Abstract
The persistence of 3 low-pathogenicity avian influenza viruses (LPAIV) (H4N6, H5N1, and H6N8) and one human influenza virus (H1N1) as well as Newcastle disease virus (NDV) and enteric cytopathogenic bovine orphan (ECBO) virus was investigated in lake sediment, duck feces, and duck meat at 30, 20, 10, and 0°C using a germ carrier technique. Virus-loaded germ carriers were incubated in each substrate, and residual infectivity of the eluted virus was quantified on cell culture after regular intervals for a maximum of 24 weeks. Data were analyzed by a linear regression model to calculate T90 values (time required for 90% loss of virus infectivity) and estimated persistence of the viruses. In general, the persistence of all of the viruses was highest in lake sediment, followed by feces, and was the lowest in duck meat at all temperatures. For the avian influenza virus subtypes, T90 values in sediment ranged from 5 to 11, 13 to 18, 43 to 54, and 66 to 394 days at 30, 20, 10, and 0°C, respectively, which were 2 to 5 times higher than the T90 values of the viruses in the feces and meat. Although the individual viruses vary in tenacity, the survival time of influenza viruses was shorter than that of NDV and ECBO virus in all substrates. The results of this study suggest that lake sediment may act as a long-term source of influenza viruses in the aquatic habitat, while the viruses may remain infectious for extended periods of time in duck feces and meat at low temperatures, allowing persistence of the viruses in the environment over winter.
INTRODUCTION
Wild aquatic birds of the genera Anseriformes and Charadriiformes are primary reservoirs of the avian influenza viruses (AIV) and play an important role in the epidemiology of these viruses (32). AIV replicate primarily in the respiratory and gastrointestinal tracts of the birds and are excreted from the nares, mouth, conjunctiva, and cloaca of infected birds (7, 35). Experimentally infected Muscovy ducks are known to shed larger amounts of virus via feces than via nasal secretions for a period of 6 to 7 days (43). However, virus shedding through the cloaca can be prolonged for a period of 28 days (15). Alternatively, for high-pathogenicity avian influenza viruses (HPAIV), higher viral titers were recorded in oropharyngeal swabs than in cloacal swabs (4, 34). These infectious excretions lead to heavy contamination of the animate and inanimate environment. In addition to direct contact with infected birds, the contaminated environment plays a vital role in the indirect transmission of AIV to susceptible birds (32, 35). Likewise, large amounts of the viruses can be detected in the meat and internal organs of birds infected with HPAIV (36, 38). Thus, consumption of carcasses by predation and cannibalism is another source of virus transmission to susceptible birds and other animals (35).
Waterborne transmission of AIV is well established (14, 21), but information on survival of these viruses in aquatic habitats is not sufficient to completely understand the epidemiology of these viruses (33). Lake sediments can harbor influenza viruses in the environment (19), but no information is available on the persistence of AIV in this substrate. Due to the large amount of AIV excreted through avian feces, viral persistence in this medium is of great concern for the spread of this disease (7, 44), but little is known in this regard. One report showed that infectious virus was not detectable after 13 days at 22°C in infected duck feces, while no drop in virus titer was recorded over a period of 2 weeks at 0°C (43). Another report showed that the virus was inactivated within 24 h at 25°C in chicken manure (8). The available studies are inconclusive to determine the precise length of time for which the AIV may remain infectious in fecal waste. Moreover, there is scarcity of data on the survival of these viruses in duck meat. The available information on the tenacity of AIV in various substrates is based mostly on experiments that were conducted for short periods of time and for which the temperature range selected was limited. The present study was therefore designed to assess the survival of three low-pathogenicity AIV (LPAIV) (H4N6, H5N1, and H6N8), one human influenza virus (H1N1), and two noninfluenza viruses (Newcastle disease virus [NDV] and enteric cytopathogenic bovine orphan [ECBO] virus) in lake sediment, duck feces, and duck meat at a wide range of temperatures for extended periods of time.
MATERIALS AND METHODS
Viruses and cells.
Viruses used in this study include three LPAIV (H4N6 A/Mallard/Wv1732-34/03, H5N1 A/Teal/Wv632/Germany/05, and H6N8 A/Mute Swan/Germany/R2927/07), obtained from Timm Harder, Friedrich-Loeffler- Institut, Insel Riems, Germany; one human influenza virus (H1N1 A/Puerto Rico/8/1934), provided by the Institute of Virology, JLU Giessen, Germany; and two additional viruses, Newcastle disease virus (NDV) Lasota and enteric cytopathogenic bovine orphan (ECBO) virus LCR-4, procured from the Institute of Environmental and Animal Hygiene, University of Hohenheim, Stuttgart, Germany. The two additional viruses (NDV and ECBO virus) were incorporated in this study to serve as representative enveloped and nonenveloped viruses and for direct comparison with influenza viruses, as these viruses serve as test organisms in viral tenacity studies and to measure the virucidal activity of disinfectants (10). All of the influenza viruses and NDV were propagated in 9- to 11-day-old specific-pathogen-free (SPF) chicken embryos (Lohmann, Cuxhaven, Germany) via allantoic sac route inoculation (27), while ECBO virus was propagated in Madin-Darby bovine kidney (MDBK) cells. The virus stocks were kept frozen at −80°C until use. Madin-Darby canine kidney (MDCK) cells, Vero cells, and MDBK cells were used for the titration of influenza viruses, NDV, and ECBO virus, respectively.
Germ carrier technique.
Before the tenacity studies in various substrates were begun, virus recovery from a virus-loaded substrate was assessed. These spiking trials were performed using lake sediment as a substrate. During several repeats of the experiments, it was noticed that the virus titer dropped by 3 log10 immediately after addition of the virus to the sediment (data not shown). Ten ml of amnioallantoic fluid (AAF) with the AIV H5N1 (with a virus titer of 106.25 50% tissue culture infective doses [TCID50]/ml) was mixed with 10 g of lake sediment and allowed to stand for 15 min. The supernatant collected afterwards had a virus titer of 103.0 TCID50/ml, indicating a loss of more than 99.9% of the virus titer. This phenomenon necessitated the use of an appropriate germ carrier for the tenacity studies.
The germ carriers were prepared according to a previously described method (24). In short, the positively electrocharged Zeta Plus Virosorb 1MDS filters (Cuno, Meriden, CT) were cut to circular pieces of 15-mm diameter and sterilized by autoclaving. To facilitate the adsorption of virus onto the filter discs, the virus stocks were mixed in phosphate loading buffer adjusted to pH 7.40 for influenza viruses and NDV (24) or pH 6.00 for ECBO virus (40) at a ratio of 1:10. The filter discs were placed in a sterile plastic filter holding device (Sartorius Stedium Biotech GmbH, Gottingen, Germany), and 5 ml of the virus suspension was filtered through each disc. The filter disc was then placed in a polycarbonate membrane (PCM) with a pore size of 10 nm (Pieper Filter GmbH, Bad Zwischenahn, Germany) and sealed from all sides. Several such germ carriers were prepared for each virus and always kept moist until use.
Virus persistence in lake sediment.
Freshly collected lake sediment from Lake Constance was procured from the Institut für Seenforschung, Langenargen, Germany, shortly before the beginning of each trial and stored in the laboratory at 4°C until use. The sediment was collected from the Gnadensee (latitude 47.72, longitude 9.05) part of Lake Constance partly beside the shore and partly about 1 kilometer away from the coast. A sediment sampler was used to collect the sediment from the upper 10 cm of the lake floor. The sediment was dense and pasty in consistency with a pH of 7.9 ± 0.5. About 30 to 40 ml of sediment was placed in sterile 50-ml plastic tubes, and three germ carriers were placed in each tube so that they were each surrounded by sediment. The tubes were closed to retain moisture and transferred to incubators previously adjusted to temperatures of 30, 20, 10, and 0°C. Titration of virus samples was performed at regular intervals: every 2 days at 30°C for 30 days, every 4 days at 20°C for 60 days, weekly at 10°C for 14 weeks, and every 2 weeks at 0°C for 6 months.
Virus persistence in duck feces.
Initially, the duck feces were collected from a free-range duck farm situated in Schwabisch Hall, Germany, and used for tenacity trials with H5N1 virus. Due to snowfall in the winter season, the collection of feces from that farm became impossible. Duck feces were therefore collected from a duck farm situated at Sachsenheim, Germany, for later trials with the other viruses. These ducks were kept indoors in the winter, so the feces was slightly mixed with straw and feed residues. About 30 to 40 g of duck feces was placed into each of several sterile 50-ml plastic tubes, and three germ carriers were placed in each tube. For close contact between feces and germ carrier-adsorbed virus, the germ carriers were placed separately and deeply embedded in the feces. The tubes were closed and then transferred to incubators previously adjusted to temperatures of 30, 20, 10, and 0°C. Virus titrations from the samples were performed at regular intervals: daily for influenza viruses and every 2 days for NDV and ECBO virus at 30°C for 14 days, every 2 days for influenza viruses and every 4 days for NDV and ECBO virus at 20°C for 28 days, weekly for influenza viruses and every 2 weeks for NDV and ECBO virus at 10°C for 12 weeks, and every 2 weeks for influenza viruses and monthly for NDV and ECBO virus at 0°C for 6 months.
Virus persistence in duck meat.
Duck meat was purchased as frozen whole ducks from a supermarket. The carcasses were incised to separate the breast meat, which was used for the tenacity studies. About 20 to 30 g of duck breast meat was placed in each of several sterile 50-ml plastic tubes, and three germ carriers were placed in each tube. The meat was sliced to make small pockets for the germ carriers, and each carrier was placed in a separate pocket for close contact of the filter-adsorbed virus with the meat contents. The tubes were then closed and transferred to incubators previously adjusted to temperatures of 30, 20, 10, and 0°C. Virus titrations from the samples were performed at regular intervals as described above for duck feces.
Virus elution.
Triplicate samples were tested at each time point. Sandwich germ carriers were removed from the sediment, feces, and meat samples kept in each tube. After removal from the substrate, the germ carriers were placed in a petri dish, and the outer surface of the PCM was washed with sterile distilled water and wiped with tissue paper. Then, after the PCM was torn open, the filter discs were removed with sterile forceps and placed immediately in 2 ml of elution medium (2% beef extract and 0.5 M NaCl) with a pH 7.00 for influenza viruses and NDV and with a pH 8.5 for ECBO virus, as described previously (24). Elution media with germ carriers were subjected to ultrasonication in an ice bath (40 kHz; Bandelin Electronics, Berlin, Germany) for 5 min, centrifuged at 2,000 × g for 15 min, and processed for virus titration on respective cells to measure the residual viral infectivity of the eluted virus.
Virus titration.
Virus titrations were performed by endpoint serial dilution in 96-well microtitration plates as described earlier (24). After trypsinization, the cells were transferred to the plates using growth medium (Dulbecco's modified Eagle medium [DMEM] supplemented with 5% fetal calf serum [FCS] and 1% nonessential amino acids [NEA]; all from Biochrom, Berlin, Germany) to achieve 90% confluence and used the next day for virus titration. Eluted virus suspension was serially 10-fold diluted from 101 to 108 in maintenance medium (DMEM supplemented with 2% FCS, 1% NEA, and the antimicrobial agents gentamicin sulfate [6.4 U], penicillin G [200 U], streptomycin sulfate [380 U], and amphotericin B [1 g/ml]; all from Biochrom, Berlin, Germany). Growth medium was discarded from all wells of the plates. Virus (0.1 ml, diluted from 101 to 108) was added to the wells with the cultured cells (4 wells per dilution step). For undiluted eluted virus, the wells were inoculated with 0.1 ml of the virus suspension and incubated at 37°C for 1 h; virus suspension was then removed and replaced by 0.1 ml maintenance medium, while cell control wells received 0.1 ml of maintenance medium. Growth and maintenance media for Vero cells were used without NEA. Plates were incubated for 7 days at 37°C and 5% CO2 and checked afterwards for cytopathic effects by light microscopy. A complete destruction of the cell monolayer was regarded as positive for virus growth. For influenza viruses, virus replication was confirmed by hemagglutination (HA) test using 1% chicken erythrocytes (Lohmann, Cuxhaven, Germany), performed independently for each plate by transferring cell culture supernatant into separate U-bottom plates as described previously (25). TCID50 values were calculated by the Spearman-Kärber formula (42). The minimum detectable limit of the assay was 101.75 TCID50/ml.
Statistical analysis.
In all of the substrates, triplicate germ carriers were tested each time to calculate infectivity titers as log10 TCID50. For the calculation of their averages, the logarithmic values were converted to arithmetic numbers, and the mean of the arithmetic numbers was changed once again to logarithmic values. The serial data thus obtained were analyzed by a linear regression model using Microsoft Excel (Microsoft Office Excel 2007; Microsoft Corporation, Redmond, WA). T90 values (time required for 90% loss of virus infectivity) and estimated persistence of viral infectivity with a starting viral concentration of 106.00 TCID50/ml were calculated using this model.
RESULTS
Persistence of the influenza viruses, NDV, and ECBO virus in various substrates.
The linear regression models showing the persistence of influenza and noninfluenza viruses in the lake sediment, duck feces, and duck meat at various temperatures are presented in the supplemental material.
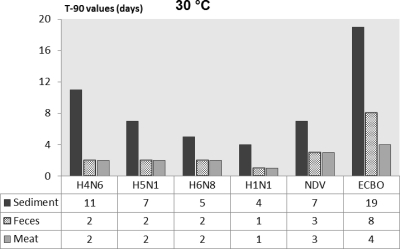
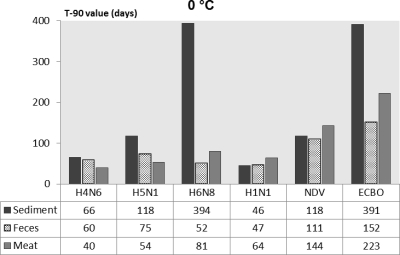
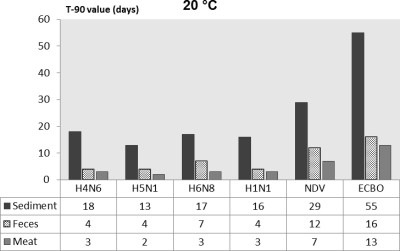
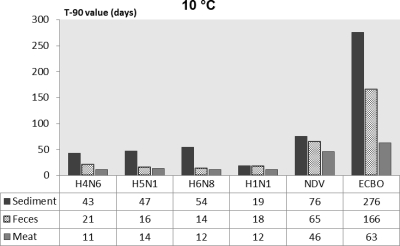
For a better comparison of persistence of influenza and noninfluenza viruses in various substrates, the T90 values calculated by the linear regression models are presented in Fig. 1 through 4. The T90 values show that in all of the substrates the persistence of the influenza viruses was highest at 0°C followed by 10, 20, and 30°C. The T90 values of individual influenza viruses vary in different substrates, but generally the viruses survived the longest in sediment, followed by duck feces, and survived for the shortest time in duck meat. The human influenza virus had slightly lower T90 values than the AIV. Both of the noninfluenza viruses had higher T90 values than the influenza viruses, and of these viruses, ECBO virus had the highest T90 values in all of the substrates at all temperatures.
Fig. 1.
Comparison of T90 values of influenza viruses, NDV, and ECBO virus in lake sediment, duck feces, and duck meat at 30°C.
Fig. 4.
Comparison of T90 values of influenza viruses, NDV, and ECBO virus in lake sediment, duck feces, and duck meat at 0°C.
Fig. 2.
Comparison of T90 values of influenza viruses, NDV, and ECBO virus in lake sediment, duck feces, and duck meat at 20°C.
Fig. 3.
Comparison of T90 values of influenza viruses, NDV, and ECBO virus in lake sediment, duck feces, and duck meat at 10°C.
DISCUSSION
Based on previous studies (3, 15, 19, 21, 26, 33), there is increasing evidence that AIV transmission within waterfowl populations is highly dependent on environmental persistence. Estimation of viral persistence in a variety of biological media and substrates is therefore of great epidemiological importance. The results of the present study indicate that in experimentally inoculated lake sediments, duck feces, and duck meat, the infectivity of various AIV subtypes can be preserved for extended periods of time at low temperatures (see the supplemental material). Of the substrates tested, viral persistence was highest in the lake sediments. Considerable differences exist in the stability and inactivation rates of viruses in biological waste not only among viruses of different families and genera but also among viruses of the same family or genus and even among similar types of viruses (29). These differences were also confirmed by the results of the present study, where virus persistence in various substrates was variable not only between the influenza and noninfluenza viruses but also between the various influenza virus subtypes tested. Similar variation in persistence between influenza virus subtypes and strains has been reported previously (5, 6, 31, 32).
The T90 values calculated for the AIV in lake sediment were much higher than those reported for the same viruses in lake water from Lake Constance (2 days at 30°C, 3 to 4 days at 20°C, 10 to 14 days at 10°C, and 31 to 35 days at 0°C) (23). In the previous study, the viruses were either suspended directly in the lake water or inoculated onto germ carriers as performed here (23, 24). This shows that AIV can survive longer in lake sediments than in lake water, which is in accordance with previous findings, where larger amounts of enteric viruses were detected in polluted estuarine sediments than in the overlying seawater (12, 17). AIV are excreted in large amounts in the feces of infected birds (43). Most probably, the fecal material passed by infected wild birds near the banks of bodies of water does not completely dissolve in the water but rather is deposited at the bottom, preserving the virus in the sediment. There is a possibility that ducks could ingest these infected materials when searching for food at the bottom of contaminated lakes. Thus, the estimation of viral persistence in lake sediment can provide valuable information for understanding the epidemiology of AIV in the aquatic habitat. Although the importance of lake sediment as a long-term environmental source of influenza viruses has been discussed before (19, 32), no experimental data on the persistence of influenza viruses in this substrate are available.
Lake sediments are largely composed of organic mud and sand (12) and have the capacity to readily adsorb viruses (16). The germ carrier technique was adopted to study the persistence of AIV in lake sediments, as in spiking trials a poor recovery of influenza viruses (0.1%) was observed after addition of virus suspension to the lake sediment. It remained unclear whether the virus was inactivated or readily adsorbed to the sediment matrix. Previous reports show that sediments can readily adsorb enteric viruses (12, 17). In one study, more than 99% of polioviruses were adsorbed after mixture of the virus suspension with estuarine sediments, and recovery of viable viruses was also possible from the sediments following elution in organic solutions (11). The elution process used in such studies requires the treatment of sediment with elution medium under extremely alkaline conditions (pH 11.00). However, while working with influenza viruses, such treatments should be used with caution, as these viruses are highly sensitive to extremes in pH (35) and can be readily inactivated. The germ carrier technique provides the best alternative that not only exposes the virus to the substrate environment but also ensures appropriate virus recovery.
Based on the T90 values and an estimated starting titer of 106 TCID50/ml in 1 g of duck feces (see the supplemental material), which is a conservative estimate of viral titer in 1 g of duck feces, the present study documents that influenza viruses may remain infectious in duck feces for periods of time ranging from a few days (at 30 and 20°C) or a few weeks (at 10°C) to several months (at 0°C). Most of the previous studies conducted to determine the survival of AIV in bird feces or fecal waste (8, 20, 43, 44) were based on quantitation of viral infectivity in the beginning and subsequent testing of residual viral infectivity after a defined period of time. The present experiments include the collection of sequential data and the calculation of a definite time (T90 values) required for the inactivation of the viruses in fecal material at a wide range of temperatures. This is helpful to determine how long viruses can remain infectious in fecal material. By incubating the fecal material in closed tubes, the effect of drying on the infectivity of the viruses was excluded from the studies. In a previous study, an influenza virus with a concentration of 2.25 to 3.75 log10 50% egg infective doses (EID50) per g of fresh duck feces became undetectable after the feces were dried overnight at room temperature (20°C), while in wet feces, the virus remained viable for 4 to 6 days at 37°C (44). The microbes present in the feces and their metabolites are hypothesized to play a role in the persistence of viruses in fecal material (20).
The survival of influenza viruses in feces is influenced by the virus strain, the type of feces (species from which the feces were obtained), the physical properties of the feces, and the temperature at which the feces is incubated (9). Similarly, the source of fecal manure can also affect the persistence of AIV in this medium, as in one study, viral persistence was higher in manure collected from SPF chickens than in manure from commercial layers (20).
Reports of the presence of AIV in bird meat are based mostly on the detection of virus from commercial poultry or duck meat following natural or experimental infection of birds (13, 22, 28, 36, 41). The estimation of viral persistence in the meat is significant, as consumption of infected meat has been linked with HPAIV disease outbreaks in backyard poultry (13). Carcasses from wild birds that have died of AIV infection pose a danger for virus transmission to susceptible birds and other animals through predation or cannibalization of infected carcasses (37). The prolonged retention of infective AIV in contaminated meat at low temperatures was confirmed by the detection of H5N1 in frozen duck carcasses in Germany (13) and the isolation of AIV from frozen duck meat that was imported from China to Korea and Japan (22, 41).
Although HPAIV have been detected in infected chicken, duck, and quail meat to a very high titer (2, 38, 39), no evidence for the presence of LPAIV in the meat of infected birds is available (30). On the other hand, LPAIV from respiratory secretions or feces can be a source of carcass surface contamination during the slaughtering process (39). In the present study, T90 values of up to 81 days for influenza viruses from germ carriers incubated in meat samples at 0°C confirm that viral infectivity can be preserved for a long duration in contaminated meat or carcasses at low temperatures.
In several past studies, naturally contaminated feces and meat were used to estimate the inactivation of AIV in these substrates (38, 39, 43). A germ carrier technique was used in the present study to assess the persistence of AIV in feces and meat. LPAIV are not naturally found in the meat of infected birds but are interesting as surrogate viruses for HPAIV in persistence studies (36, 39). Also, the uneven distribution of AIV in naturally infected feces (43) can produce inconsistent data and may lead to inappropriate results. The use of a germ carrier technique not only solves the problem of poor recovery of viruses from the substrate following addition (24) but also allows a better comparison of the results from each substrate.
As is clear from the linear regression models of all viruses under each tested condition at all temperatures (Fig. 1 to 4; also see the supplemental material), the persistence of NDV and ECBO virus was consistently higher than that of the influenza viruses. However, of the noninfluenza viruses, NDV (an enveloped virus) had shorter T90 values than ECBO virus (a nonenveloped virus), which were much closer to those of the influenza viruses at all temperatures. The T90 values calculated for the AIV in the sediment were 2 to 5 times higher at various temperatures than those in the feces and meat (Fig. 1 to 4). The higher persistence of the influenza and noninfluenza viruses in the sediments than in other substrates is hypothesized to be due to protection of the viruses from the inactivating factors present in the surrounding environment by the sediment (18). In duck feces, shorter viral persistence may be attributable to virus inactivation by the microbial metabolites and digestive enzymes present in the feces (20), while in the duck meat, the changes of rigor mortis result in a pH shift. Generally, the pH in duck breast meat reaches approximately a value of 6.00 (1). Such an acidic pH in the duck meat may explain the lower survival rate of the viruses in meat than in the other substrates.
In conclusion, our results suggest that lake sediments may act as a long-term source of influenza viruses in the aquatic habitat. AIV-contaminated feces and meat not only pose a threat of viral transmission to susceptible birds over a period of days but can also preserve viral infectivity for months at low temperatures in colder climates.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Ministerium für Ernährung und Ländlichen Raum Baden-Württemberg, Germany, under the research program “Wildvögel und Vogelgrippe.” J.N. was the recipient of a scholarship from the DAAD (Deutscher Akademischer Austausch Dienst).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Ali M. S., et al. 2008. Effect of chilling temperature of carcass on breast meat quality of duck. Poult. Sci. 87:1860–1867 [DOI] [PubMed] [Google Scholar]
- 2. Antarasena C., et al. 2006. Tissue tropism of a Thailand strain of high-pathogenicity avian influenza virus (H5N1) in tissues of naturally infected native chickens (Gallus gallus), Japanese quail (Coturnix coturnix japonica) and ducks (Anas spp.). Avian Pathol. 35:250–253 [DOI] [PubMed] [Google Scholar]
- 3. Breban R., Drake J. M., Stallknecht D. E., Rohani P. 2009. The role of environmental transmission in recurrent avian influenza epidemics. PLoS Comput. Biol. 5:e1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown J. D., Stallknecht D. E., Beck J. R., Suarez D. L., Swayne D. E. 2006. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg. Infect. Dis. 12:1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown J. D., Swayne D. E., Cooper R. J., Burns R. E., Stallknecht D. E. 2007. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 51:285–289 [DOI] [PubMed] [Google Scholar]
- 6. Brown J. D., Goekjian G., Poulson R., Valeika S., Stallknecht D. E. 2009. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 136:20–26 [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2005. Key facts about avian influenza (bird flu) and avian influenza A (H5N1) virus. http://www.cdc.gov/flu/avian/gen-info/pdf/avian_facts.pdf
- 8. Chumpolbanchorn K., Suemanotham N., Siripara N., Puyati B., Chaichoune K. 2006. The effect of temperature and UV light on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure. Southeast Asian J. Trop. Med. Public Health 37:102–105 [PubMed] [Google Scholar]
- 9. De Benedictis P., Beato M. S., Capua I. 2007. Inactivation of avian influenza viruses by chemical agents and physical conditions: a review. Zoonoses Public Health 54:51–68 [DOI] [PubMed] [Google Scholar]
- 10. Deutsche Veterinärmedizinische Gesellschaft (DVG) e.V 2007. Guidelines for the evaluation of the efficacy of chemical disinfectants. Deutsche Veterinärmedizinische Gesellschaft e.V., Verlag der DVG service GmbH, Giessen, Germany: (In German.) [Google Scholar]
- 11. Gerba C. P., Smith E. M., Melnick J. L. 1977. Development of a quantitative method for detecting enteroviruses in estuarine sediments. Appl. Environ. Microbiol. 34:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerba C. P., Goyal S. M., Smith E. M., Melnick J. L. 1977. Distribution of viral and bacterial pathogens in a coastal canal community. Mar. Pollut. Bull. 8:279–282 [Google Scholar]
- 13. Harder T. C., et al. 2009. Highly pathogenic avian influenza virus (H5N1) in frozen duck carcasses, Germany, 2007. Emerg. Infect. Dis. 15:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hinshaw V. S., Webster R. G., Turner B. 1979. Water-borne transmission of influenza A viruses? Intervirology 11:66–68 [DOI] [PubMed] [Google Scholar]
- 15. Hinshaw V. S., Webster R. G., Turner B. 1980. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 26:622–629 [DOI] [PubMed] [Google Scholar]
- 16. LaBelle R. L., Gerba C. P. 1979. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl. Environ. Microbiol. 38:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaBelle R. L., et al. 1980. Relationship between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl. Environ. Microbiol. 39:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaBelle R. L., Gerba C. P. 1980. Influence of estuarine sediment on virus survival under field conditions. Appl. Environ. Microbiol. 39:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang A. S., Kelly A., Runstadler J. A. 2008. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J. Gen. Virol. 89:509–519 [DOI] [PubMed] [Google Scholar]
- 20. Lu H., et al. 2003. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 47:1015–1021 [DOI] [PubMed] [Google Scholar]
- 21. Markwell D. D., Shortridge K. F. 1982. Possible waterborne transmission and maintenance of influenza viruses in domestic ducks. Appl. Environ. Microbiol. 43:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mase M., et al. 2005. Isolation of a genotypically unique H5N1 influenza virus from duck meat imported into Japan from China. Virology 339:101–109 [DOI] [PubMed] [Google Scholar]
- 23. Nazir J., et al. 2010. Long-term study on tenacity of avian influenza viruses in water (distilled water, normal saline and surface water) at different temperatures. Avian Dis. 54:720–724 [DOI] [PubMed] [Google Scholar]
- 24. Nazir J., Haumacher R., Abbas M. D., Marschang R. E. 2010. Use of filter carrier technique to measure the persistence of avian influenza viruses in wet environmental conditions. J. Virol. Methods 170:99–105 [DOI] [PubMed] [Google Scholar]
- 25. OIE 2009. Avian influenza. Manual of diagnostic tests and vaccines for terrestrial animals 2009. OIE, Paris, France: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2008/pdf/2.03.04_AI.pdf [Google Scholar]
- 26. Rohani P., Breban R., Stallknecht D. E., Drake J. M. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl. Acad. Sci. U. S. A. 106:10365–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senne D. A. 1998. Virus propagation in embryonating eggs, p. 235–240 In Swayne D. E., et al. (ed.), Isolation and identification of avian pathogens, 4th ed. American Association of Avian Pathologists, Kennett Square, PA [Google Scholar]
- 28. Serena Beato M., Terregino C., Cattoli G., Capua I. 2006. Isolation and characterization of an H10N7 avian influenza virus from poultry carcasses smuggled from China into Italy. Avian Pathol. 35:400–403 [DOI] [PubMed] [Google Scholar]
- 29. Sobsey M. D., Meschke J. S. 2003. Virus survival in the environment with special attention to survival in sewage droplets and other environmental media of fecal or respiratory origin. http://www.unc.edu/courses/2008spring/envr/421/001/WHO_VirusSurvivalReport_21Aug2003.pdf
- 30. Spickler A. R., Trampel D. W., Roth J. A. 2008. The onset of virus shedding and clinical signs in chickens infected with high-pathogenicity and low-pathogenicity avian influenza viruses. Avian Pathol. 37:555–577 [DOI] [PubMed] [Google Scholar]
- 31. Stallknecht E. E., Kearney M. T., Shane S. M., Zwank P. J. 1990. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis. 34:412–418 [PubMed] [Google Scholar]
- 32. Stallknecht D. E., Brown J. D. 2009. Tenacity of avian influenza viruses. Rev. Sci. Technol. 28:59–67 [DOI] [PubMed] [Google Scholar]
- 33. Stallknecht D. E., Goekjian V. H., Wilcox B. R., Poulson R. L., Brown J. D. 2010. Avian influenza virus in aquatic habitats: what do we need to learn? Avian Dis. 54:461–465 [DOI] [PubMed] [Google Scholar]
- 34. Sturm-Ramirez K. M., et al. 2005. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J. Virol. 79:11269–11279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swayne D. E., Halvorson D. A. 2003. Influenza, p. 135–179 In Saif Y. M., Barnes H. J., Glisson J. R., Fadly A. M., McDougald L. R., Swayne D. E. (ed.), Diseases of poultry, 11th ed. Iowa State University Press, Ames, IA [Google Scholar]
- 36. Swayne D. E., Beck J. R. 2005. Experimental study to determine if low-pathogenicity and high-pathogenicity avian influenza viruses can be present in chicken breast and thigh meat following intranasal virus inoculation. Avian Dis. 49:81–85 [DOI] [PubMed] [Google Scholar]
- 37. Swayne D. E. 2008. Epidemiology of avian influenza in agricultural and other man-made systems, p. 59–85 In Swayne D. E. (ed.), Avian influenza. Blackwell, Ames, IA [Google Scholar]
- 38. Thomas C., Swayne D. E. 2007. Thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. J. Food Prot. 70:674–680 [DOI] [PubMed] [Google Scholar]
- 39. Thomas C., King D. J., Swayne D. E. 2008. Thermal inactivation of avian influenza and Newcastle disease viruses in chicken meat. J. Food Prot. 71:1214–1222 [DOI] [PubMed] [Google Scholar]
- 40. Traub F., Spillmann S. K., Wyler R. 1986. Method for determining virus inactivation during sludge treatment processes. Appl. Environ. Microbiol. 52:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tumpey T. M., et al. 2002. Characterization of a highly pathogenic H5N1 avian influenza A virus isolated from duck meat. J. Virol. 76:6344–6355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Villegas P. 1998. Titration of biological suspensions, p. 248–253 In Swayne D. E., et al. (ed.), Isolation and identification of avian pathogens, 4th ed. American Association of Avian Pathologists, Kennett Square, PA [Google Scholar]
- 43. Webster R. G., Yakhno M., Hinshaw V. S., Bean W. J., Murti K. G. 1978. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization 2006. Review of latest available evidence on risks to human health through potential transmission of avian influenza (H5N1) through water and sewage. Last updated 10 October 2007. World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/emerging/avianflu/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.