Abstract
A Gram-negative, red-pigment-producing marine bacterial strain, designated S1-1, was isolated from the tidal flat sediment of the Yellow Sea, Korea. On the basis of phenotypic, phylogenetic, and genetic data, strain S1-1 (KCTC 11448BP) represented a new species of the genus Zooshikella. Thus, we propose the name Zooshikella rubidus sp. nov. Liquid chromatography and mass spectrometry of the red pigments produced by strain S1-1 revealed that the major metabolic compounds were prodigiosin and cycloprodigiosin. In addition, this organism produced six minor prodigiosin analogues, including two new structures that were previously unknown. To our knowledge, this is the first description of a microorganism that simultaneously produces prodigiosin and cycloprodigiosin as two major metabolites. Both prodigiosin and cycloprodigiosin showed antimicrobial activity against several microbial species. These bacteria were approximately 1.5-fold more sensitive to cycloprodigiosin than to prodigiosin. The metabolites also showed anticancer activity against human melanoma cells, which showed significantly more sensitivity to prodigiosin than to cycloprodigiosin. The secondary metabolite profiles of strain S1-1 and two reference bacterial strains were compared by liquid chromatography-mass spectrometry. Multivariate statistical analyses based on secondary metabolite profiles by liquid chromatography-mass spectrometry indicated that the metabolite profile of strain S1-1 could clearly be distinguished from those of two phylogenetically related, prodigiosin-producing bacterial strains.
INTRODUCTION
Traditionally, natural products have been sources for new pharmaceuticals. In the past 3 decades, research into natural products has declined in the pharmaceutical industry, in part due to the lack of compatibility with high-throughput screening (HTS) methods (22). Nevertheless, natural products are still important sources for drug research. In recent years, many researchers have focused on identifying lead compounds from marine sources. Marine microbes are prolific but underexploited producers of novel secondary metabolites with pharmaceutical potential (23).
Prodigiosin (PDG) is a red pigment produced by many bacterial species, including Serratia marcescens, Hahella chejuensis, Streptomyces variegatus, Colwellia (Vibrio) psychrerythraea, “Pseudomonas magnesiorubera,” and other eubacteria (34). The prodiginine group, of which prodigiosin is a member, is a group of structural isomers that contain a tripyrrole core with different alkyl chains. This compound has shown significant biological potential due to its antimicrobial (3), antimalarial (4), anticancer (24), and immunosuppressive (14) activities. According to a recent report, prodigiosin or its analogues have been considered effective biological control agents against harmful algae (17, 25), have been considered cell growth regulators (13), and can be used as a natural dye (1). On the other hand, it has been known that cycloprodigiosin (cPDG) is produced only as a minor compound by a few marine bacteria, including Pseudoalteromonas (Alteromonas) rubra, Pseudoalteromonas denitrificans, and Vibrio gazogenes (1, 2, 10, 19). Cycloprodigiosin hydrochloride has shown potent anticancer activity against various cancer cell lines, suggesting that cPDG may be a new class of anticancer drug (18, 34, 35, 36).
Metabolomics is a newly emerging field of research concerned with comprehensive characterization of small molecular metabolites in biological systems. One of the main strategies used in metabolomics studies (11, 31) is metabolite profiling with the use of high-throughput and highly efficient analytical methods, including mass spectrometry (MS) and nuclear magnetic resonance (NMR). Recent innovations in high-throughput technologies for measuring biological samples have made it possible to produce large-scale raw databases but have also created a paradigm shift in large-scale data processing. Currently, new tools are needed for the analysis of huge stores of biological data. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) are two common multivariate projection methods employed for large database exploration. Both methods have been very useful for deciphering systematic changes between many samples and relative differences in metabolite production (26).
Recently, a screen for potentially useful secondary metabolites produced from marine bacteria in South Korea revealed a novel red-pigment-producing bacterial strain that was isolated from marine intertidal sediment. The aim of the present study was to identify the constituents of the red pigments from this novel bacterial strain and to investigate their values for a variety of applications. In addition, we compared the metabolite profiles of various red-pigment-producing bacteria with multivariate statistical analyses of data from liquid chromatography and mass spectrometry (LC-MS).
MATERIALS AND METHODS
Bacterial strains, isolation, and cultivation.
Intertidal sediment samples were collected from the coast of Saemankum, South Korea. The samples (1 g) were serially diluted with 0.85% NaCl solution and spread onto marine agar 2216 (MA; Difco). The agar plates were incubated at 25°C, and colonies that appeared were restreaked onto fresh agar plates to obtain pure cultures. Bacterial isolates were suspended in 20% glycerol and frozen in liquid nitrogen for storage. Of the isolates, one red-pigmented bacterial strain (S1-1) was selected for further study. Hahella chejuensis KCTC 2396T and Zooshikella ganghwensis KCTC 12044T were used as reference strains for comparative study.
Identification of bacterial strain.
An amplification of the 16S rRNA gene was performed according to the method described previously with two universal primers (38); the PCR products were purified with a QIAquick PCR purification kit (Qiagen). Sequencing of the PCR products and phylogenetic analysis were performed as described by Yoon et al (39). DNA-DNA relatedness was determined by the microplate hybridization method (9) using photobiotin-labeled DNA probes. The DNA G+C content was determined by a modification of the method of Tamaoka and Komagata (30). Briefly, the DNA was hydrolyzed with nuclease P1 (Sigma) and the resultant nucleotides were analyzed by reversed-phase high-pressure liquid chromatography (HPLC). The type strain of Zooshikella ganghwensis (KCTC 12044T), obtained from the Korean Collection for Type Cultures (KCTC), Taejon, South Korea, was used as a reference strain for DNA-DNA hybridization and phenotypic characterization. Further investigation of the morphological, cultural, physiological, and biochemical characteristics of strain S1-1 was performed as described in the supplemental material.
LC-MS analysis and structure identification of red pigments.
Strain S1-1, grown on MA, was inoculated into 5 ml of marine broth 2216 (MB; Difco) and cultured for 24 h at 30°C with vigorous shaking. A portion of the culture was transferred to 200 ml of MB, further cultivated for 48 h, and centrifuged at 10,000 × g for 10 min. The supernatant was extracted with an equal volume of ethyl acetate. The pigments from the cell mass were extracted twice with 20 ml acetone. The ethyl acetate and acetone extracts were mixed and evaporated. The dried crude extract was dissolved in 50% methanol, and 10 μl was analyzed by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS) in parallel with reference compounds from Hahella chejuensis KCTC 2396T. For identification of prodigiosin, cycloprodigiosin, and prodigiosin analogues, the data obtained from the tandem mass spectrometry (MS/MS) analysis were compared with those from literature sources (1, 21). Mass Frontier 5.0 (HighChem, Ltd.) software was used to confirm the identity and structure of each compound based on observed fragmentation patterns. Accurate mass measurements were performed on the LTQ-Orbitrap XL mass spectrometer (Thermo) equipped with an electrospray ionization (ESI) source. Xcalibur software (Thermo) was used for data acquisition and processing. Additional information for LC-MS analysis is provided in the supplemental material.
Bioactivity assays of prodigiosin and cycloprodigiosin.
The process of purification of prodigiosin and cycloprodigiosin is described in the supplemental material. The antimicrobial activities of the purified prodigiosin and cycloprodigiosin were tested against Bacillus subtilis KCTC 1914, Escherichia coli KCTC 1924, Salmonella enterica serovar Typhimurium KCTC 1926, Staphylococcus aureus KCTC 1916, and Candida albicans KCTC 1940 by the disc diffusion method. Discs impregnated with 50 μg of prodigiosin and cycloprodigiosin compounds were placed onto LB plates spread with cultures of the above test organisms, respectively. The plates were incubated for 24 h at 37°C, and the inhibition zone around the disc was measured, including disc diameter (6 mm).
Two human melanoma cell lines used in this study, SK-MEL-28 (ATCC HTB-72) and A375P (ATCC CRL-1619), were obtained from the American Type Culture Collection (ATCC) and maintained in Roswell Park Memorial Institute 1640 medium (Invitrogen) and Dulbecco's modified Eagle's medium (Invitrogen), both of which were supplemented with 10% heat-inactivated fetal bovine serum (FBS), respectively. The proliferation assay was performed as described previously (15). Briefly, the two cell lines were suspended in the two media and seeded at a density of 5,000 cells per well in 96-well plates. After incubation for 24 h at 37°C, cells were replenished with fresh complete medium containing either test compounds or 0.1% dimethyl sulfoxide (DMSO). After incubation for 48 h at 37°C, the cell proliferation reagent WST-1 (Dojindo Laboratories Co.) (29) was added to each well. The formation of formazan by mitochondrial dehydrogenase was quantitatively measured at 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad Laboratories Inc.).
Metabolite profiling and statistical analysis.
For comparisons, three red-pigment-producing bacterial strains (S1-1, Zooshikella ganghwensis KCTC 12044T, and Hahella chejuensis KCTC 2396T) were cultivated under identical growth conditions and then subjected to metabolite profiling. Triplicate cultures for the three strains were prepared for metabolite profiling analysis, and each culture sample was analyzed with three separate injections into the LC-MS. The detailed sample extraction and LC-MS analysis were performed as described for the previous LC-MS analysis and in the supplemental material. Peak identification and quantification of selective ion traces were accomplished with Xcalibur 2.0. An individual base peak chromatogram was plotted for each of the ions that corresponded to the bacterial metabolites. The data were then analyzed by principal component analysis (PCA) using SPSS 12.0 (SPSS Inc.). Hierarchical component analysis (HCA) was performed using Cluster 3.0 with a complete linkage clustering method (7).
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain S1-1 has been deposited in the GenBank database under accession number FJ217962.
RESULTS
Taxonomic identification of bacterial strain S1-1.
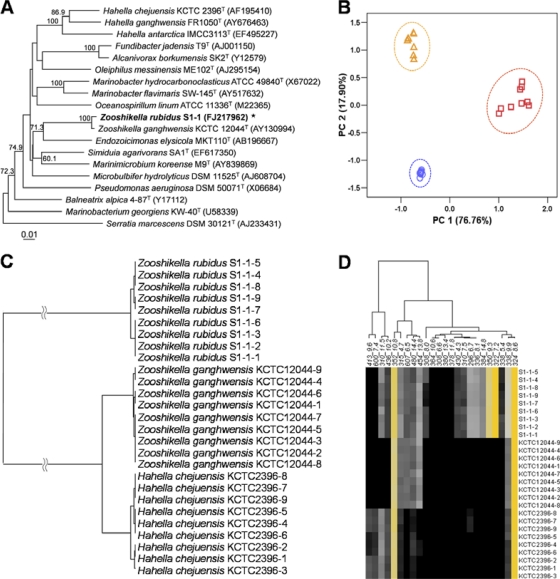
Strain S1-1 was aerobic and Gram negative and formed slightly curved rods with motility by means of a single polar flagellum. The optimal growth conditions were 30 to 37°C, pH 7.0, in the presence of 2% NaCl. The morphological, cultural, physiological, and biochemical characteristics of strain S1-1 are shown in Table S1 in the supplemental material. In the phylogenetic tree based on the neighbor-joining algorithm, strain S1-1 joined the type strain of Zooshikella ganghwensis, a member of the Gammaproteobacteria, by a bootstrap resampling value of 100% (see Fig. 4A). Strain S1-1 exhibited the highest 16S rRNA gene sequence similarity (99.4%) to Zooshikella ganghwensis KCTC 12044T. The 16S rRNA gene sequence similarity was less than 91.4% for strain S1-1 compared to other members of the Gammaproteobacteria. The DNA-DNA relatedness was 28.9% between strain S1-1 and Zooshikella ganghwensis KCTC 12044T. Ubiquinone-9 was the predominant isoprenoid quinone detected in both strain S1-1 and Zooshikella ganghwensis KCTC 12044T (37). The cellular fatty acid profile of strain S1-1 was similar to that of Zooshikella ganghwensis KCTC 12044T, although there were differences in the proportions of some fatty acids (see Table S2 in the supplemental material). The DNA G+C content of strain S1-1 was 41 mol%. The phenotypic properties of strain S1-1 demonstrate that this strain is different from Zooshikella ganghwensis, the single recognized species of the genus Zooshikella (see Table S1 in the supplemental material). On the basis of the data presented, strain S1-1 represents a novel species of the genus Zooshikella; thus, we propose to name it Zooshikella rubidus sp. nov. The description of Zooshikella rubidus sp. nov. is as follows: Zooshikella rubidus (ru′bi.dus. L. masc. adj. rubidus, dark red).
Fig. 4.
The distinctiveness of Zooshikella rubidus S1-1. (A) Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences showing the positions of Zooshikella rubidus S1-1, Zooshikella ganghwensis KCTC 12044T, and other related taxa. Bootstrap values (expressed as percentages of 1,000 replications) that were greater than 50% are shown at branch points. Bar, 0.01 substitutions per nucleotide position. (B) Principal component analysis (PCA) score plot of LC-MS profiles of three prodigiosin-producing strains. The PCA shows differences in metabolite profiles among Hahella chejuensis KCTC 2396T (▵), Zooshikella rubidus S1-1 (□), and Zooshikella ganghwensis KCTC 12044T (○) in marine broth 2216 (n = 9). (C) Dendrogram based on hierarchical cluster analysis of metabolite profiles. (D) Thumbnail image of metabolite array.
Identification of red-pigment structures.
The red pigments extracted from strain S1-1 cultures were analyzed by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS). Two major peaks occurred at 7.08 min and 7.86 min on the mass chromatogram (Fig. 1). The two major peaks had molecular weights of 321 (m/z 322, [M + H]+) and 323 (m/z 324, [M + H]+) and were identified as cycloprodigiosin and prodigiosin, respectively (Fig. 1). The structures of the two major compounds were elucidated by tandem mass spectrometry (MS/MS) analysis and by comparison with data from the literature (1, 21). The two major compounds were further confirmed as cycloprodigiosin and prodigiosin on a high-resolution electrospray ionization mass spectrometer (HR-ESI-MS). The molecular formulae of cycloprodigiosin and prodigiosin, respectively, were established by HR-ESI-MS as C20H24N3O (m/z 322.1891 [M + H]+, calculated for 322.1919) and C20H26N3O (m/z 324.2068 [M + H]+, calculated for 324.2076). In the initial LC-MS/MS analyses of the red pigments extracted from strain S1-1, minor peaks were detected before and after the major peaks (Fig. 2). These appeared to be composed of six separate compounds, with parent ions m/z 296 (6.24 min), m/z 310 (7.39 min), m/z 338 (9.62 min), m/z 352 (10.60 min), m/z 366 (11.74 min), and m/z 380 (12.95 min). Each peak appeared with a retention time slightly different from that of prodigiosin (m/z 324, 8.09 min). The differences in m/z values and peak shifts of the parent ions were in accordance with the sequential decreases or increases in the alkyl chain length on prodigiosin. All prodigiosin analogues had a common fragment ion at m/z 252 that was derived from the loss of the alkyl chain. Moreover, the prodigiosin analogues showed the same dissociation fragment patterns, for example, −15 amu and −32 amu, and these fragments were derived by the loss of a methyl ([M + H-CH3]+) and a methoxyl ([M-OCH3]+) group, respectively. Interestingly, a similar tendency was also reported in Hahella chejuensis KCTC 2396T (21). However, the 2-methyl-3-octyl prodiginine (m/z 366) and 2-methyl-3-nonyl prodiginine (m/z 380) detected in this study were new compounds that have not been described in other organisms, including Hahella chejuensis KCTC 2396T. The structures of the two new prodigiosin analogues were distinguished from that of prodigiosin (C5) by differences in alkyl chain length (C8-C9) detected by tandem mass spectrometry (Fig. 2). The molecular formulae of m/z 366 and m/z 380 were established by high-resolution mass spectrometry (resolution 30,000) as C23H32ON3 (m/z 366.2547 [M + H]+, calculated for 366.2545, Δ1.99 ppm) and C24H34ON3 (m/z 380.2705 [M + H]+, calculated for 380.2702, Δ2.37 ppm), respectively.
Fig. 1.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) results of the extracts from Zooshikella rubidus S1-1 were compared to those from Zooshikella ganghwensis KCTC 12044T. (A) LC chromatogram of extracts from Zooshikella rubidus S1-1 (top) and Zooshikella ganghwensis KCTC 12044T (bottom). The major compounds were separated by HPLC at the indicated retention times of 7.08 min (m/z 322, cycloprodigiosin) and 7.86 min (m/z 324, prodigiosin). (B) Mass spectra (top) and MS/MS fragmentation patterns (bottom) of cycloprodigiosin (left) and prodigiosin (right). (C) Molecular structures of cycloprodigiosin (left) and prodigiosin (right) identified by the MS/MS fragmentation study.
Fig. 2.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses and identified structures of prodigiosin and six prodigiosin analogues in the extract of Zooshikella rubidus S1-1. The extracted mass chromatogram (first column), mass spectrum (second column), and MS/MS spectrum (third column) of prodigiosin (C) and six prodigiosin analogues (A, B, D, E, F, and G) were separated by HPLC at the indicated retention times. The predicted molecular structures are shown in the last column.
Production of prodigiosin.
The level of prodigiosin production by Zooshikella rubidus S1-1 increased dramatically following the middle log phase (18 to 24 h), and the strain's maximum production of prodigiosin (47.8 mg/liter) occurred after cellular multiplication ceased (see Fig. S1 in the supplemental material). The amount of prodigiosin produced by Zooshikella rubidus S1-1 was 1.7 and 3.1 times higher than those produced by Hahella chejuensis KCTC 2396T and Zooshikella ganghwensis KCTC 12044, respectively (see Fig. S1 in the supplemental material).
Antimicrobial activity.
The antimicrobial activities of the prodigiosin and cycloprodigiosin extracted from strain S1-1 are summarized in Fig. 3 A. Both prodigiosin and cycloprodigiosin showed antimicrobial activity against Bacillus subtilis, Escherichia coli, Salmonella serovar Typhimurium, Staphylococcus aureus, and Candida albicans. It was found that cycloprodigiosin had higher antimicrobial activity than did prodigiosin for all test organisms. Recently, there has been increasing demand for integrating antimicrobial properties into textiles. Consumers worldwide are looking for clothing that provides greater comfort and durability (12). Therefore, we tested the ability of an extract of strain S1-1 to serve as a natural antimicrobial dye on silk and cotton fabrics. Our results showed that when silk and cotton fabrics were dyed with 0.02% (wt/vol) and 0.1% (wt/vol) red-pigment extract solution, the growth rates of E. coli KCTC 1924 and S. aureus KCTC 1916 were reduced by 91.37 to 96.98% and 96.62 to 99.98%, respectively (see Table S3 in the supplemental material).
Fig. 3.
Effects of prodigiosin and cycloprodigiosin on microorganisms and cancer cell lines. Comparison of the antimicrobial activities of prodigiosin and cycloprodigiosin purified from Zooshikella rubidus S1-1 (A). DMSO was used as a vehicle solvent and negative control. Human melanoma cancer cells were treated with prodigiosin (B) and cycloprodigiosin (C) at various concentrations or with vehicle solvent (0.1% DMSO). All assays were performed in triplicate, and error bars represent standard deviations from the means.
Anticancer activity.
Two human melanoma cell lines, SK-MEL-28 and A375P, were incubated with prodigiosin and cycloprodigiosin for 48 h to test whether growth could be inhibited at concentrations between 0.5 and 50 μM. Prodigiosin significantly inhibited growth of both SK-MEL-28 and A375P, with 50% growth inhibition (GI50) values of 2.8 and 6.5 μM, respectively (Fig. 3B). Cycloprodigiosin also significantly inhibited growth of both melanoma cell lines with GI50 values of 10.1 and 10.3 μM for A375P and SK-MEL-28, respectively (Fig. 3C). The morphologies of the two melanoma cell lines changed after 48-h incubations with prodigiosin and cycloprodigiosin; at 2 μM prodigiosin and at 5 μM cycloprodigiosin, cells rounded up and floated (see Fig. S2 in the supplemental material). Morphological changes such as rounding and floating cells show that treatment with prodigiosin and cycloprodigiosin induces inhibition of cell growth and apoptosis or necrosis of human melanoma SK-MEL-28 and A375P cell lines.
Secondary metabolite profiling by LC-MS/MS.
Comprehensive metabolite profiling was performed for secondary metabolites with liquid chromatography followed by mass spectrometry (LC-MS). All spectral data were digitized and globally normalized to apply PCA and HCA. The PCA identifies new variables, PCs that are linear combinations of the original data set. The first principal component (PC1) contains most of the information in the original variables (27). In this study, the PC vectors 1 and 2 were chosen for the best visualization of strain separation. The two most significant PCs could explain 94.66% of the data variability (PC1, 76.76%; PC2, 17.90%). The PCA score plot of PC1 versus PC2 was derived from three distinct groups without any overlap between strain S1-1 and the two reference prodigiosin-producing bacterial strains (Fig. 4 B). First, two distinct groups (S1-1 versus other strains) were distinguished by PC1; this result was mainly due to prodigiosin (m/z 324), cycloprodigiosin (m/z 322), and prodigiosin analogues such as m/z 296, m/z 308, m/z 310, m/z 336, m/z 338, m/z 350, m/z 352, m/z 364, m/z 378, and m/z 380. Then, the unknown metabolites m/z 607, m/z 436, and m/z 480 had comparatively strong impacts on PC2; they clearly separated Hahella chejuensis KCTC 2396T from Zooshikella ganghwensis KCTC 12044T (see Fig. S3 in the supplemental material).
The clustering of metabolite profiles was confirmed by HCA pattern recognition, a useful approach for the identification of strain novelty based on secondary metabolite profiles. The HCA result shows differences in the metabolite profiles between the prodigiosin-producing group (Hahella chejuensis KCTC 2396T and Zooshikella ganghwensis KCTC 12044T) and the prodigiosin- and cycloprodigiosin-producing group (Zooshikella rubidus S1-1). Based on the metabolite profiles of the three strains, the HCA dendrogram showed the existence of two distinct clusters: the strain S1-1 cluster and a cluster comprising Hahella chejuensis KCTC 2396T and Zooshikella ganghwensis KCTC 12044T (Fig. 4C). The clustering result was in accordance with the thumbnail image of the metabolite array (Fig. 4D). The clusters of Hahella chejuensis KCTC 2396T and Zooshikella ganghwensis KCTC 12044T were further divided into two subclusters corresponding to minor metabolite differences. The differentiation between strains S1-1 and Zooshikella ganghwensis KCTC 12044T was based on the analysis of metabolite profiles and was in good agreement with the results of the phylogenetic analysis based on 16S rRNA gene sequences and DNA-DNA hybridization.
DISCUSSION
From the metabolite analyses with LC-MS/MS, we found that the major compounds simultaneously produced by strain S1-1 were prodigiosin and cycloprodigiosin. In addition, strain S1-1 produced six minor prodigiosin analogues, including two prodigiosin analogues with new structures that were previously unknown (Fig. 2). In contrast, another species of the same genus, Zooshikella ganghwensis, was found to produce only one major compound, prodigiosin (Fig. 1). To our knowledge, this is the first report of a microorganism that produces both prodigiosin and cycloprodigiosin simultaneously as two major metabolites.
The polyphasic taxonomic study, including phenotypic characterization, phylogenetic analysis based on 16S rRNA gene sequences, and DNA-DNA hybridization, revealed that the red-pigment-producing bacterial strain S1-1 is a new species of the genus Zooshikella. Recently, two German research groups also established matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry for bacterial classification and identification (8, 28). In this paper, we present the application of these techniques to bacterial classification with the use of secondary metabolites. LC-MS metabolite profiling combined with statistical analyses has two primary advantages: rapidity compared to conventional techniques and high specificity that allows differentiation among species and subspecies. Our study showed that metabolite profiling combined with PCA and HCA may be a useful tool for the elucidation of bacterial novelty and for discovery of bioactive metabolites. In conclusion, our study showed for the first time a direct application of metabolomics for screening novel bacterial and secondary metabolites.
Bifurcated biosynthetic pathways of prodigiosin and several prodigiosin analogues have recently been reported (5, 16, 32, 34). The biosynthesis of prodigiosin proceeds by the enzymatic condensation of the terminal products from two different pathways. The products are 2-methyl-3-n-amyl-pyrrole (MAP), a form of monopyrrole, and 4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC). The condensation of monopyrrole and MBC to produce prodigiosin and prodigiosin analogues is catalyzed by PigC and its homologues (RedH and HapC) (20, 32). Another pathway for prodigiosin analogue synthesis was predicted in Streptomyces coelicolor A3(2). In that pathway, undecylprodigiosin is formed by the condensation of 2-undecyl-pyrrole and MBC. The oxidative cyclization of undecylprodigiosin by RedG (5) produces butyl-meta-cycloheptylprodigiosin. However, some catalytic steps in the biosynthetic pathways of prodigiosin and prodigiosin analogues remain unclear. Currently, the biosynthetic pathway(s) of cycloprodigiosin is not known precisely. Zooshikella rubidus S1-1 produced cycloprodigiosin, prodigiosin, and prodigiosin analogues but did not produce undecylprodigiosin or butyl-meta-cycloheptylprodigiosin. This observation suggests that Zooshikella rubidus S1-1 does not use the pathway predicted for Streptomyces coelicolor A3(2). Because Zooshikella rubidus S1-1 produces amounts of cycloprodigiosin and prodigiosin similar to those produced by major metabolic compounds, it must have an oxidative cyclization protein(s), like RedG, and proceed through an additional catalytic step(s) for cycloprodigiosin biosynthesis. In previous reports, Hahella chejuensis KCTC 2396, Serratia sp. strain ATCC 39006, Serratia marcescens ATCC 274, and Streptomyces coelicolor A3(2) were found to have relatively high similarities in the genes involved in prodigiosin biosynthesis (20, 33). We also suggest that horizontal gene transfers might result in the production of these red pigments, as inferred by Williamson et al. (33). The assumption that lateral gene transfers may confer the ability to produce prodigiosin and its analogues in organisms that do not inherently produce these compounds requires further study.
Most synthetic dyes are dependent on petrochemical sources, and some synthetic dyes contain toxic and carcinogenic amines that are not environmentally benign. On the other hand, most natural dyes originate from plants, animals, microorganisms, and minerals and, thus, are typically renewable and environmentally benign (6). However, only a few natural dyes are commercially available and most originate from plants. In particular, dyes of microbial origin are very rare but offer great commercial availability due to the ease of mass production by fermentation. Moreover, most natural products have biological activities that correspond to recent trends in developing bioactive textiles. Interestingly, fabrics dyed with the red pigments from strain S1-1 showed better bacterial inhibition than that reported previously (1). This observation may be attributable to a synergistic effect of the combination of cycloprodigiosin, prodigiosin, and the various prodigiosin analogues. Furthermore, both of the major compounds showed low GI50 values against two cancer cell lines, A375P and SK-MEL-28. To our knowledge, the cytotoxic activity of prodigiosin and cycloprodigiosin against human melanoma cells has not been reported in any previous studies. Prodigiosin was reported as an algicidal agent to be highly effective against Cochlodinium polykrikoides, a major red tide microalga (17). In this study, the strain S1-1 showed high productivity of prodigiosin as well as various prodigiosin analogues. Therefore, we anticipate that the red-pigment extract of Zooshikella rubidus S1-1 can be effectively used for repression of blooming by red tide microalgae and for other biological purposes.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the 21C Frontier Program of Microbial Genomics and Applications from the Ministry of Education, Science and Technology (MEST) of the Republic of Korea.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Alihosseini F., Ju K.-S., Lango J., Hammock B. D., Sun G. 2008. Antibacterial colorants: characterization of prodiginines and their applications on textile materials. Biotechnol. Prog. 24:742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett J. W., Bentley R. 2000. Seeing red: the story of prodigiosin. Adv. Appl. Microbiol. 47:1–32 [DOI] [PubMed] [Google Scholar]
- 3. Boger D. L., Patel M. 1988. Total synthesis of prodigiosin, prodigiosene, and desmethoxyprodigiosin: Diels-Alder reactions of heterocyclic azadienes and development of an effective palladium(II)-promoted 2,2′-bipyrrole coupling procedure. J. Org. Chem. 53:1405–1415 [Google Scholar]
- 4. Castro A. J. 1967. Antimalarial activity of prodigiosin. Nature 213:903–904 [DOI] [PubMed] [Google Scholar]
- 5. Cerdeño A. M., Bibb M. J., Challis G. L. 2001. Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem. Biol. 8:817–829 [DOI] [PubMed] [Google Scholar]
- 6. Dedhia E. M. 1998. Natural dyes. Colourage 65:45–49 [Google Scholar]
- 7. de Hoon M. J. L., Imoto S., Nolan J., Miyano S. 2004. Open source clustering software. Bioinformatics 20:1453–1454 [DOI] [PubMed] [Google Scholar]
- 8. Dieckmann R., Helmuth R., Erhard M., Malorny B. 2008. Rapid classification and identification of Salmonellae at the species and subspecies levels by whole-cell matrix-assisted laser desorption ionization–time of flight mass spectrometry. Appl. Environ. Microbiol. 74:7767–7778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ezaki T., Hashimoto Y., Yabuuchi E. 1989. Fluorometric DNA-DNA hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Evol. Microbiol. 39:224–229 [Google Scholar]
- 10. Gerber N. N., Gauthier M. J. 1979. New prodigiosin-like pigment from Alteromonas rubra. Appl. Environ. Microbiol. 37:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. German J. B., Hammock B. D., Watkins S. M. 2005. Metabolomics: building on a century of biochemistry to guide human health. Metabolomics 1:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta D., Khare S. K., Laha A. 2004. Antimicrobial properties of natural dyes against Gram-negative bacteria. Color Technol. 120:167–171 [Google Scholar]
- 13. Haddix P. L., et al. 2008. Kinetic analysis of growth rate, ATP, and pigmentation suggests an energy-spilling function for the pigment prodigiosin of Serratia marcescens. J. Bacteriol. 190:7453–7463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han S. B., et al. 1998. T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int. J. Immunopharmacol. 20:1–13 [DOI] [PubMed] [Google Scholar]
- 15. Han D. C., et al. 2004. 2′-Benzoyloxycinnamaldehyde induces apoptosis in human carcinoma via reactive oxygen species. J. Biol. Chem. 279:6911–6920 [DOI] [PubMed] [Google Scholar]
- 16. Harris A. K. P., et al. 2004. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology 150:3547–3560 [DOI] [PubMed] [Google Scholar]
- 17. Jeong H., et al. 2005. Genomic blueprint of Hahella chejuensis, a marine microbe producing an algicidal agent. Nucleic Acids Res. 33:7066–7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamata K., et al. 2001. Cycloprodigiosin hydrochloride suppresses tumor necrosis factor (TNF) α-induced transcriptional activation by NF-κB. FEBS Lett. 507:74–80 [DOI] [PubMed] [Google Scholar]
- 19. Kawauchi K., et al. 1997. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 237:543–547 [DOI] [PubMed] [Google Scholar]
- 20. Kim D., et al. 2006. Analysis of a prodigiosin biosynthetic gene cluster from the marine bacterium Hahella chejuensis KCTC 2396T. J. Microbiol. Biotechnol. 16:1912–1918 [Google Scholar]
- 21. Kim D., et al. 2007. Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396T. J. Appl. Microbiol. 102:937–944 [DOI] [PubMed] [Google Scholar]
- 22. Koehn F. E., Carter G. T. 2005. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4:206–220 [DOI] [PubMed] [Google Scholar]
- 23. Lam K. S. 2006. Discovery of novel metabolites from marine actinomycetes. Curr. Opin. Microbiol. 9:245–251 [DOI] [PubMed] [Google Scholar]
- 24. Montaner B., Pérez-Tomás R. 2003. The prodigiosins: a new family of anticancer drugs. Curr. Cancer Drug Targets 3:57–65 [DOI] [PubMed] [Google Scholar]
- 25. Nakashima T., et al. 2006. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium γ-proteobacterium. Appl. Microbiol. Biotechnol. 73:684–690 [DOI] [PubMed] [Google Scholar]
- 26. Pohjanen E., et al. 2006. Statistical multivariate metabolite profiling for aiding biomarker pattern detection and mechanistic interpretations in GC/MS based metabolomics. Metabolomics 2:257–268 [Google Scholar]
- 27. Ringnér M. 2008. What is principal component analysis? Nat. Biotechnol. 26:303–304 [DOI] [PubMed] [Google Scholar]
- 28. Sauer S., et al. 2008. Classification and identification of bacteria by mass spectrometry and computational analysis. PLoS One 3:e2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seki Y., et al. 2006. Construction of a novel DNA decoy that inhibits the oncogenic B-catenin/T-cell factor pathway. Mol. Cancer Ther. 5:985–994 [DOI] [PubMed] [Google Scholar]
- 30. Tamaoka J., Komagata K. 1984. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol. Lett. 25:125–128 [Google Scholar]
- 31. Villas-Bôas S. G., Rasmussen S., Lane G. A. 2005. Metabolomics or metabolite profiles? Trends Biotechnol. 23:385–386 [DOI] [PubMed] [Google Scholar]
- 32. Williamson N. R., et al. 2005. Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol. Microbiol. 56:971–989 [DOI] [PubMed] [Google Scholar]
- 33. Williamson N. R., Fineran P. C., Leeper F. J., Salmond G. P. C. 2006. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 4:887–899 [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto C., et al. 1999. Cycloprodigiosin hydrochloride, a new H(+)/Cl(−) symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatology 30:894–902 [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto D., et al. 2000. Cycloprodigiosin hydrochloride, H+/Cl− symporter, induces apoptosis and differentiation in HL-60 cells. Int. J. Cancer 88:121–128 [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto D., et al. 2002. Synergistic effects induced by cycloprodigiosin hydrochloride and epirubicin on human breast cancer cells. Breast Cancer Res. Treat. 72:1–10 [DOI] [PubMed] [Google Scholar]
- 37. Yi H., Chang Y.-H., Oh H. W., Bae K. S., Chun J. 2003. Zooshikella ganghwensis gen. nov., sp. nov., isolated from tidal flat sediments. Int. J. Syst. Evol. Microbiol. 53:1013–1018 [DOI] [PubMed] [Google Scholar]
- 38. Yoon J.-H., Lee S. T., Park Y.-H. 1998. Inter- and intraspecific phylogenetic analysis of the genus Nocardioides and related taxa based on 16S rDNA sequences. Int. J. Syst. Evol. Microbiol. 48:187–194 [DOI] [PubMed] [Google Scholar]
- 39. Yoon J.-H., Kim I.-G., Shin D.-Y., Kang K. H., Park Y.-H. 2003. Microbulbifer salipaludis sp. nov., a moderate halophile isolated from a Korean salt marsh. Int. J. Syst. Evol. Microbiol. 53:53–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.