Abstract
Bacterial biofilms are confined communities that are encapsulated in protective layers of extracellular polymeric substances. Microscopic evaluation of biofilms of diverse bacterial strains on various substrata reveals that, in general, the percentage of viable bacteria decreases with the total number of bacteria in a biofilm.
TEXT
Biofilms are complex three-dimensional structures comprised of bacteria encapsulated in extracellular polymeric substances (EPS) that form on virtually all surfaces exposed to the natural environment, including the human body. Biofilm formation on biomedical implants in the human body constitutes the main reason for their failure, because of the low susceptibility of organisms in a biofilm mode of growth to antimicrobials and the host immune system (2). The resistance of bacteria in a biofilm to antibiotics has been attributed to the EPS matrix, acting as a protective barrier (3) and preventing nutrients from penetrating into the deeper layers of a biofilm. The availability of nutrients in deeper layers of the biofilm is further limited by the metabolic degradation by organisms residing in the upper layers, thereby leaving bacteria in the deeper layers of a biofilm in a dormant state (2, 4, 5, 8). This suggests that a relationship exists between the thickness or amount of biofilm on a substratum surface and the percentage of viable bacteria within the biofilm, but there is no study directly supporting the existence of such a relationship. Therefore, we evaluated the possible relationship between the number of bacteria in biofilms formed on different surgical meshes and their viability, as evaluated with the BacLight LIVE/DEAD staining kit.
Five bacterial strains were used in this study: Staphylococcus aureus Xen29, S. aureus Xen36, Escherichia coli Xen14, Pseudomonas aeruginosa PA14, and coagulase-negative staphylococcus (CNS) DN7334. All strains were originally cultured from cryopreservative beads (Protect Technical Surface Consultants Ltd., United Kingdom) onto blood agar plates at 37°C in ambient air. Before each experiment, one colony per strain was used to inoculate 5 ml of tryptone soya broth (TSB; Oxoid, Basingstoke, Great Britain), which was cultured at 37°C for 24 h in ambient air. One hundred microliters of each culture was used to inoculate 50 ml of medium from which single-species biofilms were grown on different 0.8-cm-diameter surgical meshes at 37°C with continuous shaking at 60 rpm. The meshes were kept submerged in the medium with a stainless steel hook for a total incubation period of 3 days. TSB was refreshed every day. After 3 days, the meshes were collected, dipped once in sterile 0.9% NaCl, and gently dried by a minimal touch with soft tissue to wash off planktonic organisms and redundant TSB. Five mesh types were employed: Prolene, Ultrapro, and Proceed (all from Ethicon Inc., Sommerville, NJ), Bard Composix (C.R. Bard Inc., Helsingborg, Sweden), and Surgisis Biodesign (Cook Biotech Inc., Bloomington, IN). All experiments were executed 6 to 8 times, which generated 161 separate results from 25 substratum-bacterium combinations evaluated. After growth, biofilms were first dispersed from the meshes by sonication in an Eppendorf tube containing 0.5 ml 0.9% NaCl three times, 10 s each time, with 30-s intervals on ice-chilled water using a probing tip (Vibra-Cell; Sonics & Materials, Inc., Newton, CT). Next, a 15-μl droplet of the dispersion was placed on a glass slide and stained for 15 min in the dark at room temperature with 15 μl SYTO 9 dye and propidium iodide in a 1:1 ratio (Molecular Probes, Leiden, The Netherlands). The cellular membrane is permeable to SYTO 9 and stains both live and dead bacteria, yielding green fluorescence. Propidium iodide can enter only through damaged membranes, where it replaces SYTO 9 from the matrix of the nucleolus due to its greater affinity (6, 7), yielding red fluorescence of dead or damaged cells. Finally, a coverslip was placed over the stained dispersion and fluorescence microscopy (Leica DM4000, Heidelberg, Germany) was used to determine the percentage of viable bacteria. The total number of dispersed bacteria (live and dead together) was determined in a Bürker-Türk counting chamber using phase-contrast microscopy (Olympus BH, Zoeterwoude, The Netherlands).
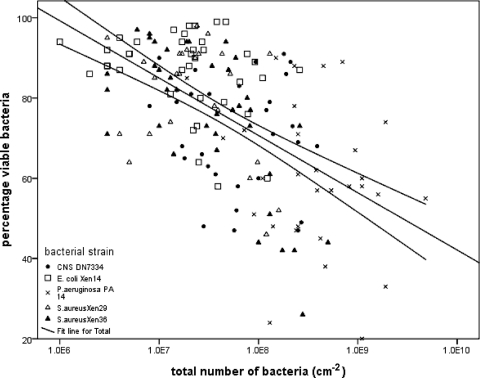
In Fig. 1, the percentage of viable bacteria is related to the total number of bacteria in the biofilms for all bacterial strains and mesh types, showing a significant relationship between the numbers of bacteria in a biofilm and the percentage of viable bacteria. This relationship can be further inferred from a cluster analysis of all data (Table 1) and by relating the properties of the clusters identified with the total number of bacteria and the percentage of viable bacteria in the biofilms, as shown in Fig. 2. Clearly, a cluster with a high total number of bacteria has a low percentage of viable bacteria and vice versa. The clusters could be fully separated on the basis of the different bacterial strains involved, while all mesh types appear within a cluster. This suggests that the total number of bacteria in a biofilm is determined to a greater extent by the bacterial strain involved than by the mesh type, although the mesh type has a considerable influence on the total amount of biofilm formed. Interestingly, no clear relationship between the total number of bacteria in the biofilm and the viability of the bacteria is observed within a single strain. Since most studies in the literature involve only one or at most two strains (1), this might be the reason why the hypothesis that bacterial viability in a biofilm is affected by the total amount of biofilm on a substratum has never been solidly confirmed. However, since our study comprises five strains on five different substrata, the results illustrated in Fig. 1 allow one to draw the generalized conclusion that the viability of organisms in a biofilm decreases significantly with the number of bacteria in the biofilm.
Fig. 1.
Percentage of viable bacteria in biofilms on different surgical meshes for the different bacterial strains indicated as a function of the total number of bacteria per cm2. The straight line indicates a significantly (R2 = 0.323, P < 0.001) negative linear correlation between the two variables (SPSS, Inc., Chicago, IL), while the 95% confidence limits, indicated by the curved lines, indicate the region where the population mean is expected.
Table 1.
Number of strain-mesh type combinations in data clusters, separated on the basis of total numbers of bacteria per cm2 and percentages of viable bacteria for the different bacterial strains and mesh types involveda
| Strain or mesh type | Frequency (no. of occurrences in data cluster): |
||
|---|---|---|---|
| 1 | 2 | 1 and 2 combined | |
| Strains | |||
| CNS DN7334 | 0 | 30 | 30 |
| E. coli Xen14 | 38 | 0 | 38 |
| P. aeruginosa PA14 | 0 | 30 | 30 |
| S. aureus Xen29 | 25 | 0 | 25 |
| S. aureus Zen36 | 1 | 37 | 38 |
| Mesh types | |||
| Bard Composix | 13 | 20 | 33 |
| Proceed | 13 | 20 | 33 |
| Prolene | 14 | 17 | 31 |
| Surgisis Biodesign | 11 | 20 | 31 |
| Ultrapro | 13 | 20 | 33 |
A two-step cluster analysis was done based on log-likelihood distances and a Schwarz Bayesian clustering criterion (SPSS Inc., Chicago, IL). The total number of strain-mesh type combinations in the clusters is 161.
Fig. 2.
Total number of bacteria per cm2 (♦, left axis) and percentage of viable bacteria (□, right axis) in biofilms for the two clusters of bacterial strains and meshes identified in Table 1. The vertical bars indicate the 95% confidence limits, representing the region where the population mean is expected.
In summary, by using a variety of different bacterial strains and species, as well as different substratum surfaces, we have demonstrated for the first time that a generalized relationship exists between the number of bacteria in a biofilm and the viability of the bacteria in the biofilm. This is of particular significance when studying the influence of substratum surface modification and the action of antimicrobial agents on biofilm formation.
Acknowledgments
This project was partially funded by Ethicon (Johnson & Johnson Medical, Norderstedt, Germany) and the research program of the BioMedical Materials Institute (BMM project P4.01 NANTICO, cofunded by the Dutch Ministry of Economic Affairs).
Footnotes
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Bakker D. P., Postmus B. R., Busscher H. J., van der Mei H. C. 2004. Bacterial strains isolated from different niches can exhibit different patterns of adhesion to substrata. Appl. Environ. Microbiol. 70:3758–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donlan R. M., Costerton J. W. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flemming H. C., Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633 [DOI] [PubMed] [Google Scholar]
- 4. Fux C. A., Costerton J. W., Stewart P. S., Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34–40 [DOI] [PubMed] [Google Scholar]
- 5. Ito A., Taniuchi A., May T., Kawata K., Okabe S. 2009. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 75:4093–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi L., et al. 2007. Limits of propidium iodide as a cell viability indicator for environmental bacteria. Cytometry A 71:592–598 [DOI] [PubMed] [Google Scholar]
- 7. Stocks S. M. 2004. Mechanism and use of the commercially available viability stain, BacLight. Cytometry A 61:189–195 [DOI] [PubMed] [Google Scholar]
- 8. Werner E., et al. 2004. Stratified growth in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 70:6188–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]