 and Toll-like receptor 9 (TLR-9), and the expression profiles of several key proteins involved in B-cell signal transduction (i.e., NF-κB/p65, SHIP1, Lyn, CD19, CD20, CD21, CD22, CD32, CD35, CD45, CD85J, and CD152) in B cells from sarcoidosis patients and healthy controls. Our results show striking disturbances in the circulating B-cell compartments of patients with severe chronic sarcoidosis, similar to those described in patients with pSS. We also found that peripheral B cells from patients with severe chronic sarcoidosis are anergic and that the anergic state was correlated with multiple B-cell intrinsic signaling defects. These data can be of diagnostic value and can provide information of possible pathogenic relevance.
MATERIALS AND METHODS
Patients.
Twenty-two adult patients who had biopsy-proven sarcoidosis confirmed by ACCESS criteria (6) were recruited for this study. All patients had severe chronic sarcoidosis with no evidence of systemic autoimmunity or immunodeficiency diseases and were either on immunosuppressive therapy or not on therapy at the time of blood collection. The clinical features of this distinct subgroup of sarcoidosis patients have been described recently (35). Briefly, most patients were African-American woman with active sarcoidosis, multiple-organ involvement, and worsening disease. Blood samples from age- and sex-matched healthy donors, who had no history of sarcoidosis, were collected by advertising at the local university and hospital employee pool. All subjects participated in the studies after signing an informed consent approved by the local institutional review board (IRB) committee.
B-cell isolation.
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral venous blood by Ficoll-Histopaque (Sigma, St. Louis, MO) density gradient centrifugation. CD19+ B cells were purified from PBMC as untouched lymphocytes by using CD19+ B-cell isolation kit II (Miltenyi Biotec, Auburn, CA). The purified lymphocyte fractions routinely contained ≥95% CD19+ cells as measured by flow cytometry (FC).
Flow cytometry.
PBMC or purified CD19+ cells (1 × 106) were stained for IgD, CD19, CD20, CD21, CD25, CD69, CD85J, CD152 (BD Biosciences, San Diego, CA), CD27, CD32, CD35, CD38, CD45, CD86, CD95, or HLA-DR (eBiosciences, San Diego, CA) using marker-specific fluorescent antibodies (Abs). After incubation on ice for 10 min, cells were washed twice with a buffer (phosphate-buffered saline [PBS] containing 1% bovine serum albumin [BSA] and 0.01% NaN3) and were resuspended in 0.5 ml of the same buffer for FC analysis. Sample acquisition was performed using a FACScan (Becton Dickinson, San Jose, CA) flow cytometer, and data were analyzed with CellQuest software (Becton Dickinson). An Fc receptor-blocking reagent (human immunoglobulins) was used to reduce nonspecific staining as recommended by the manufacturer (Miltenyi Biotec). Isotype-matched fluorochrome-labeled Abs and cells that were not stained were used as controls. A dual-platform FC method was used to generate absolute cell counts as previously described (40).
Immunoblotting.
Purified fractions of CD19+ cells and K562 cells were extracted in 1% Triton X-100 lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 μM NaCl, 5 mM EDTA, 10% glycerol, 1 mM sodium orthovanadate, 10 mM β-glycerolphosphate, and a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Protein extracts were obtained by centrifugation at 15,000 rpm for 15 min at 4°C and were stored at −80°C. Protein concentrations were determined by a standard colorimetric assay (Bio-Rad Laboratories, Richmond, CA). Twenty micrograms of purified total protein was loaded and separated by 10 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes and were immunoblotted with monoclonal Abs to NF-κB/p65, actin, Src homology domain 2-containing inositol phosphatase 1 (SHIP), or Lyn (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer's recommendations.
B-cell activation, proliferation, and differentiation assays.
Fractions of CD19+ cells either were labeled with 10 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE; Invitrogen/Molecular Probes, Eugene, OR) as previously described (38) or were left unlabeled. Cells were suspended in RPMI 1640 medium (GIBCO BRL, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, and 1 mM sodium pyruvate with antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) and were then plated in 24-well culture plates (0.5 × 106/ml) containing 50 ng/ml CD40 ligand (CD40L; Miltenyi Biotec), 10 μg/ml CpG oligodeoxynucleotides (CpG-ODN; Imgenex, San Diego, CA), 20 U/ml interleukin 2 (IL-2), 50 ng/ml IL-10, and 10 ng/ml IL-15 (Peprotech, Rocky Hill, NJ). Cultures incubated at 37°C under humidified air plus 5% CO2 for 1 (activation assays) or 5 (proliferation assays) days were harvested, and the cells were stained for CD19, CD20, CD21, CD25, CD32, CD35, CD45, CD69, CD85J, CD86, CD95, CD152, or HLA-DR for FC analysis. To induce plasmacytic differentiation, CD19+ cells, stimulated for 4 days as described above, were harvested, washed, and recultured (1 × 106/ml) in the presence of 20 U/ml IL-2, 50 ng/ml IL-6, 50 ng/ml IL-10, and 10 ng/ml IL-15 (Peprotech) for an additional 3 days. For intracellular staining, cells were fixed in 4% paraformaldehyde and were incubated with fluorescein isothiocyanate (FITC)-labeled anti-IgG (BD Biosciences, San Diego, CA) in a buffer containing 0.1% saponin. Samples were analyzed by FC.
Statistical analysis.
Statistical comparisons were performed using the nonparametric Mann-Whitney U test (GraphPad Prism, version 5.0; GraphPad Software, Inc., La Jolla, CA), and significance was defined as a P value of <0.05.
RESULTS
Decreased number and frequency of blood memory B cells in sarcoidosis.
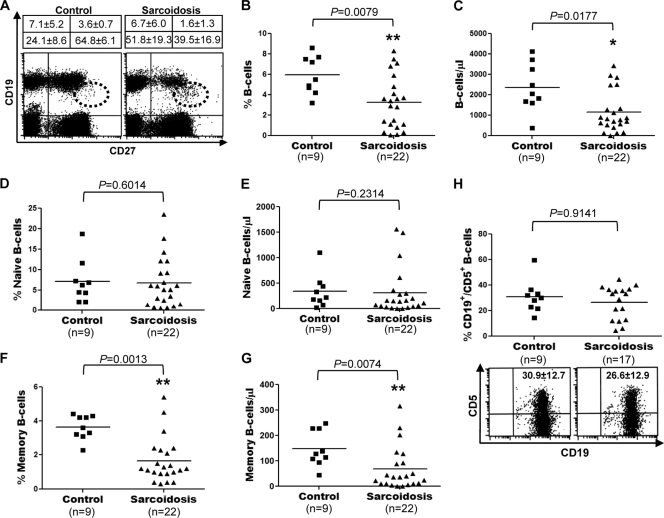
The relative composition of the blood B-cell compartment in patients with sarcoidosis has not been investigated. To examine possible disease-associated changes in the B-cell populations of sarcoidosis patients, we determined and compared the median percentages and median absolute numbers of total, naïve, and memory B cells in sarcoidosis patients and healthy controls by using dual-platform FC. A representative example of the double immunofluorescent staining for CD19 and CD27 is shown in Fig. 1A. Both the median frequency (5.9% ± 1.8% versus 3.2% ± 2.5%; P = 0.0079) and the median absolute number (2,346 ± 1,178 versus 1,149 ± 1,018 cells/μl; P = 0.0177) of total B cells (CD19+) were significantly decreased in sarcoidosis patients (Fig. 1B and C). There were no significant differences in the median frequency (7.1% ± 5.2% versus 6.7% ± 6.0%; P = 0.6014) and median total number (337.3 ± 328.9 versus 312.0 ± 460.3 cells/μl; P = 0.2314) of naïve B cells (CD19+ CD27−) between sarcoidosis patients and healthy controls (Fig. 1D and E). In contrast, the median frequency (3.6% ± 0.7% versus 1.6% ± 1.3%; P = 0.0013) and the median absolute number (148.3 ± 70.4 versus 68.3 ± 85.5 cells/μl; P = 0.0074) of memory B cells (CD19+ CD27+) were significantly reduced in patients with sarcoidosis (Fig. 1F and G). A comparative analysis of CD5 expression in CD19+ cells isolated from sarcoidosis patients and healthy controls revealed no significant differences (30.9% ± 12.7% versus 26.6% ± 12.9%; P = 0.9141) in the proportion of CD5+ B cells between sarcoidosis patients and healthy controls (Fig. 1H). The reduced frequency of peripheral blood memory B cells in patients with sarcoidosis did not reflect an increase in the peripheral naïve B-cell population, since the total numbers of naïve B cells in these patients were similar to those observed in normal controls.
Fig. 1.
Decreased numbers and frequency of memory B cells in sarcoidosis patients. (A) Representative example of CD19 and CD27 expression on PBMC. The frequencies of naïve and memory B cells in sarcoidosis patients and healthy controls are compared. Results are given as percentages (means ± standard deviations). Plasmablasts (CD19+/− CD27++) are shown within dotted circles. (B through G) Frequencies of total (CD19+) (B), naïve (CD19+ CD27−) (D), or memory (CD19+ CD27+) (F) B cells and absolute numbers of total (C), naïve (E), and memory (G) B cells were determined by dual-platform FC of PBMC from patients with sarcoidosis and healthy controls. (H) Analysis of CD19 and CD5 expression in blood B cells purified from sarcoidosis patients and healthy controls by magnetic-activated cell sorting. Horizontal lines indicate median percentages and median absolute numbers of the different populations.
Decreased number and frequency of unswitched memory B cells and increased frequency of activated naïve B cells and plasmablasts in sarcoidosis.
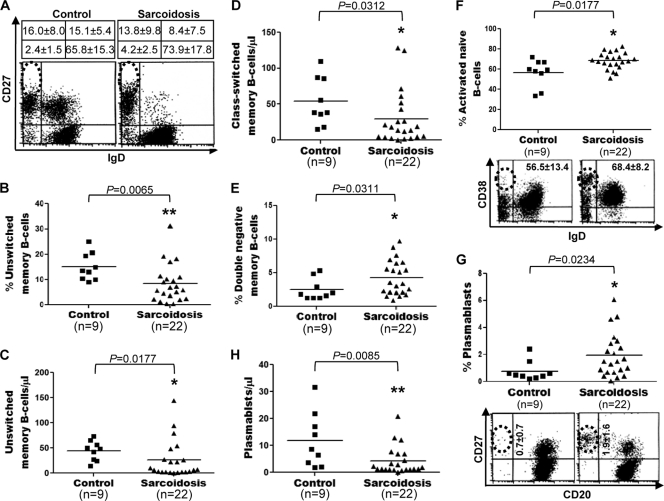
The human peripheral memory B-cell compartment is heterogeneous and comprises a variety of memory cell types (58). Consequently, the observed reduction in the memory (CD27+) B-cell population of sarcoidosis patients might reflect either a decrease in a specific memory B-cell type or a reduction of all memory B-cell subpopulations. To distinguish between these two possibilities, we determined and compared the median percentages and median absolute numbers of various memory B-cell subtypes in sarcoidosis patients and healthy controls by using dual-platform FC. A representative example of the triple immunofluorescent staining for CD19, IgD, and CD27 is shown in Fig. 2A. Both the median frequency (15.1% ± 5.4% versus 8.4% ± 7.5%; P = 0.0065) and the median absolute number (44.5 ± 19.7 versus 25.7 ± 37.2 cells/μl; P = 0.0177) of unswitched memory B cells (CD19+ CD27+ IgD+) were significantly decreased in sarcoidosis patients (Fig. 2B and C). The median frequency (16.0% ± 8.0% versus 13.8% ± 9.8%; P = 0.3496) of class-switched memory B cells (CD19+ CD27+ IgD−) did not differ significantly between sarcoidosis patients and healthy controls (data not shown), but the median absolute number (53.8 ± 33.2 versus 29.7 ± 37.2 cells/μl; P = 0.0312) of class-switched memory B cells was significantly reduced in patients with sarcoidosis (Fig. 2D). Compared with healthy controls, sarcoidosis patients had a significantly increased median frequency (2.4% ± 1.5% versus 4.2% ± 2.5%; P = 0.0311) (Fig. 2E), but normal median total numbers (14.8 ± 10.9 versus 10.3 ± 10.7 cells/μl; P = 0.2149) (data not shown), of double-negative memory B cells (CD19+ CD27− IgD−). A comparative analysis of CD38 expression in the PBMC of sarcoidosis patients and healthy controls revealed a significantly increased proportion (56.5% ± 13.4% versus 68.4% ± 8.2%; P = 0.0177) of activated (CD38+) cells within the naïve B-cell (CD19+ IgD+) compartment of patients with sarcoidosis (Fig. 2F). Additionally, sarcoidosis patients exhibited a significantly increased median frequency (0.7% ± 0.7% versus 1.9% ± 1.6%; P = 0.0234) (Fig. 2G), but reduced median absolute numbers (11.9 ± 10.1 versus 4.1 ± 5.1 cells/μl; P = 0.0085) (Fig. 2H), of circulating plasmablasts (CD19+/− CD20− CD27++). These results suggest altered memory B-cell homeostasis, increased B-cell activation, and accelerated differentiation of B cells into plasma cells in patients with sarcoidosis.
Fig. 2.
Decreased numbers and frequency of unswitched memory B cells and increased frequency of activated naïve B cells and plasmablasts in sarcoidosis patients. (A) Representative example of CD27 and IgD expression on CD19+ B cells. The frequencies of B-cell subpopulations in sarcoidosis patients and healthy controls are compared. Results are given as percentages (means ± standard deviations). (B through H) Frequencies of unswitched memory (CD19+ CD27+ IgD+) (B), double-negative memory (CD19+ CD27− IgD−) (E), and activated naïve (CD19+ CD38+ IgD+) (F) B cells and plasmablasts (CD19+/− CD20− CD27++) (G) and absolute numbers of unswitched (C) and class-switched (CD19+ CD27+ IgD−) (D) memory B cells and plasmablasts (H) were determined by dual-platform FC of peripheral blood CD19+ B cells from patients with sarcoidosis and healthy controls. Horizontal lines indicate median percentages and median absolute numbers of the different populations. CD38 and IgD (F, bottom) or CD20 and CD27 (G, bottom) expression within the blood CD19+ subset of sarcoidosis patients and healthy controls was analyzed. (Plasmablasts are shown within dotted circles.) Dot plots were gated on CD19+ events.
Impaired responsiveness of sarcoid blood B cells to TLR9- and CD40-mediated stimulation.
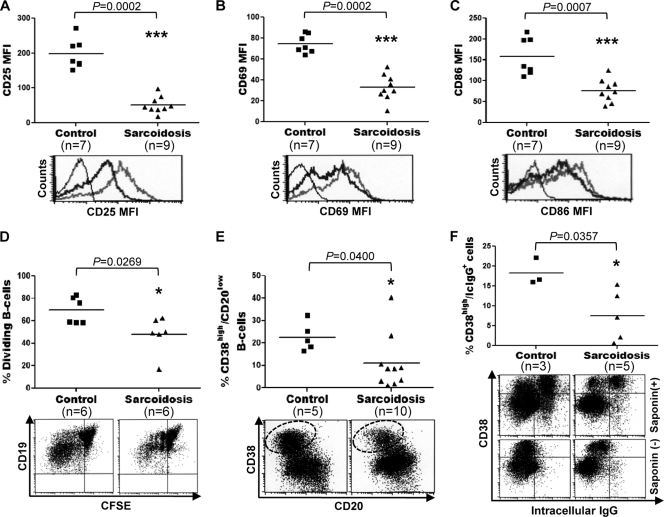
To investigate the possibility that the activated phenotype and features of plasmacytic differentiation exhibited by peripheral blood B cells from patients with sarcoidosis resulted from increased responsiveness of these lymphocytes to stimulation, the effects of TLR9 and CD40 ligation on the expression of activation markers, proliferation, and differentiation were examined. Purified preparations of CD19+ cells from sarcoid patients and healthy controls were stimulated for 24 h with CD40L, CpG-ODN, IL-2, IL-10, and IL-15, and the cells were then analyzed by FC for levels of expression of CD25, CD69, CD86, CD95, and HLA-DR. Each of these surface markers is known to be upregulated by B cells upon antigenic stimulation (48). In comparison to B cells from healthy controls, stimulation of sarcoid B cells with CD40L, CpG-ODN, IL-2, IL-10, and IL-15 resulted in significantly reduced expression of CD25 (mean fluorescence intensity [MFI], 197.7 ± 42.2 versus 51.4 ± 23.4; P = 0.0002), CD69 (MFI, 74.5 ± 8.8 versus 32.9 ± 12.5; P = 0.0002), and CD86 (MFI, 158.4 ± 44.4 versus 76.8 ± 27.3; P = 0.0007) (Fig. 3A, B, and C, respectively). After CD40L, CpG-ODN, and IL-2 stimulation, no significant differences in the levels of expression of CD95 (18.2 ± 5.3 versus 14.7 ± 4.4; P = 0.1113) and HLA-DR (1,169 ± 139.3 versus 1,035 ± 241.4; P = 0.2523) were observed between B cells from sarcoidosis patients and those from healthy controls (data not shown). After 5 days of culture, the CD19+ cells were analyzed by FC for proliferation by use of CFSE staining. Cells that have divided exhibit less CFSE fluorescence due to intracellular dilution of the stain (38). Stimulation of sarcoid B cells with CD40L, CpG-ODN, IL-2, IL-10, and IL-15 was associated with significantly less cell proliferation (69.3% ± 11.8% versus 47.9% ± 16.3%; P = 0.0269) than that observed for B cells from healthy controls (Fig. 3D). The reduced proliferation of sarcoid B cells was not due to a higher fraction of naïve B cells, which divide considerably less than memory B cells (61); we did not observe any significant difference between the frequencies of naïve B cells in cultures of B cells from sarcoidosis patients versus those from healthy controls (Fig. 1D). To evaluate the capacity of sarcoid B cells to differentiate into plasma cells, purified preparations of CD19+ cells from sarcoid patients and healthy controls were stimulated for 4 days with CD40L, CpG-ODN, IL-2, IL-10, and IL-15, followed by restimulation with IL-2, IL-6, IL-10, and IL-15 for an additional 3 days. On day 7, the frequency of CD20low CD38high cells appearing in cultures of sarcoid B cells was significantly lower (22.6% ± 6.3% versus 10.9% ± 12.1%; P = 0.0400) than that in cultures of healthy control B cells (Fig. 3E). Subsequent staining for intracellular IgG, a marker of plasmacytic differentiation (3), established that a considerable proportion of the CD20low CD38high cells in control cultures exhibited intense cytoplasmic IgG staining. Stimulation of sarcoid B cells, however, resulted in a significantly lower frequency (18.2% ± 3.4% versus 7.5% ± 6.3%; P = 0.0357) of IgG-containing CD20low CD38high cells than that observed for cultures of B cells from healthy controls (Fig. 3F). The reduced frequency of IgG-containing cells in cultures of sarcoid B cells was not due to a lower fraction of IgG-containing plasma cell precursors (2); we observed no significant difference (16.0% ± 8.0% versus 13.8% ± 9.8%; P = 0.3496) between the frequencies of class-switched memory B cells in cultures of sarcoidosis versus healthy-control B cells. No significant differences in cell viability (92.9% ± 3.9% versus 92.1% ± 8.5%; P = 0.1375), as determined by a trypan blue exclusion assay, were found between B-cell cultures from sarcoid patients and healthy controls for as long as 7 days of observation (data not shown). These results indicate that peripheral blood B cells from patients with sarcoidosis are anergic and differentiate poorly into plasma cells after polyclonal stimulation.
Fig. 3.
Impaired activation, proliferation, and differentiation of sarcoid B cells. (A to C) CD19+ lymphocytes, purified from PBMC of sarcoid patients and healthy controls by magnetic-activated cell sorting, were incubated for 24 h with CD40L, CpG-ODN, IL-2, IL-10, and IL-15. (Top) The cells were then analyzed by FC for levels of expression of CD25 (A), CD69 (B), and CD86 (C). Horizontal lines indicate median MFI values. (Bottom) Representative histograms. Thin lines, isotype control; thick black lines, sarcoidosis cells; thick gray lines, control cells. (D and E) Blood CD19+ cells, purified either from sarcoidosis patients or from healthy controls, were labeled with CFSE and were stimulated with CD40L, CpG-ODN, IL-2, IL-10, and IL-15 for 5 days. The frequency of dividing B cells (D) and plasma cells (CD20low CD38high) (E) were determined by FC in the CD19+ population. Representative dot plots are shown below the graphs. Plasma cells are shown within dotted ovals in the dot plot in panel E. (F) Purified preparations of CD19+ cells from sarcoid patients and healthy controls were first stimulated for 4 days as described for panels D and E and then restimulated with IL-2, IL-10, and IL-15 for 3 days. At day 7, the frequency of IgG-containing cells in these cultures was determined by intracellular staining using FC. Upon saponin permeabilization, CD20low CD38high cells were specifically labeled for intracellular (Ic) IgG (top). No intracellular IgG staining was observed in CD20low CD38high cells without saponin permeabilization (bottom).
Peripheral blood B cells from patients with sarcoidosis exhibit decreased expression of costimulatory and coinhibitory receptors.
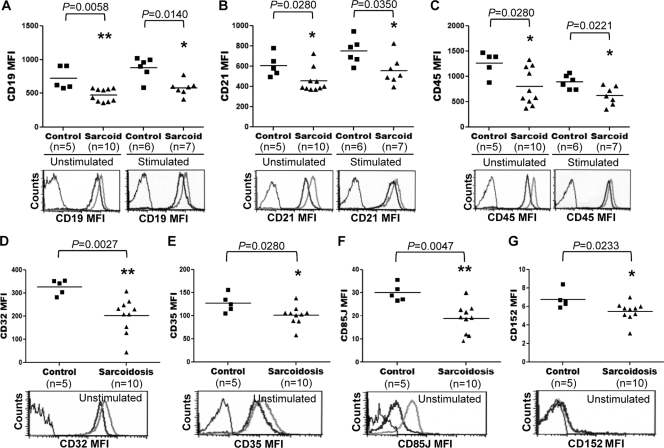
To explore whether the impaired activation, proliferation, and differentiation of sarcoid B cells resulted from defects in the expression of costimulatory receptors, the expression of CD19, CD20, CD21, and CD45 in blood B cells from patients with sarcoidosis was examined. Purified preparations of CD19+ cells from sarcoid patients and healthy controls were analyzed by FC for levels of expression of CD19, CD20, CD21, and CD45 after these cells were cultured with or without CD40L, CpG-ODN, IL-2, IL-10, and IL-15. Compared with levels in control B cells, significantly reduced levels of CD19 were found in both resting (MFI, 723.8 ± 170.0 versus 477.2 ± 94.7; P = 0.0058) and activated (MFI, 879.8 ± 161.6 versus 580.4 ± 107.1; P = 0.0140) B cells from sarcoidosis patients (Fig. 4A). The levels of CD20 (MFI, 677.6 ± 224.7 versus 421.5 ± 198.0; P = 0.0272) (data not shown), CD21 (MFI, 608.0 ± 111.5 versus 453.5 ± 117.8; P = 0.0280), and CD45 (MFI, 1,266 ± 239.6 versus 799.3 ± 364.4; P = 0.0280) expressed by unstimulated sarcoid B cells were also significantly lower than those expressed by unstimulated B cells from healthy controls (Fig. 4B and C). In comparison with no stimulation of B cells, stimulation with CD40L, CpG-ODN, IL-2, IL-10, and IL-15 increased the levels of CD21 and decreased the levels of CD45 expressed by B cells from both sarcoid patients and healthy controls (Fig. 4B and C). When sarcoid B cells were stimulated with CD40L, CpG-ODN, IL-2, IL-10, and IL-15, however, significantly lower levels of CD20 (MFI, 136.5 ± 33.1 versus 93.5 ± 32.2; P = 0.0379) (data not shown), CD21 (MFI, 750.5 ± 128.1 versus 558.0 ± 140.4; P = 0.0350), and CD45 (MFI, 890.5 ± 137.7 versus 620.0 ± 184.3; P = 0.0221) were detected in these cells than in identically stimulated B cells from healthy controls (Fig. 4B and C). The impaired responsiveness of sarcoid B cells could not be attributed to increased expression of the coinhibitory receptors CD22, CD32, CD35, CD85J, and CD152 on these cells, since baseline levels of these molecules in sarcoid B cells were either equivalent (CD22 [MFI, 70.1 ± 11.2 versus 76.7 ± 21.4; P = 0.6419]) (data not shown) to or significantly lower (CD32 [MFI, 327.2 ± 32.1 versus 202.8 ± 75.8; P = 0.0027], CD35 [MFI, 127.5 ± 19.7 versus 101.1 ± 20.4; P = 0.0280], CD85J [MFI, 29.9 ± 3.7 versus 18.7 ± 6.3; P = 0.0047], and CD152 [MFI, 6.7 ± 0.9 versus 5.4 ± 1.0; P = 0.0233]) than those in control cells (Fig. 4D, E, F, and G). Taken together, these results suggest defective expression of multiple costimulatory and coinhibitory receptors in B cells from patients with sarcoidosis.
Fig. 4.
Expression levels of costimulatory (CD19, CD21, and CD45) and coinhibitory (CD32, CD35, CD85J, and CD152) receptors in sarcoid B cells. CD19+ cells, purified from PBMC of sarcoid patients and healthy controls by magnetic-activated cell sorting, were either left unstimulated or stimulated for 24 h with CD40L, CpG-ODN, IL-2, IL-10, and IL-15. The cells were then analyzed by FC for levels of expression of CD19 (A), CD21 (B), CD45 (C), CD32 (D), CD35 (E), CD85J (F), and CD152 (G). Horizontal lines indicate the median MFI for each of the indicated markers. Representative histograms are shown at the bottoms of all panels. Thin lines, isotype control; thick black lines, sarcoidosis cells; thick gray lines, control cells.
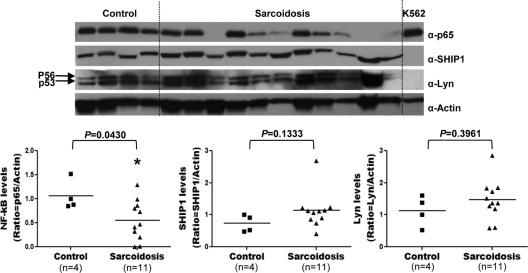
Peripheral blood B cells from patients with sarcoidosis exhibit decreased levels of NF-κB/p65.
To investigate whether defects in NF-κB signaling were associated with the peripheral B-cell anergy observed in sarcoidosis patients, whole-protein lysates of peripheral blood CD19+ cells isolated from sarcoid patients or controls were immunoblotted using a monoclonal Ab specific to NF-κB/p65. We found that p65 protein levels were significantly reduced or absent (p65/actin ratio, 1.0 ± 0.3 versus 0.5 ± 0.4; P = 0.0430) in 65% of the sarcoid patients analyzed (Fig. 5). The intensity of the p65 band in the remaining 35% of sarcoid patients was quantitatively comparable to that of normal controls. In contrast, levels of the protein tyrosine phosphatase SHIP1 (SHIP1/actin ratio, 0.7 ± 0.2 versus 1.1 ± 0.5; P = 0.1333) and the protein tyrosine kinase Lyn (Lyn/actin ratio, 1.1 ± 0.4 versus 1.4 ± 0.6; P = 0.3961) in these sarcoid B cells were equivalent to levels found in normal controls. These results suggest that a considerable proportion of patients with severe chronic sarcoidosis exhibit a specific defect in the expression of p65 in their blood B cells.
Fig. 5.
Reduced levels of NF-κB/p65 in sarcoid B cells. Purified CD19+ cells isolated from PBMC of sarcoidosis patients and healthy controls by magnetic-activated cell sorting were lysed, and protein lysates were electrophoretically separated, transferred to membranes, and immunoblotted with monoclonal Abs to p65, SHIP1, Lyn, and actin. (Top) The two major Lyn isoforms are shown (arrows). Lysates from K562 cells were used as controls. (Bottom) The median optical density of each protein band was quantified by densitometry, and results are expressed in arbitrary units as the ratio of the optical density of the target protein to that of actin. Horizontal lines indicate the median optical density of each protein band.
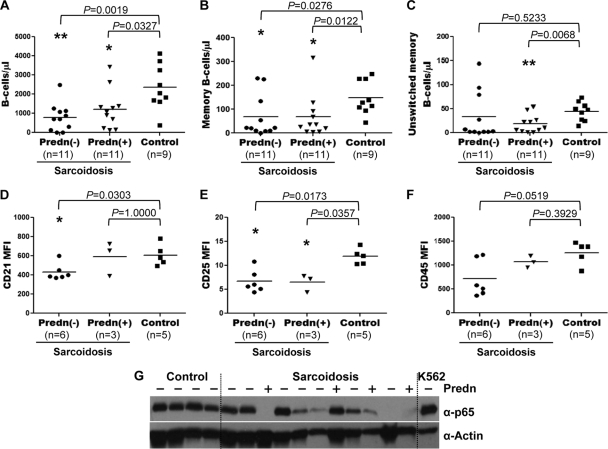
B-lymphocyte variables and treatment status.
Because corticosteroids can affect various aspects of B-cell function (49), it is essential to determine whether steroid therapy was associated with (i) the memory B-cell population imbalance, (ii) the hyporesponsiveness of sarcoid B cells, or (iii) the observed deficiency of NF-κB/p65. We found no associations between the use of prednisone and decreased absolute numbers of total, memory, and unswitched memory B cells (Fig. 6A, B, and C) or between the use of prednisone and reduced levels of CD21, CD25, and CD45 expression (Fig. 6D, E, and F). Western blot analysis revealed that the reduced levels of p65 protein in sarcoid B cells did not correlate with steroid therapy (Fig. 6G). Furthermore, no associations were found between the use of hydroxychloroquine or methotrexate and the laboratory variables analyzed above (data not shown). These results suggest that therapy, for the most part, cannot be held accountable for the molecular and cellular defects observed in sarcoid B cells.
Fig. 6.
Relationship between B-cell variables and steroid therapy. Associations between the use of prednisone (Predn) and the numbers of total (A), memory (B), and unswitched memory (C) B cells and between the use of prednisone and the levels of CD21 (D), CD25 (E), CD45 (F), and NF-κB/p65 (G) in patients with sarcoidosis were investigated. The absolute numbers of the B-cell populations and the levels of CD21, CD25, CD45, and p65 were determined by dual-platform FC analysis and Western blotting, respectively, as described in the legends to Fig. 1 and 5. Horizontal lines in panels A to C indicate the median absolute numbers of total, memory, and unswitched memory B cells. Lysates from K562 cells were used as controls.
DISCUSSION
Patients with sarcoidosis exhibit clinical, serologic, and pathological features resembling systemic autoimmunity (67). However, because B cells and plasma cells are rarely found in sarcoid granulomas, it is generally assumed that B cells play no role in the pathogenesis of sarcoidosis (28). Here we demonstrate that the peripheral B-cell compartment of patients with severe chronic sarcoidosis is significantly altered. These results represent the first analysis of the distribution of B-cell populations in sarcoidosis patients and show for the first time that the peripheral B-cell anergy observed with sarcoidosis is associated with multiple intrinsic signaling defects of B cells.
Immunophenotyping analysis revealed that patients with severe chronic sarcoidosis generally had absolute B-cell lymphopenia and exhibited a significantly decreased frequency and number of memory B cells. Since there were no differences in the total number and percentage of naïve B cells between severe sarcoidosis patients and healthy controls, a predominant reduction in the numbers and frequency of memory B cells accounted for the B-cell deficiency found in these patients. Further characterization of the B-cell compartment of patients with severe chronic sarcoidosis revealed that the decreased numbers and frequency of memory B cells resulted from a combined reduction in the total numbers of class-switched and unswitched memory B cells.
The pattern of peripheral B-cell populations found in severe chronic sarcoidosis clearly contrasted with those described for SLE and RA (8, 26, 46) but was strikingly similar to that reported for pSS (9, 26). Sarcoidosis and pSS are characterized by intense Th1 infiltrates with fewer B cells at sites of disease activity (23, 26), and HLA-DR3 and -DQ2 are important risk factors for the development of these two diseases (11, 31). Sarcoidosis, however, was not associated with the increased frequency of autoreactive CD5+ B cells that has been observed in pSS patients (12). The close relationship between sarcoidosis and pSS might not be casual; the two diseases may share a immunopathogenic mechanism (55).
The peripheral B-cell subset distribution observed in patients with severe chronic sarcoidosis was also similar to that described for a subgroup of common variable immunodeficiency (CVID) patients who exhibit autoimmune manifestations (16). This patient subgroup has low numbers of circulating memory B cells and often presents granulomatous lymphocytic interstitial lung disease, a chronic inflammatory condition characterized by the development of granulomas identical to those of sarcoidosis (1, 4). This suggests that a lack of memory B cells may be associated with the altered genetic, cellular, or cytokine environment that supports granuloma formation. Interestingly, several abnormalities associated with CVID, such as decreased CD154 expression in activated CD4+ T cells (22), increased proportions of B cells expressing low levels of CD19 and CD21 (32, 54), and deficient TLR9-mediated B-cell activation and differentiation (17), were also seen in patients with severe chronic sarcoidosis.
In healthy individuals, the percentage of memory B cells increases gradually with age (15). This is in stark contrast to our observations for patients with severe chronic sarcoidosis, who exhibited a significantly reduced memory B-cell compartment. Since naïve B cells differentiate into either memory B cells or plasma cells (36), the reduced numbers and frequency of memory B cells found in sarcoidosis could be explained by a skewing toward plasma cell differentiation, resulting in depletion of memory B cells. The increased proportions of activated naïve B cells and plasmablasts observed in the blood of patients with severe chronic sarcoidosis support this notion. However, since the number of antigen-specific B cells in sarcoidosis patients may be low (60), it is unlikely that a general decrease in the population of memory B cells is due to plasmacytic differentiation of B cells that have repeatedly encountered their specific antigens. In fact, we found that B cells from patients with severe chronic sarcoidosis did not exhibit increased levels of CD85J and CD152, two inhibitory receptors that should be upregulated in B cells upon continual antigenic stimulation (41).
In the absence of specific antigenic stimulation, differentiation of memory B cells into plasma cells could have occurred as a result of B cells receiving bystander T-cell help (7, 28). Since patients with severe chronic sarcoidosis have an increased population of effector-memory T cells that constitutively express CD134 at high levels (our unpublished observation), it is possible that upregulation of CD134 in these lymphocytes facilitates plasma cell differentiation by promoting bystander activation of B cells through CD134–CD252 interactions (43). Our previous study on the kinetics of CD154 expression revealed significantly reduced expression of CD154 in activated T cells from patients with severe chronic sarcoidosis (35). Since continuous interactions between CD154 and CD40 provide inhibitory signals for B-cell differentiation into plasma cells (56), down-modulation of CD154 expression in sarcoid T cells has the potential to interrupt those inhibitory signals, thereby permitting B-cell differentiation into plasma cells.
B cells accumulate in the pulmonary lesions of patients with sarcoidosis (23), an increase that may be determined by overexpression of B-cell-attracting chemokines in the inflamed lungs (13). If memory B cells are preferentially attracted by these chemokines (44), then selective recruitment of memory B cells to the lungs could explain the reduction of the blood memory B-cell pool observed in sarcoidosis. Within the lungs, memory B cells may be activated by local T cells to differentiate into plasma cells, a process that would eventually lead to polyclonal hypergammaglobulinemia and perhaps to elevated levels of circulating immune complexes (28). Under physiological conditions, immune complexes provide important inhibitory feedback signals to B cells via Fcγ receptors (FcγRs) and complement receptors (30, 52). Since B cells from patients with severe chronic sarcoidosis exhibited significantly reduced levels of CD32 (FcγRII) and CD35 (CR1) expression, it is possible that down-modulation of these two receptors could have disrupted those inhibitory signals, thus contributing to the B-cell hyperactivation observed in sarcoidosis. In spite of this, we found that peripheral blood B cells from patients with severe chronic sarcoidosis were anergic. These results support the notion that in sarcoidosis, enhanced immunoglobulin synthesis occurs at sites of disease activity (28).
Although the B-cell anergy associated with sarcoidosis was recognized long ago, previous studies evaluating the function of B cells in sarcoidosis were performed using unfractionated PBMC (5, 34). Thus, these studies failed to differentiate between B-cell-intrinsic and -extrinsic defects as the primary mechanism involved in this anergy. Using purified preparations of B cells from patients with sarcoidosis, we now demonstrate that these lymphocytes exhibit multiple intrinsic signal abnormalities (i.e., low levels of CD19, CD20, CD21, CD45, and NF-κB/p65 expression) that are associated with decreased lymphocyte responses upon CD40 and TLR-9 stimulation (i.e., activation, proliferation, and differentiation). Since CD19, CD20, CD21, and CD45 are positive regulators of BCR signal transduction (27), a deficiency of any of these molecules could have resulted in anergy induction in these lymphocytes. In fact, B cells from mice deficient in CD19, CD20, CD21, or CD45 exhibit a higher threshold for activation and thus are hyporesponsive to transmembrane stimulation (14, 21, 47, 64). Since sarcoid B cells displayed reduced levels of NF-κB/p65, and because NF-κB/p65 is essential for initiating the transcriptional response to CD40 and TLR-9 ligation (20, 65), it is possible that low levels of NF-κB/p65 could be responsible, at least in part, for the anergy exhibited by these cells. The impaired responses displayed by B cells from NF-κB/p65-deficient mice demonstrate that a lack of NF-κB/p65 could induce an anergic state in B lymphocytes (19). Whether reduced levels of CD19, CD20, CD21, CD45, and NF-κB/p65 in sarcoid B cells were directly responsible for the B-cell anergy observed in sarcoidosis is currently unclear. Restoration experiments are under way in our laboratory to demonstrate a direct cause-effect relationship between reduced levels of these signaling molecules and the anergic state of sarcoid B cells.
Preliminary studies in our laboratory indicated that NF-κB/p65-deficient sarcoid B cells produced very low levels of IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) following stimulation. However, the levels of IL-7 (a survival factor for CD4+ T cells) (25) and IL-16 (a chemoattractant for CD4+ T cells) (33) were notably increased in these sarcoid lymphocytes. Thus, NF-κB/p65-deficient sarcoid B cells could play an important role in attracting and providing survival signals to pathogenic Th1 cells at sites of disease activity.
In summary, our study identified disturbed homeostasis and multiple intrinsic signaling defects in the peripheral blood B-cell compartments of patients with severe chronic sarcoidosis. The altered distribution of the B-cell populations in these patients resembled that found in pSS and CVID and may be related to conditions of bystander stimulation of memory B cells by activated Th1 cells in the affected organs. The significance of the lack of memory B cells for the pathogenesis of sarcoidosis is unclear. Since memory B cells play important regulatory roles by producing suppressive cytokines, such as IL-10 and transforming growth factor β (TGF-β) (37), deficient production of these cytokines could have favored granuloma formation in sarcoidosis. Likewise, if an effective B-cell response is essential for clearing pathogenic antigens in sarcoidosis, then a reduction in memory B-cell numbers may induce a defective Ab response that cannot eliminate antigens that induce granuloma development (42). This could result in antigen persistence and chronic inflammation. To clarify these important issues, it will be necessary to investigate the phenotype and function of B cells found at sites of disease activity in patients with different clinical forms of sarcoidosis, particularly those who have not yet received treatment.
ACKNOWLEDGMENTS
We are indebted to Rudolf A. Manz and Marc A. Judson for critical reading of the manuscript, to Patricia Cannon for excellent secretarial assistance, and to Irene Marshall, Sue A. Joyner, Renuka A. Kadali, and Mary C. Cashion for help with the recruitment of research subjects.
This work was supported by startup funds from East Carolina University to Sergio Arce.
Footnotes
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Agematsu K., et al. 2002. Absence of memory B cells in patients with common variable immunodeficiency. Clin. Immunol. 103:34–42 [DOI] [PubMed] [Google Scholar]
- 2. Agematsu K., et al. 1997. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 27:2073–2079 [DOI] [PubMed] [Google Scholar]
- 3. Arce S., et al. 2004. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J. Leukoc. Biol. 75:1022–1028 [DOI] [PubMed] [Google Scholar]
- 4. Ardeniz O., Cunningham-Rundles C. 2009. Granulomatous disease in common variable immunodeficiency. Clin. Immunol. 133:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barth J., Falsafi-Amin R., Petermann W., Kekow J., Gross W. L. 1992. B-lymphocyte response in peripheral blood of patients with pulmonary sarcoidosis. Sarcoidosis 9:49–53 [PubMed] [Google Scholar]
- 6. Baughman R. P., et al. 2001. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 164:1885–1889 [DOI] [PubMed] [Google Scholar]
- 7. Bernasconi N. L., Traggiai E., Lanzavecchia A. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199–2202 [DOI] [PubMed] [Google Scholar]
- 8. Bohnhorst J. O., Bjorgan M. B., Thoen J. E., Natvig J. B., Thompson K. M. 2001. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J. Immunol. 167:3610–3618 [DOI] [PubMed] [Google Scholar]
- 9. Bohnhorst J. O., Thoen J. E., Natvig J. B., Thompson K. M. 2001. Significantly depressed percentage of CD27+ (memory) B cells among peripheral blood B cells in patients with primary Sjogren's syndrome. Scand. J. Immunol. 54:421–427 [DOI] [PubMed] [Google Scholar]
- 10. Bolland S., Ravetch J. V. 2000. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity 13:277–285 [DOI] [PubMed] [Google Scholar]
- 11. Bolstad A. I., Jonsson R. 2002. Genetic aspects of Sjogren's syndrome. Arthritis Res. 4:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brennan F., Plater-Zyberk C., Maini R. N., Feldmann M. 1989. Coordinate expansion of ‘fetal type' lymphocytes (TCR γδ+ T and CD5+ B) in rheumatoid arthritis and primary Sjogren's syndrome. Clin. Exp. Immunol. 77:175–178 [PMC free article] [PubMed] [Google Scholar]
- 13. Busuttil A., et al. 2009. CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis. Eur. Respir. J. 34:676–686 [DOI] [PubMed] [Google Scholar]
- 14. Carter R. H., Spycher M. O., Ng Y. C., Hoffman R., Fearon D. T. 1988. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J. Immunol. 141:457–463 [PubMed] [Google Scholar]
- 15. Colonna-Romano G., et al. 2003. B cells in the aged: CD27, CD5, and CD40 expression. Mech. Ageing Dev. 124:389–393 [DOI] [PubMed] [Google Scholar]
- 16. Cunningham-Rundles C. 2008. Autoimmune manifestations in common variable immunodeficiency. J. Clin. Immunol. 28(Suppl. 1):S42–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunningham-Rundles C., et al. 2006. TLR9 activation is defective in common variable immune deficiency. J. Immunol. 176:1978–1987 [DOI] [PubMed] [Google Scholar]
- 18. Daniele R. P., McMillan L. J., Dauber J. H., Rossman M. D. 1978. Immune complexes in sarcoidosis: a correlation with activity and duration of disease. Chest 74:261–264 [DOI] [PubMed] [Google Scholar]
- 19. Doi T. S., Takahashi T., Taguchi O., Azuma T., Obata Y. 1997. NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J. Exp. Med. 185:953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elgueta R., et al. 2009. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229:152–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engel P., et al. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3:39–50 [DOI] [PubMed] [Google Scholar]
- 22. Farrington M., et al. 1994. CD40 ligand expression is defective in a subset of patients with common variable immunodeficiency. Proc. Natl. Acad. Sci. U. S. A. 91:1099–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fazel S. B., Howie S. E., Krajewski A. S., Lamb D. 1992. B lymphocyte accumulations in human pulmonary sarcoidosis. Thorax 47:964–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flores-Borja F., Kabouridis P. S., Jury E. C., Isenberg D. A., Mageed R. A. 2007. Altered lipid raft-associated proximal signaling and translocation of CD45 tyrosine phosphatase in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 56:291–302 [DOI] [PubMed] [Google Scholar]
- 25. Guimond M., Fry T. J., Mackall C. L. 2005. Cytokine signals in T-cell homeostasis. J. Immunother 28:289–294 [DOI] [PubMed] [Google Scholar]
- 26. Hansen A., Lipsky P. E., Dorner T. 2007. B cells in Sjogren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasler P., Zouali M. 2001. B cell receptor signaling and autoimmunity. FASEB J. 15:2085–2098 [DOI] [PubMed] [Google Scholar]
- 28. Hunninghake G. W., Crystal R. G. 1981. Mechanisms of hypergammaglobulinemia in pulmonary sarcoidosis. Site of increased antibody production and role of T lymphocytes.. J. Clin. Invest. 67:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iannuzzi M. C., Rybicki B. A., Teirstein A. S. 2007. Sarcoidosis. N. Engl. J. Med. 357:2153–2165 [DOI] [PubMed] [Google Scholar]
- 30. Isaak A., et al. 2008. Physiological up-regulation of inhibitory receptors FcγRII and CR1 on memory B cells is lacking in SLE patients. Int. Immunol. 20:185–192 [DOI] [PubMed] [Google Scholar]
- 31. Judson M. A. 2003. The etiologic agent of sarcoidosis: what if there isn't one? Chest 124:6–8 [DOI] [PubMed] [Google Scholar]
- 32. Kanegane H., et al. 2007. Novel mutations in a Japanese patient with CD19 deficiency. Genes Immun. 8:663–670 [DOI] [PubMed] [Google Scholar]
- 33. Kaser A., et al. 2000. B lymphocyte-derived IL-16 attracts dendritic cells and Th cells. J. Immunol. 165:2474–2480 [DOI] [PubMed] [Google Scholar]
- 34. Katz P., Fauci A. S. 1978. Inhibition of polyclonal B-cell activation by suppressor monocytes in patients with sarcoidosis. Clin. Exp. Immunol. 32:554–562 [PMC free article] [PubMed] [Google Scholar]
- 35. Lee N. S., et al. 2011. Low levels of NF-κB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin. Vaccine Immunol. 18:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Y. J., Banchereau J. 1997. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin. Immunol. 9:235–240 [DOI] [PubMed] [Google Scholar]
- 37. Lund F. E. 2008. Cytokine-producing B lymphocytes—key regulators of immunity. Curr. Opin. Immunol. 20:332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lyons A. B., Parish C. R. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131–137 [DOI] [PubMed] [Google Scholar]
- 39. Mackay M., et al. 2006. Selective dysregulation of the FcγIIB receptor on memory B cells in SLE. J. Exp. Med. 203:2157–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mandy F., Janossy G., Bergeron M., Pilon R., Faucher S. 2008. Affordable CD4 T-cell enumeration for resource-limited regions: a status report for 2008. Cytometry B Clin. Cytom. 74(Suppl. 1):S27–S39 [DOI] [PubMed] [Google Scholar]
- 41. Moir S., Fauci A. S. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moller D. R., Chen E. S. 2002. Genetic basis of remitting sarcoidosis: triumph of the trimolecular complex? Am. J. Respir. Cell Mol. Biol. 27:391–395 [DOI] [PubMed] [Google Scholar]
- 43. Morimoto S., et al. 2000. CD134L engagement enhances human B cell Ig production: CD154/CD40, CD70/CD27, and CD134/CD134L interactions coordinately regulate T cell-dependent B cell responses. J. Immunol. 164:4097–4104 [DOI] [PubMed] [Google Scholar]
- 44. Muehlinghaus G., et al. 2005. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105:3965–3971 [DOI] [PubMed] [Google Scholar]
- 45. Nishizumi H., et al. 1995. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 3:549–560 [DOI] [PubMed] [Google Scholar]
- 46. Odendahl M., et al. 2000. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165:5970–5979 [DOI] [PubMed] [Google Scholar]
- 47. Pani G., Siminovitch K. A., Paige C. J. 1997. The motheaten mutation rescues B cell signaling and development in CD45-deficient mice. J. Exp. Med. 186:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parker D. C. 1993. T cell-dependent B cell activation. Annu. Rev. Immunol. 11:331–360 [DOI] [PubMed] [Google Scholar]
- 49. Paterson R. L., Or R., Domenico J. M., Delespesse G., Gelfand E. W. 1994. Regulation of CD23 expression by IL-4 and corticosteroid in human B lymphocytes. Altered response after EBV infection. J. Immunol. 152:2139–2147 [PubMed] [Google Scholar]
- 50. Pettersson T. 1997. Rheumatic features of sarcoidosis. Curr. Opin. Rheumatol. 9:62–67 [DOI] [PubMed] [Google Scholar]
- 51. Potter K. N., et al. 2002. Disturbances in peripheral blood B cell subpopulations in autoimmune patients. Lupus 11:872–877 [DOI] [PubMed] [Google Scholar]
- 52. Pritchard N. R., Smith K. G. 2003. B cell inhibitory receptors and autoimmunity. Immunology 108:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pugh-Bernard A. E., Cambier J. C. 2006. B cell receptor signaling in human systemic lupus erythematosus. Curr. Opin. Rheumatol. 18:451–455 [DOI] [PubMed] [Google Scholar]
- 54. Rakhmanov M., et al. 2009. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc. Natl. Acad. Sci. U. S. A. 106:13451–13456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramos-Casals M., Brito-Zeron P., Garcia-Carrasco M., Font J. 2004. Sarcoidosis or Sjogren syndrome? Clues to defining mimicry or coexistence in 59 cases Medicine (Baltimore) 83:85–95 [DOI] [PubMed] [Google Scholar]
- 56. Randall T. D., et al. 1998. Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity 8:733–742 [DOI] [PubMed] [Google Scholar]
- 57. Rose N. R., Bona C. 1993. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol. Today 14:426–430 [DOI] [PubMed] [Google Scholar]
- 58. Sanz I., Wei C., Lee F. E., Anolik J. 2008. Phenotypic and functional heterogeneity of human memory B cells. Semin. Immunol. 20:67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sohn H. W., Pierce S. K., Tzeng S. J. 2008. Live cell imaging reveals that the inhibitory FcγRIIB destabilizes B cell receptor membrane-lipid interactions and blocks immune synapse formation. J. Immunol. 180:793–799 [DOI] [PubMed] [Google Scholar]
- 60. Song Z., et al. 2005. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J. Exp. Med. 201:755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tangye S. G., Avery D. T., Deenick E. K., Hodgkin P. D. 2003. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 170:686–694 [DOI] [PubMed] [Google Scholar]
- 62. Torralba K. D., Quismorio F. P., Jr 2009. Sarcoidosis and the rheumatologist. Curr. Opin. Rheumatol. 21:62–70 [DOI] [PubMed] [Google Scholar]
- 63. Townsend M. J., Monroe J. G., Chan A. C. 2010. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol. Rev. 237:264–283 [DOI] [PubMed] [Google Scholar]
- 64. Uchida J., et al. 2004. Mouse CD20 expression and function. Int. Immunol. 16:119–129 [DOI] [PubMed] [Google Scholar]
- 65. Vallabhapurapu S., Karin M. 2009. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27:693–733 [DOI] [PubMed] [Google Scholar]
- 66. Weinberg I., Vasiliev L., Gotsman I. 2000. Anti-dsDNA antibodies in sarcoidosis. Semin. Arthritis Rheum. 29:328–331 [DOI] [PubMed] [Google Scholar]
- 67. Wiesenhutter G. W., Sharma O. P. 1979. Is sarcoidosis an autoimmune disease? Report of four cases and review of the literature Semin. Arthritis Rheum. 9:124–144 [DOI] [PubMed] [Google Scholar]