Abstract
This study demonstrated, for the first time, that immunity genes licFGEHI are not essential for self-protection and production of the two-component lantibiotic lichenicidin in the Gram-negative heterologous host Escherichia coli BLic5. Additionally, it was experimentally demonstrated that lichenicidin lantibiotics are active against the E. coli imp4213 strain, a mutant strain possessing a permeable outer membrane.
TEXT
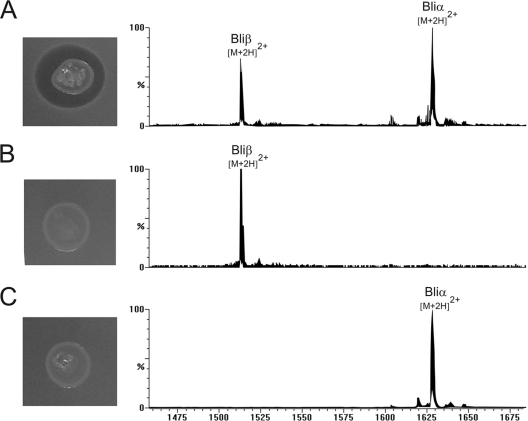
Lichenicidin is a two-component lantibiotic consisting of the peptides Bliα and Bliβ, produced by Bacillus licheniformis, with activity against several clinical important Gram-positive strains such as methicillin-resistant Staphylococcus aureus (MRSA). All the machinery involved in the expression, modification, transport, and regulation of the two peptides Bliα and Bliβ are encoded in 14 contiguous open reading frames (ORFs; lic gene cluster) on the B. licheniformis chromosome. Recently, the lic gene cluster was cloned and successfully expressed in Escherichia coli (BLic5 strain). This constituted the first report of fully active lantibiotic production totally in vivo by a Gram-negative host (3). This expression system was further exploited to investigate the role of all the ORFs of the lic gene cluster through the deletion of each of the genes, except those putatively involved in immunity. Lantibiotic self-protection mechanisms of the producer strain usually involve an individual immunity protein (LanI) and/or an ABC transporter, usually composed of two or three subunits (LanFE[G]). Also, an ancillary protein for the assembly of a functioning ABC transporter has been described (LanH) (5). These five proteins are putatively represented in the lic gene cluster by the licFGEHI genes (9, 13). Herein, the role of the five ORFs in lichenicidin self-protection and production by E. coli BLic5 was investigated. Lichenicidin peptides interact synergistically at a ratio of 1:1 within the nanomolar concentration range to exert their full activity (11). Individually, the action of each peptide requires much higher concentrations (11). Thus, the replacement of the licFGEHI ORFs by an apramycin resistance cassette was performed in the pLic5 fosmid (containing the complete lichenicidin gene cluster) in which either licA1 or licA2 structural genes were deleted (pLic5ΔA1 and pLic5ΔA2, respectively) to reduce the possibility of bacterial self-killing by the lichenicidin peptides. For this purpose, the λ Red redirect system was used as previously described by Caetano et al. (3). The constructed fosmids pLic5ΔA1ΔFGEHI and pLic5ΔA2ΔFGEHI conferring resistance to chloramphenicol (12.5 μg/ml) and apramycin (50 μg/ml) were transformed into chemically competent E. coli BL21-Gold (DE3) (Agilent). The obtained strains, E. coli BLic5ΔA1ΔFGEHI and E. coli BLic5ΔA2ΔFGEHI (Table 1), were characterized by the absence of bioactivity against the indicator strain Micrococcus luteus ATCC 9341 (Fig. 1). These mutants were subjected to cross-feeding agar diffusion experiments, either with Bliα (through the strain BLic5ΔA2) or Bliβ (through the strain BLic5ΔA1) as previously described by Caetano et al. (3). Bioactivity was completely restored, confirming that E. coli BLic5ΔA1ΔFGEHI retained the capacity to produce Bliβ and that E. coli BLic5ΔA2ΔFGEHI retained the capacity to produce Bliα (Fig. 2). Likewise, such results were further confirmed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) analysis of butanol extracts (Fig. 1) as described in Caetano et al. (3). In order to understand if survival of these mutants retaining biosynthetic capabilities for one of the two peptides was due to the absence of the complementary peptide, Bliα or Bliβ, deletion of licFGEHI genes was performed in the pLic5 fosmid possessing both the licA1 and licA2 structural genes. The resulting plasmid, pLic5ΔFGEHI, was constructed and transformed in E. coli as described above. Remarkably, E. coli BLic5ΔFGEHI mutants producing both the Bliα and Bliβ peptides were obtained, as confirmed by bioassay (Fig. 1) and LC-ESI-MS analysis (Fig. 1). The relative abundance of both peptides was further investigated by LC-ESI-MS analysis of butanol extracts of three independent cultures of E. coli BLic5 and BLic5ΔFGEHI after 24 h of growth. The values obtained from the TIC (total ion current) peak integration of each peptide were generally higher for BLic5 cultures (Bliα, 585.7 ± 64.0 arbitrary units [AU]; Bliβ, 236.0 ± 16.6 AU) than for BLic5ΔFGEHI cultures (Bliα, 400.7 ± 169.5 AU; Bliβ, 125.7 ± 41.8 AU). These results demonstrate undoubtedly that E. coli is able to produce both the Bliα and Bliβ peptides, even if the immunity genes licFGEHI are not present. Nevertheless, it is not clear if such genetic modification can negatively affect BLic5ΔFGEHI bacterial fitness and consequently the lichenicidin production. The susceptibility of the BLic5 and BLic5ΔFGEHI strains to lichenicidin was also investigated. The agar plates containing each of these strains were prepared as done previously for those used for the bioassays of M. luteus (3). The lichenicidin sample was obtained from butanol extraction of a BLic5 culture grown for 24 h. The pellet obtained after butanol evaporation was dissolved in aqueous acetonitrile (70%) solution and used in the bioactivity test. After an overnight incubation at 37°C, no inhibition zones were observed on BLic5 and BLic5ΔFGEHI strain plates, suggesting again that licFGEHI are not essential for the lichenicidin self-protection mechanism in Gram-negative E. coli.
Table 1.
List of E. coli strains used in the present study
| Strain | Fosmid | Observation(s)a |
|---|---|---|
| BLic5 | pLic5 | E. coli BL21-Gold (DE3) cells transformed with the pLic5 fosmid containing the complete lichenicidin gene cluster; production of Bliα and Bliβ |
| BLic5ΔA1 | pLic5ΔA1 | Deletion of licA1 gene in the pLic5 fosmid; single production of Bliβ |
| BLic5ΔA2 | pLic5ΔA2 | Deletion of licA2 gene in the pLic5 fosmid; single production of Bliα |
| BLic5ΔFGEHI | pLic5ΔFGEHI | Replacement of licFGEHI genes in the pLic5 fosmid by an Aprar cassette; production of Bliα and Bliβ |
| BLic5ΔA1ΔFGEHI | pLic5ΔA1ΔFGEHI | Replacement of licFGEHI genes in the pLic5ΔA1 fosmid by an Aprar cassette; single production of Bliβ |
| BLic5ΔA2ΔFGEHI | pLic5ΔA2ΔFGEHI | Replacement of licFGEHI genes in the pLic5ΔA2 fosmid by an Aprar cassette; single production of Bliα |
| BWLic5ΔFGEHI | pLic5ΔFGEHI | E. coli BW25113 cells transformed with the pLic5ΔFGEHI fosmid |
| BWΔtolCLic5ΔFGEHI | pLic5ΔFGEHI | E. coli BW25113ΔtolC::kan cells transformed with the pLic5ΔFGEHI fosmid |
Aprar, apramycin resistance.
Fig. 1.
Bioassay of E. coli mutants BLic5ΔFGEHI (A), BLic5ΔA1ΔFGEHI (B), and BLic5ΔA2ΔFGEHI (C) against the M. luteus indicator strain. LC-ESI-MS analyses of butanol extracts from the respective mutant liquid culture are also shown.
Fig. 2.
Cross-feeding agar diffusion assay of the E. coli BLic5ΔA1ΔFGEHI and BLic5ΔA2ΔFGEHI mutants with the Bliα (BLic5ΔA2) and Bliβ (BLic5ΔA1) peptides. The bacterial activity restored between BLic5ΔA1 and BLic5ΔA2ΔFGEHI suggested that this mutant was able to produce the Bli〈 peptide. Similarly, the M. luteus inhibition zone observed between BLic5ΔA2 and BLic5ΔA1ΔFGEHI suggested that this mutant was able to produce the Bliβ peptide.
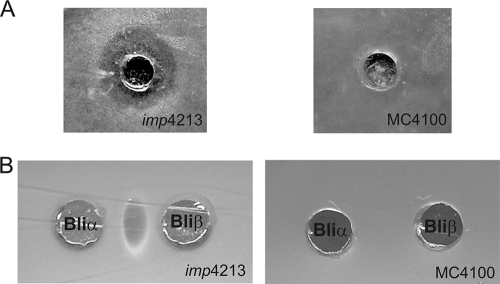
In analogy with the mechanism of action of haloduracin and lacticin 3147 reported previously (8, 14), it is believed that lichenicidin exerts its activity by inhibiting the cell wall biosynthesis accompanied by pore formation in the cytoplasmic membrane. Lantibiotics such as nisin are generally not active against Gram-negative bacteria. It has been shown that this is due to the presence of the outer membrane (OM), since the alteration of its permeability by chelators or some physical treatments resulted in nisin sensitivity by several Gram negatives (1, 6, 12). Likewise, an E. coli strain with a permeable OM should be sensitive to the extracellular presence of a mixture of Bliα and Bliβ peptides. To prove this hypothesis, a culture of E. coli BLic5 in medium M (3) was performed, and its cell-free supernatant (containing both Bliα and Bliβ) was bioassayed against the E. coli imp4213 strain and also its wild-type strain E. coli MC4100. The E. coli imp4213 strain possesses a mutation on the essential lptD (imp) gene, which causes an OM permeability defect (2) and thus sensitivity to a variety of antibiotics, including vancomycin, which is otherwise not active against Gram-negative strains (10, 15). An inhibition zone caused by the BLic5 supernatant was observed on the agar plates containing the imp4213 strain, contrary to what was registered in the plates inoculated with the wild-type MC4100 strain, where no inhibition of growth was observed (Fig. 3). The activity of the individual peptides on these two strains was also examined. To that end, E. coli BLic5ΔA1 (producing exclusively Bliβ) and E. coli BLic5ΔA2 (producing exclusively Bliα) cell-free supernatants were bioassayed side by side on plates containing either the E. coli imp4213 strain or the E. coli MC4100 strain. It was observed that E. coli imp4213 strain growth was affected only if both Bliα and Bliβ peptides were present (Fig. 3). These findings proved that in fact, similarly to nisin, once both the Bliα and Bliβ peptides reach the E. coli periplasmic space, they could exert their activity. It was recently demonstrated that the TolC OM protein is related with the presence of the active lichenicidin peptides in the E. coli BLic5 supernatant (3). However, it was not determined whether their transport should involve a periplasmic sublocation. Together, our results suggest that either the peptides are exported through a type I system or at least one of the lichenicidin peptides needs to be inactive in the periplasmic space. In this case, the cooccurrence of Bliα and untrimmed Bliβ′ can be considered, where Bliβ′ would require extracellular processing by the LicP protease to achieve its Bliβ active form (3). The fact that it was possible to transform a tolC-deficient E. coli BW25113 with the pLic5ΔFGEHI fosmid (BWΔtolCLic5ΔFGEHI strain) (Table 1) also supports this hypothesis. After the bioassay, BWΔtolCLic5ΔFGEHI was not able to inhibit M. luteus growth, in contrast with the control strain (BWLic5ΔFGEHI).
Fig. 3.
Bioassay of lichenicidin peptides against the E. coli imp4213 and MC4100 strains. (A) The E. coli imp4213 strain is susceptible to E. coli BLic5 cell-free supernatant, containing both the Bliα and Bliβ peptides. (B) E. coli imp4213 strain growth is inhibited only if both peptides are present. The extracts of the BLicΔA1 and BLicΔA2 strains include only Bliβ or Bli〈, respectively.
Currently, little information is available concerning the lantibiotics' immunity systems. Nevertheless, for lacticin 3147, it was demonstrated that LtnI is an essential protein for the Lactococcus lactis immune phenotype most probably by preventing the insertion of the bacteriocins in the membrane or by interacting directly with the peptides, inactivating them (7). More recently, it was shown that both components of the ABC transporter, LtnFE, are also involved in lacticin 3147 self-protection. In this case, LtnFE should be involved in the transport of Ltnα and Ltnβ peptides that have crossed the cell wall of the Gram-positive producer (4). The application of such models to the E. coli lichenicidin expression system could help to explain the results herein reported. If Bliα and Bliβ are not exported simultaneously through the periplasmic space and once outside the cell, the peptides are unable to enter the cell, then at least the LicFEI proteins would be dispensable in such a system. Therefore, BLic5 and BLic5ΔFGEHI lichenicidin self-protection is more likely due to the Gram-negative cell wall structure rather than to the expression of licFGEHI immunity genes.
Acknowledgments
Tânia Caetano was supported by a Fundação para a Ciência e Tecnologia and Medinfar Pharmaceuticals SA grant (SFRH/BDE/15559/2005). The work was supported by the Centre for Environmental and Marine Studies (CESAM) of the University of Aveiro and the Cluster of Excellence “Unifying Concepts in Catalysis” granted by the DFG, coordinated by TU Berlin.
We acknowledge José C. Duarte for supplying the B. licheniformis I89 strain, Thomas J. Silhavy from Princeton University for supplying the E. coli MC4100 and E. coli imp4213 strains, and Klaus Hantke from the University of Tübingen for supplying the E. coli BW25113 and BW25113ΔtolC::kan strains.
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Boziaris I. S., Adams M. R. 2000. Transient sensitivity to nisin in cold-shocked Gram negatives. Lett. Appl. Microbiol. 31:233–237 [DOI] [PubMed] [Google Scholar]
- 2. Braun M., Silhavy T. J. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol. Microbiol. 45:1289–1302 [DOI] [PubMed] [Google Scholar]
- 3. Caetano T., Krawczyk J. M., Mösker E., Süssmuth R. D., Mendo S. 2011. Heterologous expression, biosynthesis and mutagenesis of type II lantibiotics from Bacillus licheniformis in Escherichia coli. Chem. Biol. 18:90–100 [DOI] [PubMed] [Google Scholar]
- 4. Draper L. A., et al. 2009. Cross-immunity and immune mimicry as mechanisms of resistance to the lantibiotic lacticin 3147. Mol. Microbiol. 71:1043–1054 [DOI] [PubMed] [Google Scholar]
- 5. Draper L. A., Ross P., Hill C., Cotter P. D. 2008. Lantibiotic immunity. Curr. Protein Pept. Sci. 9:39–49 [DOI] [PubMed] [Google Scholar]
- 6. Kalchayanand N., Hanlin M. B., Ray B. 1992. Sublethal injury makes Gram-negative and resistant Gram-positive bacteria sensitive to the bacteriocins, pediocin AcH and nisin. Lett. Appl. Microbiol. 15:239–243 [Google Scholar]
- 7. McAuliffe O., Hill C., Ross R. P. 2000. Identification and overexpression of ltnI, a novel gene which confers immunity to the two-component lantibiotic lacticin 3147. Microbiology 146:129–138 [DOI] [PubMed] [Google Scholar]
- 8. Oman T. J., van der Donk W. A. 2009. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem. Biol. 4:865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rey M., et al. 2004. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol. 5:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruiz N., Falcone B., Kahne D., Silhavy T. J. 2005. Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121:307–317 [DOI] [PubMed] [Google Scholar]
- 11. Shenkarev Z. O., et al. 2010. Isolation, structure elucidation, and synergistic antibacterial activity of a novel two-component lantibiotic lichenicidin from Bacillus licheniformis VK21. Biochemistry 49:6462–6472 [DOI] [PubMed] [Google Scholar]
- 12. Stevens K. A., Sheldon B. W., Klapes N. A., Klaenhammer T. R. 1991. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl. Environ. Microbiol. 57:3613–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veith B., et al. 2004. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J. Mol. Microbiol. Biotechnol. 7:204–211 [DOI] [PubMed] [Google Scholar]
- 14. Wiedemann I., et al. 2006. The mode of action of the lantibiotic lacticin 3147—a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61:285–296 [DOI] [PubMed] [Google Scholar]
- 15. Wu T., et al. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235–245 [DOI] [PubMed] [Google Scholar]