Abstract
Bacillus cereus spores are surrounded by a loose-fitting layer called the exosporium, whose distal part is mainly formed from glycoproteins. The role played by the exosporium glycoproteins of B. cereus ATCC 14579 (BclA and ExsH) was investigated by considering hydrophobicity and charge, as well as the properties of spore adhesion to stainless steel. The absence of BclA increased both the isoelectric point (IEP) and hydrophobicity of whole spores while simultaneously reducing the interaction between spores and stainless steel. However, neither the hydrophobicity nor the charge associated with BclA could explain the differences in the adhesion properties. Conversely, ExsH, another exosporium glycoprotein, did not play a significant role in spore surface properties. The monosaccharide analysis of B. cereus ATCC 14579 showed different glycosylation patterns on ExsH and BclA. Moreover, two specific glycosyl residues, namely, 2-O-methyl-rhamnose (2-Me-Rha) and 2,4-O-methyl-rhamnose (2,4-Me-Rha), were attached to BclA, in addition to the glycosyl residues already reported in B. anthracis.
INTRODUCTION
The food-borne pathogen Bacillus cereus has been extensively isolated in the form of spores from various environments, including food contact surfaces (26, 33). Spores of the B. cereus group (closely related species, such as B. cereus, B. anthracis, or B. thuringiensis) are characterized by the presence of an outer layer called the exosporium, which surrounds the spore and is suspected of playing a major role in the interface phenomena. Indeed, the exosporium has been shown to play a role in spore adhesion to abiotic surfaces (14) and to professional phagocytic cells (25), in the escape of spores from macrophages (27), and in spore germination (16). As revealed by electron microscopy, the exosporium is made of an external hair-like nap on top of a paracrystalline basal layer (18). The hair-like nap is mainly composed of the collagen-like glycoprotein BclA in B. anthracis (34). The BclA protein contains three domains: the N-terminal domain (NTD) of 44 amino acids (aa) is involved in targeting and anchoring the protein in the exosporium (38); the C-terminal domain (CTD) is composed of multiple β-strands (28); and the collagen-like region (CLR), with a triple-helix conformation (34), is composed of GXX repeats, mostly GPT, whose threonine residues provide putative glycosylation sites (8). The filament length is proportional to the number of GXX repeats (35). The presence of BclA at the surfaces of B. anthracis spores has been shown to have an impact on the overall hydrophobicity (6) and charge (7) of the spores. Furthermore, the presence of BclA affects the germination of B. anthracis spores and is involved in specific interaction with immune cells (4, 24) and surfactants (28). Genes of other collagen-like proteins are also present in the B. anthracis genome (22, 39), as well as in other Bacillus species, such as exsH, which encodes a protein with an NTD of 145 aa with putative helical secondary structures, or exsJ (40). However, their localization, composition, and role in spore properties have been poorly investigated, except for BclB (38).
The monosaccharide composition of BclA has so far been described only in B. anthracis. Two oligosaccharides have been characterized: a trisaccharide composed of 3-O-methyl-rhamnose (3-Me-Rha), l-rhamnose (Rha), and N-acetyl-galactosamine (GalNAc) residues and a pentasaccharide composed of one anthrose residue, three Rha residues, and a GalNAc residue (5, 8). Rha derivatives and GalNAc residues were also found in the BclB proteins of B. anthracis (42). While 3-Me-Rha, glucosamine (GlcNH2), and galactosamine (GalNH2) residues were found in the B. cereus exosporium (12), the reported results suggest significant differences in glycosylation patterns within Bacillus species and/or strains of B. cereus. First, 2-Me-Rha and 2,4-Me-Rha (12, 15), as well as fucose (15), absent from B. anthracis spores (8, 15), were identified in some B. cereus and B. thuringiensis strains, including B. cereus ATCC 14579. Elsewhere, anthrose was detected in B. anthracis and two B. thuringiensis spores but not in several B. cereus strains, including ATCC 14579 (8, 9). Lastly, Tamborrini et al. (36) showed that an antibody targeting the B. anthracis BclA tetrasaccharide reacted with spore extracts of some B. cereus strains, but not with B. cereus ATCC 14579. However, the glycosylation patterns of specific exosporium-associated glycoproteins have yet to be characterized in B. cereus.
The aim of this study was to investigate the influence of the BclA and ExsH glycoproteins on the ultrastructure of the B. cereus ATCC 14579 spore exosporium and on its physicochemical properties and adhesion to stainless steel.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Escherichia coli strains and the B. cereus ATCC 14579 wild-type strain and its related mutants are listed in Table 1. Spores were produced as previously described (14). First, sporulation was carried out on plates for 10 days in order to obtain more than 95% mature spores. Then, the spores were scraped off the plates, washed 5 times with water, and stored at 4°C in order to kill the last few vegetative cells. The spores were used within 2 months. Before each experiment, two additional washing steps were carried out to remove most of the cell debris, and spore aggregates were disrupted by a sonication step (bath sonicator; 1 min 30 s twice at 42 kHz). All experiments were carried out on at least two independent spore batches. PCR using specific primers (Table 2) was performed on heated spores to verify the presence of the different alleles used in this study. The antibiotic resistance of the germinated spores was also checked for each batch. The E. coli K-12 TG1 strain was used for cloning experiments, and the E. coli ET12567 strain was used to generate unmethylated plasmid DNA prior to B. cereus electroporation. The following antibiotic concentrations were used when necessary: 100 μg/ml ampicillin and 20 μg/ml kanamycin for E. coli and 10 μg/ml tetracycline, 200 μg/ml kanamycin 116, and 10 μg/ml erythromycin for B. cereus.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source |
|---|---|---|
| B. cereus | ||
| ATCC 14579 | Wild-type strain | |
| ATCC 14579 ΔbclA | Kanr; bclA::kan deletion | This study |
| ATCC 14579 ΔexsH | Tetr; exsH::tet deletion | This study |
| ATCC 14579 ΔbclA ΔexsH | Kanr Tetr; bclA::kan and exsH::tet deletions | This study |
| E. coli K-12 | ||
| TG1 | Δ(lac-proAB) supE thi hsdΔ5 (F′ traD36 proA+proB+lacIqlacZΔM15) | |
| ET12567 | F′ dam-13::Tn9 dcm-6 hsdM hsdR recF143 zjj-202::Tn10 glaK2 galT22 ara14 pacY1 xyl-5 leuB6 thi-1 | |
| Plasmids | ||
| pHT304 | Ampr Eryr; shuttle vector for E. coli and B. cereus | 1 |
| pRN5101 | Thermosensitive vector | 20 |
| pDG786 | Source of the Kanr resistance cassette | 17 |
| pHTS2 | Source of the Tetr resistance cassette | 30 |
| pRN5101 ΔbclA ATCC 14579 Kanr | Kanr; plasmid used to delete bclA | This study |
| pRN5101 ΔexsH ATCC 14579 Tetr | Tetr; plasmid used to delete exsH | This study |
| pHT304 bclA ATCC 14579 | Ampr Eryr; plasmids containing the bclA gene and 317 bp upstream and 138 bp downstream; used for complementation | This study |
| pHT304 exsH ATCC 14579 | Ampr Eryr; plasmids containing the exsH gene and 465 bp upstream and 914 bp downstream; used for complementation | This study |
| pYL304 | Ampr Eryr,; plasmids expressing a BclA protein of 189 aa with the CTD deleted; bclA-ΔCT | This study |
| pYL306 | Ampr Eryr; plasmid expressing a BclA protein of 45 aa with the CLR and CTD deleted; bclA-ΔCTCLR | This study |
Kanr, kanamycin resistance; Ampr, ampicillin resistance; Tetr, tetracycline resistance; Eryr, erythromycin resistance.
Table 2.
Primers used in this study
| Primer | Sequence | Purpose |
|---|---|---|
| BclI | 5′-CCCAAGCTTTCATAGCAATCTCCTAAC-3′ | For amplification upstream of bclA |
| Bcll2 | 5′-AACTGCAGTAGATGCAAAACCGAAAGAAAA-3′ | |
| Bcl3 | 5′-AACGAGCTCGTTACAGGACTTGGGCTATCA-3′ | For amplification downstream of bclA |
| Bcl4 | 5′-CGGGATCCAT TGTGGATTCGTACATATC-3′ | |
| exsH1bis | 5′-CGGGATCCTATACATTTCCGATTCT-3′ | For amplification upstream of exsH |
| exsH4 | 5′-GGAATTCACACATGAAGCTTGGACCCCTTTGTATTATAG-3′ | |
| exsH71 | 5′-GCTCTAGAGCTGCAGTTGCTGGATTTGTAAGT-3′ | For amplification downstream of exsH |
| exsH8bis | 5′-CCCAAGCTTTAAAACCCGGGCAAGTTGC-3′ | |
| Bcl5 | 5′-CCGGAATTCTAAACAACGGGCTATTGTCTC-3′ | For amplification of the bclA gene |
| Bcl6 | 5′-CCCAAGCTTTCCATATTTGTGCCTCCTGC-3′ | |
| exsI7 | 5′-GCTCTAGAATAAATGGTTGAATGATAGGAAT-3′ | For amplification of the exsH gene |
| exsH20 | 5′-TAATGACGTAATAACCCCTGCTCT-3′ | |
| exsH19 | 5′-GCTCTTGCACCATTCTTCTTCTC-3′ |
Plasmids and mutant strains.
The bclA and exsH genes of B. cereus ATCC 14579 were disrupted by inserting a kanamycin and a tetracycline resistance cassette, respectively. The thermosensitive plasmid pRN5101 was used to exchange the genes by homologous recombination as described previously (21). Briefly, the upstream and downstream regions of bclA and exsH were amplified using primers listed in Table 2. PCR fragments and a gene-resistant cassette (a Kanr resistance cassette for bclA deletion and a Tetr resistance cassette for exsH deletion) were cloned into the BamHI-HindIII site of pRN5101 (Table 1). Unmethylated plasmids were electroporated into the B. cereus ATCC 14579 strain, and Kanr or Tetr resistant clones were selected for the deletion of bclA and exsH, respectively. Double recombinants were selected by screening for Kanr and Tetr clones sensitive to erythromycin. The double recombinant was verified by PCR. The resulting strains were designated ATCC 14579 ΔbclA and ATCC 14579 ΔexsH, respectively. The double-mutant strain ATCC 14579 ΔbclA ΔexsH was constructed by electroporating the ATCC 14579 ΔbclA strain with pRN5101 ΔexsH ATCC 14579 Tetr. To perform the complementation, a 1.2-kb EcoRI-HindIII fragment containing the bclA gene and its own promoter was amplified by PCR and cloned into the digested EcoRI-HindIII pHT304 vector. pHT304 is a shuttle vector (E. coli-B. cereus) with a low copy number in B. cereus (∼4 copies per equivalent chromosome) (1) (Table 1). The entire exsH gene with its own promoter was amplified in two steps. First, two overlapping fragments were generated using the exsI7-exsH20 and exsH19-exsH8bis primers (Table 2). The two fragments were used as primers to each other in a second PCR in order to generate the exsH gene, which was cloned into the digested XbaI-HindIII pHT304 vector (Table 1).
Characterization of the glycoprotein oligosaccharide moieties. For the determination of the monosaccharide composition of exosporium fractions, spores were washed twice in water, resuspended in water, and subjected to four successive passages through a French press at 20,000 lb/in2. After elimination of spores by centrifugation (3,000 × g; 30 min; 4°C), insoluble fractions of exosporia were pelleted by ultracentrifugation (120,000 × g; 30 min; 4°C). The monosaccharide composition of the exosporium fraction was established by gas chromatography (GC) and GC-mass spectrometry (MS) as alditol-acetate derivatives. Briefly, samples were hydrolyzed in 4 M trifluoroacetic acid (TFA) for 4 h at 100°C and then reduced with sodium borohydride in 0.05 M NaOH for 4 h. Reduction was stopped by dropwise addition of acetic acid until pH 6 was reached, and borate salts were codistilled by repetitive evaporation in dry methanol. Peracetylation was performed in acetic anhydride at 100°C for 2 h. A galactosyl residue (Gal) was always detected in B. cereus exosporium samples, but the large quantitative batch-to-batch variability strongly suggested it originated, at least partly, from ubiquitous agarose contamination, and it was therefore excluded from further glycosyl residue dosages.
Transmission electron microscopy (TEM). Whole spores were adsorbed onto Formvar-coated grids (Formvar 15/95; Euromedex, EMS, Hatfield, PA) and examined after negative staining with 1% uranyl acetate (VWR, Fontenay-sous-Bois, France) on a Hitachi H600 electron microscope at a 75-kV accelerated voltage (12). In order to allow the observation of nanofeatures, ultrathin sections were also observed by TEM after sectioning. Since the external part of exosporia is mainly composed of glycoproteins, a ruthenium red staining procedure previously described (41), and slightly modified in our laboratory, was performed (14). The lengths of filaments were measured using ImageJ software.
MATH.
A kinetic microbial affinity to hydrocarbon (MATH) method previously described (2) was slightly modified. Hexadecane (Sigma) was chosen as an apolar solvent. Briefly, spores were resuspended in 3 ml of NaCl (0.9% [wt/vol]) at an absorbance of 0.6 at 600 nm in glass tubes. Hexadecane (500 μl) was added to the spore suspension, and the glass tubes were vortexed for different times ranging from 5 to 150 s. Then, the glass tubes were left to settle for 30 min to reach complete separation of the two phases. The absorbance of the aqueous phase was measured at 600 nm (At). Log [(At/A0) × 100] was plotted against the vortexing time. The initial slope represents the initial removal rate (R0 [min−1]) of spores from the aqueous suspension and is related to the hydrophilic/hydrophobic spore character.
Measurements of spore electrical properties.
The electrical properties of spores were measured by microelectrophoresis using a Zeta Compact zetameter (CAD Instruments, Les Essarts-le-Roi, France). The electrophoretic motilities were determined using the Helmotz-Smoluchowski equation. Solutions at pH 2.8, 3, 4, 5, 6, 7, 8, and 9 were obtained by combining KOH, KNO3, and HNO3 solutions (1 mM). Spores were suspended in each solution (50 ml) to obtain 40 to 60 spores per reading.
Detachment of spores from stainless steel slides using normal stress.
Spores were suspended (103 to 105 spores/ml) in 300 ml of distilled water (the exact concentration of spores was determined by plating a serial dilution of the suspension on nutrient agar plates). Stainless steel slides (304L; bright annealed; 70 mm by 65 mm) were vertically immersed in the spore suspension for 4 h, allowing spores to adhere. The slides were then rinsed five times in water to eliminate loosely attached spores and dried at 50°C for 45 min. Using an ATL plate applicator, 10 contact agar plates (nutrient agar plus triphenyl tetrazoliumchloride [TTC]; ATL) were successively applied to each stainless steel slide for 10 s at a force of 2,451 Pa (according to ATL's instructions) and lifted off to remove spores. After the 10 contacts, the slide was covered with a solid layer of TTC-agar (2 to 3 mm wide). The contact agar plates and stainless steel slides covered with TTC-agar were incubated at 30°C for 48 h. The colonies formed on each contact agar plate corresponded to the number of spores (Ni) removed at the contact (i). The number of spores remaining on the slide after the 10 contacts (Nr) was determined by the number of colonies developed in the TTC-agar layer covering the stainless steel slides. The equation was plotted. The initial number of adherent spores, N0, was calculated as the sum of the colonies on each contact agar plate plus Nr. The number of contacts required to remove 50% of the spores was calculated in order to compare the adhesion properties of mutant spores. Experiments were repeated at least 3 times with two different batches of spores.
Nucleotide sequence accession number.
The new sequence of the bclA gene of the B. cereus ATCC 14579 strain is available in the NCBI database under accession no. HM071986.1.
RESULTS AND DISCUSSION
Characterization of the exosporium glycoproteins of B. cereus ATCC 14579.
The bclA open reading frame (ORF) (bc1207; GenBank accession number AAPO8192.1) annotated in the B. cereus ATCC 14579 genome of the NCBI database predicts a 128-aa protein with very short CLR and CTD compared to the BclA of B. anthracis. Our cloning and sequencing of the bclA gene of the B. cereus ATCC 14579 strain showed an 888-bp-long ORF. A 295-amino-acid protein (27.7 kDa) was predicted to have an NTD identical to that of B. anthracis Sterne BclA, a CLR containing 41 GXX collagen-like motifs (shorter than in B. anthracis BclA), and a CTD with 89% identity to that of B. anthracis Sterne BclA.
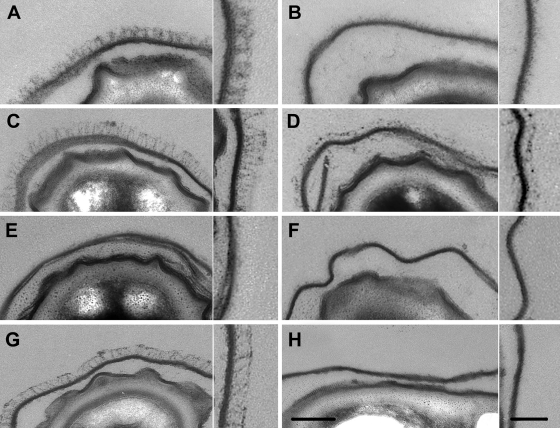
A B. cereus ATCC 14579 strain with the bclA gene deleted was created to characterize the role of BclA in the exosporium integrity and spore surface properties. Ultrathin sections of wild-type spores observed by TEM showed a dense and regular hair-like nap with 36- ± 1-nm-long filaments (Fig. 1A). Conversely, spores with bclA deleted exhibited only a little material, which was unevenly distributed at the exosporium surface (Fig. 1B), but apart from the lack of the well-structured hair-like nap, the exosporium did not seem to be affected by the bclA deletion.
Fig. 1.
Ultrathin sections of B. cereus ATCC 14579 and B. anthracis 9131 spores and the related mutants visualized by transmitted electron microscopy (scale bar = 100 nm). For each strain, the inset is an enlargement of a representative part of the corresponding exosporium (scale bar = 50 nm). (A to F) B. cereus ATCC 14579. (A) Wild type. (B) ΔbclA. (C) ΔbclA pHT304 bclA. (D) ΔexsH. (E) ΔbclA ΔexsH. (F) ΔbclA ΔexsH pHT304 exsH. (G and H) B. anthracis 9131. (G) Wild type. (H) ΔbclA.
The complementation of the bclA mutation restored a hair-like nap similar to that of the wild-type strain (Fig. 1C). The material around the ΔbclA spores was observed whatever the spore batch and could be another glycoprotein component of the nap or fragments of the inner part of the exosporium, which would slough off in the absence of BclA. It could also be supposed that the material comes from the vegetative cell or even the growth medium and adhered to the spore surface due to unusual adhesive properties of the spore surface revealed following bclA deletion. Elsewhere, the presence of such material unevenly distributed at the exosporium surface has not previously been reported on B. anthracis spores with bclA deleted (3, 34). In order to check if the presence of this material was due to the experimental conditions used in this study, we performed similar observations on the avirulent strain B. anthracis 9131 (10), lacking both the pXO1 and pXO2 plasmids, and its mutant with bclA deleted (35), both provided by the Institut Pasteur (Paris, France) (Fig. 1G and H, respectively). Observation by TEM revealed no material around the spores, thus suggesting quite different compositions of the external layers of B. cereus ATCC 14579 and B. anthracis 9131 spores.
The B. cereus ATCC 14579 genome contains other genes encoding BclA-like glycoproteins. One of them, exsH, encodes a protein with a 145-aa NTD containing putative helical secondary structures absent from the 44-aa NTD of BclA. We therefore investigated the role of this protein in the exosporium properties by deleting the exsH ORF. The deletion of exsH did not cause any visible alterations to the exosporium, which remained intact and surrounded with a hair-like nap (Fig. 1D) similar to that observed around the wild-type spores. This observation suggests that ExsH does not play a major role in the exosporium structure and that BclA is the major protein of the hair-like nap, as in B. anthracis (34). In order to verify that BclA did not mask any minor modifications to the exosporium structure following exsH deletion, the ATCC 14579 ΔbclA ΔexsH double mutant was created. The exsH gene under the control of its own promoter was expressed on a multicopy plasmid in the double mutant. Its expression was confirmed by analysis of the glycosyl moieties of the different mutants. Some material was still observed by TEM at the spore surface of the double mutant (Fig. 1E) and of the double mutant complemented with exsH (Fig. 1F). There was no significant difference in the material at the exosporium surfaces of the three mutants with bclA deleted. This result indicates that the material was not made of ExsH but did not permit us to draw definitive conclusions about the origin of the material (spore, vegetative cell, or growth medium).
Lastly, the presence of the long appendages, about 10 nm wide (12, 13), was not affected by either bclA or exsH deletion (data not shown).
Identification of the monosaccharide composition of BclA and ExsH glycoproteins.
As shown recently (12), along with Rha, GalNH2, and GlcNH2, the major rhamnose derivatives found in the exosporium of B. cereus ATCC 14579 were 2-Me-Rha, 3-Me-Rha, and 2,4-diMe-Rha residues. Rha, GalNH2, GlcNH2, and 3-Me-Rha have been previously identified in B. anthracis (8), while 2-Me-Rha and 2,4-diMe-Rha have been described in a few B. cereus and B. thuringiensis strains (12). The method used here to analyze monosaccharide composition did not permit us to discriminate NH2 from N-acetyl groups and, thus, GalNH2-GlcNH2 from GalNAc-GlcNAc. The identification of GalNAc, rather than a GalNH2 residue, in B. anthracis spores strongly suggested that the GalNH2 and GlcNH2 residues identified in B. cereus strains also derived from N-acetyl-galactosamine and N-acetyl-glucosamine (8). The monosaccharide compositions of bclA and exsH mutants were analyzed to investigate their relative contributions to the global glycosylation of the B. cereus exosporium. The quantities of all Rha derivatives and GalNH2 and GlcNH2 residues were below the detection level in exosporia of strains with bclA alone deleted and with both bclA and exsH genes deleted (Fig. 2). In comparison, the deletion of exsH had no significant influence on the overall monosaccharide content of the exosporium. Complementation of the bclA deletion restored a profile similar to that of the wild type. These results established that the exosporium glycosylation was mainly BclA borne, irrespective of the monosaccharide residue considered. In order to identify the monosaccharide composition of ExsH, otherwise masked by the dominant glycosylation moieties associated with BclA, the exsH gene was expressed on a multicopy plasmid in the B. cereus ATCC 14579 ΔbclA ΔexsH double mutant. GalNH2, GlcNH2, Rha, and 3-Me-Rha residues were detected, but 2-Me-Rha and 2,4-Me-Rha residues were not (Fig. 3). These results suggested that the ExsH protein was indeed glycosylated and exhibited a glycosylation pattern different from that of B. cereus ATCC 14579 BclA but similar to that of B. anthracis BclA (8). This result also confirmed that exsH was expressed from the multicopy plasmid in the bcla exsH mutant background but must be a minor protein in the exosporium, not observed by TEM. It is noteworthy that the Rha derivatives in B. anthracis are involved in specific recognition of the macrophage receptor CD14 (5, 24). The presence of several surface glycoproteins differently glycosylated on the B. cereus ATCC 14579 exosporium suggests specific recognition of different cell receptors. In particular, the specific glycosyl residues on BclA of B. cereus ATCC 14579 might mediate the recognition of host cell receptors different from the ones recognized by B. anthracis.
Fig. 2.
Monosaccharide contents of exosporium fractions. The main monosaccharides in the B. cereus exosporium were identified and quantified as alditol-acetates in gas chromatography from the wild-type B. cereus ATCC 14579 and the related mutants with the bclA gene and the exsH gene deleted. The ΔbclA and the double ΔbclA ΔexsH mutants were complemented with plasmids pHT304 bclA ATCC 14579 and pTH304 exsH ATCC 14579, respectively.
Fig. 3.
Comparison of properties of spore adhesion to stainless steel. The numbers of contacts required to remove 50% of spores from stainless steel slides (304L, bright annealed) are represented by bars. One-way analysis of variance (ANOVA) was performed using Bonferroni's multiple-comparison test. The five-pointed asterisk represents a significant difference between a mutant and the ATCC 14579 wild type, the six-pointed asterisk represents a significant difference between a mutant and ATCC 14579 ΔexsH, and the eight-pointed asterisk represents a significant difference between a mutant and ATCC 14579 ΔbclA pHT304 bclA. One symbol represents a P value of <0.05 and two symbols a P value of <0.01. The error bars indicate standard deviations.
Influence of BclA and ExsH glycoproteins on both hydrophobic and electrical properties of the whole spores.
Due to their external localization, BclA and ExsH proteins are good candidates for modulating spore surface properties. Thus, the hydrophobic character of bclA and exsH mutant spores was estimated from the initial removal rate (R0) measured with the MATH assay (2, 23). The R0 of wild-type B. cereus ATCC 14579 spores was measured at −4.39 ± 0.16 s−1, indicating that wild-type spores were mostly hydrophobic. The R0 of ΔbclA spores was even more negative than that of the wild type, revealing an increase in the spore hydrophobicity (Table 3). The complementation of the bclA mutation only partially restored the initial hydrophobicity, and the difference could not be attributed to the presence of the pHT304 vector. Indeed, the insertion of the empty pHT304 vector in the wild-type strain did not modify spore hydrophobicity (data not shown). Lastly, the deletion of exsH in the wild type or in the bclA mutant did not cause any significant change in spore hydrophobicity compared to the parental strains (Table 3).
Table 3.
Physicochemical properties of sporesa
| Strain | Hydrophobicity |
IEP |
||||
|---|---|---|---|---|---|---|
| R0 (min−1) | SD | P | Value | SD | P | |
| ATCC 14579 | −4.39 | 0.16 | 3.05 | 0.05 | ||
| ATCC 14579 ΔbclA | −6.33 | 0.43 | <0.001 | 3.35 | 0.05 | <0.01 |
| ATCC 14579 ΔexsH | −4.47 | 0.28 | ND | 3.06 | 0.08 | ND |
| ATCC 14579 ΔbclA ΔexsH | −6.32 | 0.08 | <0.001 | 3.56 | 0.05 | <0.001 |
| ATCC 14579 ΔbclA pHT304 bclA | −5.16 | 0.14 | ND | 3.10 | 0.06 | ND |
| ATCC 14579 ΔbclA pHT304 ΔCT-bclA | −7.01 | 0.18 | <0.001 | 3.42 | 0.04 | <0.05 |
| ATCC 14579 ΔbclA pHT304 ΔCT-CLR-bclA | −6.40 | 0.14 | <0.05 | 3.62 | 0.07 | <0.001 |
The hydrophobicity was estimated by least-square fitting, yielding a negative initial removal rate (R0 [min−1]). Statistical analyses comparing the wild type to each mutant were performed using Bonferroni's multiple-comparison test. ND, not statistically different from the control. SD, standard deviation.
Despite the increased hydrophobicity of the whole spores resulting from the bclA deletion, the GRAVY score (0.100) calculated from the BclA sequence predicted a hydrophobic character (20). This result suggested that the overall hydrophobicity of B. cereus spores is driven by underlying hydrophobic proteins and/or lipids (2) rather than by the external BclA. The opposite results have been reported for B. anthracis spore hydrophobicity following bclA deletion (7) in spite of the similar methods used in the two studies to estimate the spore hydrophobicity (affinity to hexadecane and brief contact). One may hypothesize that the underlying layers of the two species are quite different, at least in their hydrophobic characters.
The influence of bclA and exsH mutations on the electrical properties of spores was measured using a zetameter. The isoelectric point (IEP) was used to compare strains. The IEP of spores statistically increased when the bclA gene was deleted (Table 3). The complementation of the bclA mutation restored the IEP, similar to the wild type. In comparison, the deletion of exsH in the wild type or in the bclA mutant did not significantly change the IEP compared to their parental strains. These results showed that BclA, but not ExsH, affected the overall charge of B. cereus spores. The variation in the BclA sequence and glycan moieties observed between species of the B. cereus group may influence the overall charge of spores.
Influences of BclA and ExsH glycoproteins on adhesion properties of B. cereus ATCC 14579 spores to stainless steel.
We then investigated the influence of bclA and exsH deletions on the properties of B. cereus ATCC 14579 spore adhesion to stainless steel slides. The results showed that the absence of BclA in the hair-like nap significantly reduced (P < 0.05) adherent spores' resistance to detachment (Fig. 3). The complementation of the bclA mutation restored the adhesion properties to a level similar to that of the wild-type spores. Therefore, BclA increased the interaction force between spores and stainless steel. The deletion of the exsH gene did not cause any significant changes in the adhesion properties of spores compared to the parental strains.
We then investigated if the reduced resistance to detachment could be attributed to differences in spore surface physicochemistry (increased hydrophobicity and IEP). Numerous works have reported the role of hydrophobicity in spore interaction with materials. However, although it is generally admitted that spore hydrophobicity promotes spore adhesion to inert hydrophobic surfaces (11, 12, 29, 32), published studies failed to demonstrate any influence of spore hydrophobicity on their resistance to detachment (37). Furthermore, recent results obtained in our laboratory on 16 Bacillus strains from 7 Bacillus species, characterized by different surface hydrophobicities, showed that the mechanical detachment of adherent spores was not affected by their surface hydrophobicity (data not shown). We then investigated the role of electrostatic charge on the removal of adherent spores by comparing spore behavior at pH 7.0 and at pH 3.0. Indeed, spores of B. cereus ATCC 14579 and its mutants are negatively charged at pH 7.0, and their IEP is close to pH 3.0. As most materials are also negatively charged at neutral pH, the electrostatic repulsion occurring at pH 7.0 would limit spore adhesion. Indeed, previous studies have shown that a higher number of Bacillus spores adhere to stainless steel at pH 3.0 than at pH 7.0 (19, 31). Conversely, we showed that the resistance to detachment of adherent spores of both strains (the wild type and the ΔbclA strain) increased by only about 1.25-fold at pH 3.0 compared to pH 7.0. This result suggested that the spore charge poorly affected spore resistance to detachment. Therefore, the BclA-borne charges exhibit a marginal influence on the electrostatic repulsion of spores by stainless steel.
In conclusion, the study showed that BclA was the major glycoprotein of the exosporium hair-like nap of B. cereus strain ATCC 14579. The removal of BclA affected both the overall hydrophobicity and charge of spores, as well as their adhesion properties, but it did not affect exosporium integrity. The presence of BclA filaments of the hair-like nap plays a major role in spore interaction with materials, contrary to overall spore physicochemistry, probably by providing a larger contact surface with stainless steel. The monosaccharide composition of glycoproteins in the exosporium of B. cereus ATCC 14579 was different from that of the B. anthracis exosporium. Variations in the glycan moieties inside the B. cereus group may be responsible for different specificities of binding to host cells. The identification of the critical domains and amino acids of BclA involved in spore adhesion would help in the development of new compounds and processes to remove B. cereus spores from surfaces and host cells. Minor glycoproteins, such as BclB in B. anthracis and ExsH in B. cereus, as well as the multitude of putative glycoprotein genes present in the B. cereus group genomes (22, 40), could be involved in the differentiation of ligands at the surfaces of host cells. Conversely, we showed in this study that the ExsH protein is not involved in exosporium integrity, in the overall surface properties of spores, or in their adhesion properties.
ACKNOWLEDGMENTS
We thank Martine Clarisse, Etienne Dewailly, Véronique Lebret, Sylviane Parent, and Annette Ronse for their valuable assistance.
This work has been financed by the Agence Nationale de la Recherche under the Programme National de Recherche en Alimentation et Nutrition Humaine, project ANR-05-PNRA-013, B. cereus, and project ANR-07-PNRA-009, InterSpore.
Footnotes
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Arantes O., Lereclus D. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115–119 [DOI] [PubMed] [Google Scholar]
- 2. Bos R., Busscher H. J. 1999. Role of acid-base interactions on the adhesion of oral streptococci and actinomyces to hexadecane and chloroform. Influence of divalent cations and comparison between free energies of partitioning and free energies obtained by extended DLVO analysis. Coll. Surf. B Biointerfaces 14:169–177 [Google Scholar]
- 3. Boydston J. A., Chen P., Steichen C. T., Turnbough C. L. 2005. Orientation within the exosporium and structural stability of the collagen-like glycoprotein BclA of Bacillus anthracis. J. Bacteriol. 187:5310–5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bozue J., et al. 2007. Bacillus anthracis spores of the bclA mutant exhibit increased adherence to epithelial cells, fibroblasts, and endothelial cells but not to macrophages. Infect. Immun. 75:4498–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bozue J. A., et al. 2005. Construction of a rhamnose mutation in Bacillus anthracis affects adherence to macrophages but not virulence in guinea pigs. Microb. Pathog. 38:1–12 [DOI] [PubMed] [Google Scholar]
- 6. Brahmbhatt T. N., et al. 2007. Bacillus anthracis exosporium protein Bc1A affects spore germination, interaction with extracellular matrix proteins, and hydrophobicity. Infect. Immun. 75:5233–5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen G., Driks A., Tawfiq K., Mallozzi M., Patil S. 2010. Bacillus anthracis and Bacillus subtilis spore surface properties and transport. Coll. Surf. B Biointerfaces 76:512–518 [DOI] [PubMed] [Google Scholar]
- 8. Daubenspeck J. M., et al. 2004. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J. Biol. Chem. 279:30945–30953 [DOI] [PubMed] [Google Scholar]
- 9. Dong S. L., et al. 2008. Anthrose biosynthetic operon of Bacillus anthracis. J. Bacteriol. 190:2350–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etiennetoumelin I., Sirard J. C., Duflot E., Mock M., Fouet A. 1995. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J. Bacteriol. 177:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faille C., et al. 2002. Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can. J. Microbiol. 48:728–738 [DOI] [PubMed] [Google Scholar]
- 12. Faille C., et al. 2010. Morphology and physico-chemical properties of Bacillus spores surrounded or not with an exosporium: consequences on their ability to adhere to stainless steel. Int. J. Food Microbiol. 143:125–135 [DOI] [PubMed] [Google Scholar]
- 13. Faille C., et al. 2010. Viability and surface properties of spores subjected to a cleaning-in-place procedure. Consequences on their ability to contaminate surfaces of equipment. Food Microbiol. 27:769–776 [DOI] [PubMed] [Google Scholar]
- 14. Faille C., Tauveron G., Le Gentil-Lelièvre C., Slomianny C. 2007. Occurrence of Bacillus cereus spores with a damaged exosporium: Consequences on the spore adhesion on surfaces of food processing lines. J. Food Prot. 70:2346–2353 [DOI] [PubMed] [Google Scholar]
- 15. Fox A., et al. 2003. Carbohydrates and glycoproteins of Bacillus anthracis and related bacilli: targets for biodetection. J. Microbiol. Methods 54:143–152 [DOI] [PubMed] [Google Scholar]
- 16. Giorno R., et al. 2007. Morphogenesis of the Bacillus anthracis spore. J. Bacteriol. 189:691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerout-Fleury A. M., Shazand K., Frandsen N., Stragier P. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336 [DOI] [PubMed] [Google Scholar]
- 18. Henriques A. O., Moran C. P. 2007. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 61:555–588 [DOI] [PubMed] [Google Scholar]
- 19. Hüsmark U., Rönner U. 1992. The influence of hydrophobic, electrostatic and morphologic properties on the adhesion of Bacillus spores. Biofouling 5:335–344 [Google Scholar]
- 20. Kyte J., Doolittle R. F. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132 [DOI] [PubMed] [Google Scholar]
- 21. Lereclus D., Vallade M., Chaufaux J., Arantes O., Rambaud S. 1992. Expansion of insecticidal host range of Bacillus thuringiensis by in vivo genetic recombination. Biotechnology 10:418–421 [DOI] [PubMed] [Google Scholar]
- 22. Leski T. A., et al. 2009. Identification and classification of bcl genes and proteins of Bacillus cereus group organisms and their application in Bacillus anthracis detection and fingerprinting. Appl. Environ. Microbiol. 75:7163–7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lichtenberg D., Rosenberg M., Sharfman N., Ofek I. 1985. A kinetic approach to bacterial adherence to hydrocarbon. J. Microbiol. Methods 4:141–146 [Google Scholar]
- 24. Oliva C., Turnbough C. L., Kearney J. F. 2009. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc. Natl. Acad. Sci. U. S. A. 106:13957–13962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliva C. R., et al. 2008. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. Proc. Natl. Acad. Sci. U. S. A. 105:1261–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajkovic A., Uyttendaele M., Courtens T., Heyndrickx M., Debevere J. 2006. Prevalence and characterisation of Bacillus cereus in vacuum packed potato puree. Int. J. Food Sci. Technol. 41:878–884 [Google Scholar]
- 27. Ramarao N., Lereclus D. 2005. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell Microbiol. 7:1357–1364 [DOI] [PubMed] [Google Scholar]
- 28. Rety S., et al. 2005. The crystal structure of the Bacillus anthracis spore surface protein BclA shows remarkable similarity to mammalian proteins. J. Biol. Chem. 280:43073–43078 [DOI] [PubMed] [Google Scholar]
- 29. Rönner U., Hüsmark U., Henriksson A. 1990. Adhesion of Bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol. 69:550–556 [DOI] [PubMed] [Google Scholar]
- 30. Sanchis V., Agaisse H., Chaufaux J., Lereclus D. 1996. Construction of new insecticidal Bacillus thuringiensis recombinant strains by using the sporulation non-dependent expression system of cryIIIA and a site specific recombination vector. J. Biotechnol. 48:81–96 [DOI] [PubMed] [Google Scholar]
- 31. Seale R. B., Bremer P. J., Flint S. H., McQuillan A. J. 2010. Characterization of spore surfaces from a Geobacillus sp. isolate by pH dependence of surface charge and infrared spectra. J. Appl. Microbiol. 109:1339–1348 [DOI] [PubMed] [Google Scholar]
- 32. Simmonds P., Mossel B. L., Intaraphan T., Deeth H. C. 2003. Heat resistance of Bacillus spores when adhered to stainless steel and its relationship to spore hydrophobicity. J. Food Prot. 66:2070–2075 [DOI] [PubMed] [Google Scholar]
- 33. Svensson B., et al. 2006. Occurrence of emetic toxin producing Bacillus cereus in the dairy production chain. Int. Dairy J. 16:740–749 [Google Scholar]
- 34. Sylvestre P., Couture-Tosi E., Mock M. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169–178 [DOI] [PubMed] [Google Scholar]
- 35. Sylvestre P., Couture-Tosi E., Mock M. 2003. Polymorphism in the collagen-like region of the Bacillus anthracis BclA protein leads to variation in exosporium filament length. J. Bacteriol. 185:1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamborrini M., et al. 2009. Immuno-detection of anthrose containing tetrasaccharide in the exosporium of Bacillus anthracis and Bacillus cereus strains. J. Appl. Microbiol. 106:1618–1628 [DOI] [PubMed] [Google Scholar]
- 37. Tauveron G., Slomianny C., Henry C., Faille C. 2006. Variability among Bacillus cereus strains in the spore surface properties and influence on their ability to contaminate food surface equipment. Int. J. Food Microbiol. 110:254–262 [DOI] [PubMed] [Google Scholar]
- 38. Thompson B. M., Stewart G. C. 2008. Targeting of the BclA and BclB proteins to the Bacillus anthracis spore surface. Mol. Microbiol. 70:421–434 [DOI] [PubMed] [Google Scholar]
- 39. Thompson B. M., Waller L. N., Fox K. F., Fox A., Stewart G. C. 2007. The BclB glycoprotein of Bacillus anthracis is involved in exosporium integrity. J. Bacteriol. 189:6704–6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Todd S. J., Moir A. J., Johnson M. J., Moir A. 2003. Genes of Bacillus cereus and Bacillus anthracis encoding proteins of the exosporium. J. Bacteriol. 185:3373–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waller L. N., Fox N., Fox K. F., Fox A., Price R. L. 2004. Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore of Bacillus anthracis and Bacillus subtilis. J. Microbiol. Methods 58:23–30 [DOI] [PubMed] [Google Scholar]
- 42. Waller L. N., et al. 2005. Identification of a second collagen-like glycoprotein produced by Bacillus anthracis and demonstration of associated spore-specific sugars. J. Bacteriol. 187:4592–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]