Abstract
The processes responsible for producing and maintaining the diversity of natural arbuscular mycorrhizal (AM) fungal communities remain largely unknown. We used natural CO2 springs (mofettes), which create hypoxic soil environments, to determine whether a long-term, directional, abiotic selection pressure could change AM fungal community structure and drive the selection of particular AM fungal phylotypes. We explored whether those phylotypes that appear exclusively in hypoxic soils are local specialists or widespread generalists able to tolerate a range of soil conditions. AM fungal community composition was characterized by cloning, restriction fragment length polymorphism typing, and the sequencing of small subunit rRNA genes from roots of four plant species growing at high (hypoxic) and low (control) geological CO2 exposure. We found significant levels of AM fungal community turnover (β diversity) between soil types and the numerical dominance of two AM fungal phylotypes in hypoxic soils. Our results strongly suggest that direct environmental selection acting on AM fungi is a major factor regulating AM fungal communities and their phylogeographic patterns. Consequently, some AM fungi are more strongly associated with local variations in the soil environment than with their host plant's distribution.
INTRODUCTION
A central aim in ecology is to quantify the mechanisms that regulate the diversity of natural communities. However, most research examining species abundance patterns and the underlying mechanisms controlling diversity has focused on conspicuous macroorganisms and largely overlooked microbial communities. This bias is of fundamental concern given the important roles many microbes play in terrestrial ecosystem functions, such as nutrient cycling, and in the interactions that affect plant community dynamics. The arbuscular mycorrhizal (AM) fungi are one such functionally important microbial group with poorly understood community ecology. AM fungi are obligatory biotrophic plant root endosymbionts, a ubiquitous functional group in soils, and are estimated to colonize around two-thirds of plant species (9). In return for photosynthates, AM fungi provide host plants with enhanced phosphorus and nitrogen uptake (38), highlighting the central role AM fungi play in terrestrial ecosystem processes (32). Although AM fungi are clearly an important component of terrestrial ecosystems, the mechanisms that control the composition and diversity of their communities are mostly unknown.
Molecular studies have revealed high taxon richness of AM fungi in natural systems (5, 43) but have focused mainly on relating the diversity of AM fungi in planta to host plant species, and the effect of other environmental factors on AM fungal communities has been neglected (15, 44). The debate about AM fungal diversity therefore has centered on whether host diversity drives AM fungal diversity or vice versa (17, 45), rather than the ecological and environmental niches of AM fungi. However, AM fungi produce an extensive extraradical mycelial network in soil and therefore will be subject to strong selection pressures from the abiotic soil environment. Recent studies suggesting that dry, waterlogged, or disturbed environments have distinct AM fungal communities provide evidence of environmental selection differentiating these communities (7, 13, 16, 18, 19, 51).
It is challenging to study the effect of the soil environment on natural microbial communities in situ due to the heterogeneous and dynamic nature of soil ecosystems. We have identified natural CO2 springs (mofettes) as systems where a severe and relatively constant selection pressure is maintained across a small spatial scale. In mofettes, CO2 of geological origin reaches the soil surface, and this has been widely used to research the impacts of long-term elevated CO2 on ecosystems, with the main focus being on plant responses to an environment enriched with atmospheric CO2 (3, 29, 30). However, atmospheric CO2 concentrations may show considerable variation, and the more consistent environmental impact is the displacement of the soil atmosphere by CO2, which leads to localized hypoxia. At the Stavešinci mofette (northeast Slovenia), we used soil air CO2 concentrations as a more stable and precise measure to determine the exposure of plant and other biota to different concentrations of geological CO2 (21, 22, 29, 46, 48). The soil gas regime at the Stavešinci mofette has been very well described, both spatially and temporally (47, 49), and additional variables have been extensively documented, including soil chemistry, temperature, and water content (49), along with plant responses such as photosynthesis (28, 48) and root respiration (21).
The primary impact of geological CO2 in a mofette is on the soil, but the consequences of these impacts on below-ground processes within mofette areas remain sparsely researched (21). The raised concentration of soil CO2 reduces O2 availability, leading to hypoxia (49), but the only study focusing on the biodiversity of soil microbes from mofette sites is on the diversity of CO2-fixing bacteria in grassland soils from the Stavešinci mofette (46). Rillig et al (31) reported a linear correlation between atmospheric CO2 concentration and AM fungal root colonization, soil hyphal length, and glomalin concentration in a mofette, but they did not examine AM fungal community composition. This omission reflects the lack of research on AM fungal diversity in extreme natural environments, which currently is limited to studies on geothermal (1) and serpentine soils (34). Quantifying the response of natural communities to extreme environmental variation will aid robust predictions about the impacts of environmental change on biodiversity.
The objectives of the present study were (i) to quantify AM fungal colonization in roots of several plant species in the extreme environment of the Stavešinci mofette; (ii) to analyze AM fungal communities in roots exposed to extreme soil CO2 concentrations within this ecosystem; (iii) to determine whether long-term abiotic selective pressures could drive the evolution of a unique AM fungal community; and (iv) to explore whether these phylotypes are exclusive to soils with extremely high geological CO2 concentrations or merely widespread generalists that can tolerate hypoxic conditions.
MATERIALS AND METHODS
Site description and plant sampling.
The study was conducted at Stavešinci, northeast Slovenia (47) (for a detailed site description, see Fig. S4 in the supplemental material). The site is a flat, postagricultural area where very pure CO2 of ambient temperature, without traces of sulfur compounds, CH4, or CO, is released into the atmosphere via several vents (48). The existing vegetation at the study area consists of C3 and C4 grasses, grassland herb species, and some ruderals (28, 48). Because atmospheric CO2 concentrations vary, depending on weather and wind conditions, from ambient to at least 1% at 0.5 m above ground (47), soil CO2 concentration and CO2 efflux are better estimates of plant exposure to geological CO2.
Plants were sampled from June to August 2007 around two separate CO2 springs (referred to as mofette 1 and mofette 2) ca. 40 m apart. An area of ∼100 m2 was defined at each mofette, which allowed sampling at high and control CO2 concentrations from the center of the mofette up to the control sites in the surrounding soil (see Fig. S4 in the supplemental material). To select precise locations of plants exposed to high and control geological CO2 concentrations within each of the selected locations, soil CO2 flux was measured using an LI-6400-09 soil CO2 flux chamber (Licor, Lincoln, NE) (49). Fluxes were measured at least 2 h before sampling in the immediate vicinity of each plant sampled. Plants sampled within mofette 1 were exposed to extremely high CO2 concentrations (efflux level, >400 μmol CO2 m−2 s−1); efflux levels in mofette 2 were lower (228.0 ± 50.4 μmol CO2 m−2 s−1). These two areas are defined as high CO2. The efflux levels in control (low-concentration) CO2 soils, distant from the vents, were <16.8 ± 4.6 μmol CO2 m−2 s−1. Soil CO2 concentration is positively correlated with CO2 flux (46) and negatively correlated with soil O2 concentration (49), and CO2 concentrations higher than 50% and up to 99.9% can be measured at a soil depth of 20 cm (29, 47), where soil CO2 flux is high (49). Thus, locally hypoxic or even anoxic soil conditions are found at locations that are highly enriched in geological CO2, as is the case for mofettes 1 and 2.
To ensure sufficient replicates (individual plants), both at high and control soil CO2 concentrations, only the most abundant plant species were sampled, where at least three individual plants could be located in both the mofette and the surrounding control soil. For mofette 1, these included Setaria pumila (Pior.) Roem & Schult, Echinochloa crus-galli (L.) PB, Juncus effusus L., and Solidago gigantea Aiton. For mofette 2, these included Dactylis glomerata L., Poa pratensis L., Phleum pratense L., and Plantago lanceolata L. (Plants are herein referred to by their generic names.) Since the locations with extremely high soil CO2 concentrations are limited, individual plants were sampled within a distance of 1 to 6 m. All plants were sampled during their flowering period and at the same time for high and control CO2 concentrations for each species. Roots were separated from the soil within 2 days of sampling. Half of the root material was dried at 40°C and stored in plastic bags for molecular analyses, and the other half was stored in 80% ethanol for the assessment of AM fungal root colonization. Plant shoots were sampled simultaneously with roots, dried at 80°C, and stored for further analyses of mineral nutrient content (see Fig. S1 in the supplemental material). For mofette 1, soil samples were taken from three sampling points for high CO2 exposure and three from the surrounding low-CO2 soil (see Table S2 in the supplemental material). For mofette 2, data on the soil chemistry for the same sampling points as those used in our study (at a maximum distance of 15 cm) were available from one of our recent studies (46) (see Table S2).
AM fungal colonization.
Roots from three individual plants for each plant species, sampled from high- and low-CO2 areas, were cleared with hot 10% KOH and acidified with 1N HCl. The fungal tissue inside roots was stained with 0.05% trypan blue in lactoglycerol. The AM fungal root colonization was evaluated by the grid line intersection method (11). In addition, for Setaria plants that were exposed to the highest level of geological CO2 during growth, a more detailed estimation of AM fungal structures (abundance of arbuscules) in roots was assessed by following the methods of Trouvelot et al. (42) using an Olympus Provis AX70 microscope.
Molecular analyses.
To target the active AM fungal community in planta, DNA was extracted from 50 mg of root sample from three individual plants per species from high-CO2 and control CO2 sites using a PowerPlant DNA isolation kit (Mo Bio) according to the manufacturer's instructions. Partial small subunit (SSU) ribosomal RNA gene fragments (ca. 550 bp) were amplified using Taq DNA polymerase with the universal eukaryotic primer NS31 (37) and the AM fungal primer AM1 (12). The NS31/AM1 primer pair amplifies a consistent subset of the dominant AM fungal taxa, but it does exclude some taxa from certain rarer groups (7), notably the Glomus group B and Archaeospora. Other primer combinations and multiple primer sets may increase taxonomic coverage, but these methods generally require nested PCR (1, 2), making them unsuitable for the quantitative analysis of communities. Subsequently, as our main aim is to quantitatively examine the turnover of AM fungal taxa between high-CO2 and control CO2 soils, we chose to focus on a specific subset of taxa rather than conduct large-scale species inventorying.
PCRs for every DNA extract were carried out in the presence of 0.2 mM deoxynucleoside triphosphates (dNTPs) (Invitrogen), 20 pmol of each primer, and the manufacturer's reaction buffer in 25-μl reaction mixtures (PCR conditions were the following: 95°C for 2 min; 10 cycles at 95°C for 1 min, 58°C for 1 min, and 72°C for 2 min; 19 cycles at 95°C for 30 s, 58°C for 1 min, and 72°C for 3 min; and 1 cycle at 95°C for 30 s, 58°C for 1 min, and 72°C for 10 min) on a Gradient96 Robocycler (Stratagene). PCR amplicons were gel extracted and purified using the QIAquick gel extraction kit (Qiagen) by following the manufacturer's instructions. Purified PCR products then were cloned into pGEM-T Easy (Promega) and transformed into Escherichia coli (DH5α) (Invitrogen). Clone libraries were constructed for each of the six individual plants of Setaria sampled. However, from the other plants (Dactylis, Plantago, and Poa), clone libraries were constructed from pooled PCR products separately for each plant species and CO2 exposure. Putative positive transformants were screened using standard SP6-T7 amplification. For the initial clone screening, 162 clone library colonies each from high-CO2 and low-CO2 locations were picked for Setaria, and 54 colonies each from high- and low-CO2 locations were picked for each of the other three plant species. Thus, a total of 648 clones were screened across both mofette sites. All positive clones were restriction digested using HinfI (Fermentas), Hsp92II, and RsaI (Promega) enzymes by following the manufacturer's instructions. PCR products of 131 clones screened from Setaria and 279 clones from the other plant species were amplified using standard SP6-T7 amplification, purified using the Qiaquick (Qiagen) purification kit, and sequenced (Macrogen Inc.) (sequencing was performed using BigDye terminator cycling conditions and run using an ABI3730xl automatic sequencer).
Data analyses.
SSU rRNA gene sequence chromatograms were checked using Chromas. ClustalX (41) was used for multiple alignments and the calculation of neighbor-joining phylogeny (33), using Geosiphon pyriformis (10) as a specific outgroup to the AM fungi and Corallochytrium limacisporum, a choanozoan, as a general outgroup to all fungi (43). Phylogenetic support was calculated using nonparametric bootstrapping (8) with 10,000 pseudoreplicates. Clone numbers were used to calculate the abundance of each distinct phylotype. We considered sequences with pairwise similarities of <97% to be separate phylotypes.
The true replication of mofette sites, i.e., sites with the same plant community and soil conditions that differ only in CO2 flux, is not possible, due to the rarity of comparable vents at each location and the environmental variation within and among sites. Thus, comparisons of the mofette rhizosphere communities of high-CO2 and control CO2 sites need to be based on examining a null hypothesis that the observed difference between communities could not have happened by chance given the composition of the local species pool. To test this null hypothesis statistically, we measured changes in α and β diversity between high-CO2 and control CO2 sites. First we calculated three indices of α diversity—Margalef's index (species richness), Shannon-Wiener's index (diversity), and the complement of Simpson's index (1 − D; species evenness)—and calculated the complement of Morisita-Horn's similarity index to examine β diversity, such that an increase in the index value corresponds to an increase in β diversity. Diversity indices were calculated using clone numbers as a proxy for abundance (14, 25) and following the methods of Magurran (23). We used bootstrapping methods to calculate confidence intervals for each index (39). Observed values of α and β diversity were then compared to values derived from a null model, which randomly partitioned the combined species abundance matrices of the high-CO2 and control CO2 sites into two artificial communities (40). Differences in α diversity and β diversity between the two artificial communities were recorded and compared to the original observed difference between high-CO2 and control CO2 sites; this process was repeated 10,000 times, and P values were computed based on the number of times that the difference between the artificial communities was greater than or equal to that observed (40). This method statistically tests whether, given the local species pool, the observed difference between sites could happen by chance, and it is commonly employed in studies where pseudoreplicates are not independent and true replication is logistically difficult (6). In our case, all the plants were sampled close to the CO2 vents, and because they all were sampled within a small spatial scale they were not spatially independent samples. Thus, all standard statistical approaches run the risk of increased type 1 statistical errors associated with spatial autocorrelation within the species abundance data. The randomization approach used treats the entire community as a single data set (reducing errors associated with spatial autocorrelation), and it is an absolute statistical measure that does not require replication to produce probabilities.
Analysis of α diversity was conducted using the computer program PISCES, and randomization tests for β diversity were coded in C++. Rarefied phylotype accumulation curves were calculated using rarefaction to check for differences in sampling intensity between sites, and analysis of covariance (ANCOVA) was used to test that rates of accumulation were not significantly different between sites. Student's t test was used to compare the means of soil chemistry data between high-CO2 and control CO2 soils and root colonization from different plant species (Statgraphic Plus for Windows 5.1).
Nucleotide sequence accession numbers.
All new sequences have been submitted to the European Molecular Biology Laboratory (EMBL) nucleotide sequence database (accession numbers from FN869884 to FN869903).
RESULTS
AM fungal root colonization and chemical analysis of soil and plant samples.
Root colonization was significantly lower (P ≤ 0.003) in the high-CO2 mofette soils than in control soils in two plant species from mofette 1 (Setaria and Echinochloa) and two plant species from mofette 2 (Plantago and Poa) (Fig. 1). Echinochloa exhibited no signs of AM fungal colonization in high-CO2 sites, whereas Setaria plants still were highly mycorrhizal at extreme CO2 exposure, with 56% ± 6% of root length colonized (RLC). In mofette 1, neither Juncus nor Solidago was mycorrhizal, and consequently Setaria was the only mycorrhizal plant species at high CO2 exposure. Although hyphal root colonization decreased significantly in Setaria (P = 0.003) (Fig. 1), colonization by arbuscules decreased but remained high, with 33% ± 5% and 76% ± 5% of the root cortex colonized in high-CO2 and control CO2 soils, respectively, implying a physiologically active symbiosis in this species. In contrast, in mofette 2, while Poa and Plantago had lower RLCs than control plants, both Dactylis and Phleum had similar RLCs irrespective of soil CO2 concentration, and all four species sampled remained mycorrhizal at high CO2.
Fig. 1.
Root colonization with AM fungi, measured in eight plant species, growing in the Stavešinci mofette area and exposed to high and control concentrations of geological CO2. Dark columns indicate high-CO2 samples, and light columns indicate control samples. NM, nonmycorrhizal. Averages ± standard errors are shown (n = 3). Significant differences between high-CO2 and control samples are indicated above columns (***, P = 0.001; **, P = 0.01; *, P = 0.05).
Phosphorus, nitrogen, and potassium concentrations in leaves (data and statistical tests are presented in Fig. S1 in the supplemental material) generally were higher in the control samples than in those taken from high-CO2 sites in mofette 1 but not mofette 2. Soil chemical characteristics (see Table S2 in the supplemental material) also differed between sites of low and high soil CO2 concentrations, with soil pH lower and available soil P higher at high CO2 concentrations, especially in mofette 1.
Phylogenetic relationships and community structure of AM fungi growing in high CO2 and control CO2 conditions.
In mofette 1 high-CO2 samples, DNA amplification was successful only from Setaria roots, the only plant species colonized by AM fungi in both the high-CO2 and control sites (Fig. 1), whereas three of the four plant species sampled from mofette 2 (Dactylis, Poa, and Plantago) yielded AM fungus-specific PCR products. Those four species therefore were used in further analyses. A total of 231 clones screened positive for AM fungal DNA when sampled from Setaria roots, with equal numbers from high-CO2 (116 clones) and control (115 clones) soils. Combining the three species, Dactylis, Poa, and Plantago, a total of 172 clones from both high-CO2 (89 clones) and control (83 clones) soils from mofette 2 screened positive for AM fungal DNA. Overall, 20 distinct ribosomal DNA phylotypes (sequences grouped by a dissimilarity of 3% or less using BLAST2 algorithm) were detected (Fig. 2; also see Table S3 in the supplemental material).
Fig. 2.
Neighbor-joining phylogenetic tree showing the AM fungal taxa (phylotypes) from plant roots sampled in this study. Sequences from this study are shown in boldface, and named reference sequences and closely related phylotypes from environmental samples are displayed, with accession numbers, in gray. Named sequences are from Schüßler et al (36), and closely related phylotypes from environmental samples represent the closest matches to those recorded in this study compared to the NCBI database using the BLAST algorithm. The phylotypes identify sequences with a maximum 3% sequence dissimilarity. Bootstrap values of ≥75% (10,000 replicates) are shown above the branches and before the node to which they correspond. The relative abundances of each phylotype found in this study are shown in Fig. 3 and Table S3 in the supplemental material. All of the new sequences have been submitted to the European Molecular Biology Laboratory (EMBL) nucleotide sequence database (accession numbers FN869884 to FN869903).
Glo86 was the most widespread of the phylotypes; it was found in all plant species investigated from both mofettes and was exclusive to high-CO2 sites (Fig. 2 and 3; also see Table S3 in the supplemental material). The second most abundant phylotype in the high-CO2 locations was Glo85, which, unlike Glo86, was found only in Setaria and Plantago but also was exclusive to high-CO2 sites. The dominant phylotypes in control CO2 sites were Glo8 and Glo84. Glo8 is a widespread type (24, 26), found in all four plant species in our survey, with a higher abundance in control CO2 sites. Glo84 was most abundant in Setaria but also appeared occasionally in Dactylis and Plantago in high-CO2 sites. Phylotypes Glo80, Glo22, Glo2, and Glo82 appeared exclusively in Setaria roots, and phylotype Glo81 appeared exclusively in Plantago roots. Rare phylotypes, found only once in this study, were Glo80 and Glo82. In common with many published studies (see Table S3 in the supplemental material), the phylotypes belong to Glomus group A. This could be due in part to the higher specificity of the NS31/AM1 primer pair for this group. We cannot rule out the presence of other, more abundant phylotypes, like Archaeospora and Glomus group B (1, 2), which are not amplified by AM1, but changes among samples in their relative abundance of this subset of AM fungal taxa nonetheless is a good estimator of overall β diversity.
Fig. 3.
Rank abundance plots for (a) plant species Setaria pumila, Dactylis glomerata, Poa pratensis, and Plantago lanceolata, (b) high-CO2 and control samples combined for both mofettes, (c) high-CO2 and control samples for mofette 1 (Setaria pumila), and (d) high-CO2 and control samples for mofette 2 (Dactylis glomerata, Poa pratensis, and Plantago lanceolata). The y axis indicates the number of clones assigned to each phylotype. The phylotypes with zero abundance indicate AM fungal taxa absent from each mofette site.
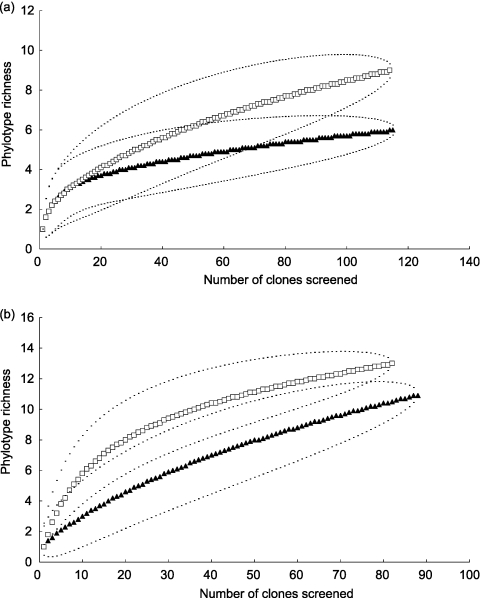
There was no significant difference between the α diversities of the AM fungal communities colonizing Setaria roots in high-CO2 and control soils (mofette 1; pairwise randomization tests; Shannon-Wiener index, δ = 0.048, P = 0.71; Simpson's index, δ = 0.063, P = 0.92) or in their phylotype richness (Margalef's index, δ = 0.63, P = 0.21) (Table 1 ). However, the examination of rarefied sequence accumulation curves showed that in mofette 1, the sequence accumulation of AM fungi from Setaria roots from high-CO2 soils was beginning to reach an asymptote, whereas in the surrounding control soils it was still rising (Fig. 4a). Thus, further sampling might have revealed differences between soil types.
Table 1.
Phylotype diversity, evenness, and richness of AM fungal communities sampled from different sets of dataa
| Mofette no. and CO2 concn | Index |
||
|---|---|---|---|
| Shannon-Wiener | Simpson | Margalef | |
| 1 | |||
| High | 1.07 ± 0.15 | 2.30 ± 0.43 | 1.05 ± 0.42 |
| Low | 1.12 ± 0.17 | 2.23 ± 0.45 | 1.69 ± 0.84 |
| 2 | |||
| High | 0.99 ± 0.23 | 1.65 ± 0.42 | 2.23 ± 1.11 |
| Low | 2.09 ± 0.11 | 6.55 ± 1.44 | 2.72 ± 0.91 |
95% confidence intervals are presented. Significant differences (P < 0.0001) are indicated in boldface.
Fig. 4.
Phylotype accumulation curves of AM fungi from (a) mofette 1 Setaria pumila and (b) mofette 2 (Dactylis glomerata, Poa pratensis, and Plantago lanceolata) growing in high-CO2 (dark triangles) and low-CO2 (light squares) soils. Data points show estimated phylotype richness (±95% confidence interval envelopes) using rarefaction.
In contrast to mofette 1, the AM fungal community from high-CO2 soils in mofette 2 was significantly less diverse and less even than in the surrounding control CO2 soils (pairwise randomization tests; Shannon-Wiener index, δ = 1.1, P < 0.0001; Simpson's index, δ = 4.9, P < 0.0001) (Table 1). However, there was no difference in species richness (Margalef's index, δ = 0.49, P = 0.35). The phylotype accumulation of AM fungi still was rising when all clones were screened from plant roots in both high-CO2 and control soils, but there was no significant difference in the rates of phylotype accumulation between soil types (Fig. 4b), as shown by the lack of an interaction between soil type and clone number in an ANCOVA, using the number of screened clones as a covariate (F1,166 = 1.83, P = 0.178). Further data collection therefore would not have quantitatively affected the results.
The examination of β diversity at the two locations revealed high levels of phylotype turnover between AM fungal communities from high-CO2 and control soils (Morisita-Horn's index for mofette 1, 0.99; mofette 2, 0.95). The high phylotype turnover between high-CO2 and control sites was significantly greater than expected by chance given the local phylotype pool (pairwise randomization tests; mofette 1 observed β diversity = 0.99 [P < 0.0001]; mofette 2, 0.95 [P = 0.0005]) and indicated an almost complete change in community phylotype composition and relative abundance between sites. For mofette 1, only about 15% of all AM fungal phylotypes present were shared between high-CO2 and control soils; for mofette 2 the figure was 50% (Fig. 2 and 3; also see Table S3 in the supplemental material).
DISCUSSION
Field-based studies of AM fungi using molecular techniques suggest that the diversity of the phylum Glomeromycota is much greater than the figure of >200 described morphospecies (http://www.lrz.de/∼schuessler/amphylo/) suggests. AM fungal biogeography is still a relatively new area of study, and as yet it is unclear what the main drivers of AM fungal distribution and diversity are at the regional and global levels. An additional complication is that the effect of soil environment often is confounded by a change in the host plant community (13). One way of resolving this is to investigate extreme but localized variations in soils, such as waterlogging (51) and temperature (1). This study highlights the response of AM fungal communities to an extreme, high soil CO2/low O2 gradient at the local scale.
The CO2 gradient imposed on the soil by the mofette is not a transient phenomenon: the measurement of the spatial pattern of CO2 fluxes and concentration at this site have been largely constant for more than 10 years (47, 48, 49). Mofettes are widely assumed to exist for hundreds of years or longer, although few historical records exist to confirm this (27). Despite the prolonged stress and the carbon cost of colonization, all but one plant species remained mycorrhizal at very high soil CO2 concentrations, although several species showed reduced or no colonization at high CO2, and two species (Juncus and Solidago species) were not mycorrhizal in any samples, although all of the sampled species have been reported to be mycorrhizal (50) (Fig. 1). Since the extraradical mycelium of the fungi is likely to be severely restricted in highly hypoxic soils, it is unclear whether the plants benefit from the symbiosis in this environment. A few other studies of AM fungal communities over short, extreme gradients show similar changes, suggesting that this is a general phenomenon. The wetland grass Phragmites australis forms diverse mycorrhizal associations in seasonally flooded zones, but plants are uncolonized at the permanently flooded lakeward front (51), most probably due to permanent hypoxia. The pattern found in this study of high AM fungal community turnover between soil types and numerical dominance by single AM fungal phylotypes in hypoxic soils also have been found in serpentine soils, which are characterized by low nutrient and high heavy metal concentrations (37), and in geothermal soils (1).
The high soil CO2 concentration has had incidental impacts on other soil characteristics in the two mofettes, notably in reducing pH and raising concentrations of available P, both of which are known to affect the structure of AM fungal communities (7). We therefore need to be confident that the observed effects were caused by the primary factor (hypoxia) rather than by these secondary impacts. Importantly, the correlative changes in soil pH and available P were much more marked in mofette 1 than in mofette 2, consequently soil pH and available P are at similar levels in the low-CO2 soil from mofette 1 and the high-CO2 soils from mofette 2 (see Table S2 in the supplemental material). However, despite the similarity in pH and P availability, these two locations have AM fungal communities with different compositions, reflecting differences in soil CO2 levels and not soil pH or available P (Fig. 3; also see Table S3 in the supplemental material). This consistent effect on community structure is good evidence that high CO2 levels and soil hypoxia are the common denominator that is primarily affecting AM fungal community structure. Thus, although it is likely that soil pH and available P are influencing the AM fungal communities recorded in this study (7), these effects are secondary compared with soil hypoxia and are likely to be acting on a distinct hypoxia-tolerant subset of the local AM fungal metacommunity.
Molecular analyses showed that the AM fungal community composition at high CO2 differed from the control population, but most importantly, that the community was not simply a subset of the control. β diversity between sites was significantly higher than values expected under a null model, and the AM fungal community in the high-CO2 locations was dominated by the Glo85 and Glo86 sister phylotypes. These phylotypes were not found in plants growing around the mofettes. The two vents (mofettes 1 and 2) are ca. 40 m apart, and we suggest that these are either phylotypes that are widespread but rare under normal (control CO2) conditions and favored in the high-CO2 soils, or that these are fungi that have adapted to these soils locally and have dispersed among the vents. These types closely match other sequences in GenBank (e.g., accession numbers FJ194512 and FJ194511; see Table S3 in the supplemental material) from other localities (35), but we have no evidence that they are functionally similar. More intensive sampling using, for example, a pyrosequencing approach (4, 5, 20, 25), may reveal these phylotypes to be rare taxa within the wider AM fungal community. The development of robust techniques to study functional traits also are required.
Despite the presence of the same host plant species in both high-CO2 and control conditions, the diversity of the AM community in mofette 1 was reduced in high-CO2 conditions. Rarefaction analysis shows that the high-CO2 community was closer to being asymptotic, suggesting that additional sampling would not have revealed many additional taxa. This was not the case in mofette 2, where the CO2 concentrations were not as extreme, but this result may also be due to the multiple host plants on which this result is based rather than the single mycorrhizal species remaining in mofette 1. The low diversity in the high-CO2 soils (with Shannon-Weiner diversity values of around 1) reflects the dominance of two phylotypes (Glo85 and Glo86) at high CO2 and shows an alteration of community structure in addition to a small reduction of diversity. The likely explanation for this is that the dominant taxa shared by the two mofettes are more effective competitors for the root niche in these extreme conditions. The functional traits that underpin this competitiveness are unknown, but this is additional evidence for functional differentiation among AM taxa that most probably is independent of the host plant (13).
The phylotypes that dominated the high-CO2 soils of the mofette were not present in the surrounding metacommunity, and may be, in this location at least, confined to this unfavorable location. We make the following predictions based on these data. (i) The mofette fungal types are not being subsidized by mycelium in surrounding soil, which would be an obvious explanation of how these aerobic fungi survive in hypoxic or anoxic soils. Even if they are present in the surrounding soils, they are not abundant, as they were not found at all despite relatively intensive sampling. They must therefore be adapted to, or at least competitive in, hypoxic conditions, presumably either tolerating low O2 or acquiring sufficient O2 from the roots; either explanation has profound implications for their biology. It follows, therefore, that some AM fungi are strongly locally adapted to the soil environment. (ii) If there is no dispersal limitation, these phylotypes should occur widely in similar habitats. Both dominant phylotypes are present in the two mofettes, which are separated by 40 m. The evolutionary history of the two dominant taxa cannot be inferred from these data, but it is highly unlikely that the same lineages have evolved independently, so dispersal between the mofettes is the likely explanation. Data from other, more-distant mofette areas will be required to determine the extent of dispersal limitation, if any, although the presence of similar phylotypes in samples from the United States (35) suggests the possibility of long-range dispersal. The fact that the two dominant phylotypes are sister taxa also supports adaptation and local dispersal as an explanation for the pattern observed at this locality. (iii) Finally, these data imply that adaptation to the abiotic environment is an important factor in controlling AM fungal distributions and community structure. We therefore predict that the systematic analysis of AM fungal biogeography will show strong phylogeographic patterns, showing, for part of the community at least, a strong interaction between local adaptation and geography that may be independent of the host plant. Indeed, at a global scale, evidence is emerging that this is the case (26).
If these predictions are tested and found to hold, then this calls into question much of our interpretation of AM fungal community patterns, which currently is dominated by the view that plant and fungal community structures are closely linked. We suggest that community assemblages of AM fungi are the result of powerful selection by and local adaptation to the soil environment. This alone could explain many of the unexplained patterns that we perceive in fungal community structure and could radically alter our view of the assembly rules for this important group of microbes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Slovenian Research Agency (project no. Z4-9295 and J4-2235) and the UK Natural Environment Research Council. Visits of I.M. to the University of York were funded by the British Council and the Royal Society. We gratefully acknowledge all of the given support.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Appoloni S., Lekberg Y., Tercek M. T., Zabinski C. A., Redecker D. 2008. Molecular community analysis of arbuscular mycorrhizal fungi in roots of geothermal soils in Yellowstone national park (U. S. A.). Microb. Ecol. 56:649–659 [DOI] [PubMed] [Google Scholar]
- 2. Baar J., et al. 2011. Molecular analysis of AMF diversity in aquatic macrophytes: A comparison of oligotrophic and utra-oligotrophic lakes. Aquat. Bot. 94:53–61 [Google Scholar]
- 3. Badiani A., Raschi A., Paolacci A. R., Miglietta F. 1999. Plant responses to elevated CO2: a prospective from natural CO2 springs. In Agrawal S. B., Agrawal M. (ed.), Environmental pollution and plant responses. CRC Press LLC, Boca Raton, FL [Google Scholar]
- 4. Buée M., et al. 2009. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 184:449–456 [DOI] [PubMed] [Google Scholar]
- 5. Dumbrell A. J., et al. 2011. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. doi:10.1111/j.1469–8137.2010.03636.x [DOI] [PubMed] [Google Scholar]
- 6. Dumbrell A. J., et al. 2008. Estimated changes in species' diversity following habitat disturbance are dependent on spatial scale: theoretical and empirical evidence. J. Appl. Ecol. 45:1469–1477 [Google Scholar]
- 7. Dumbrell A. J., Nelson M., Helgason T., Dytham C., Fitter A. H. 2010. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 4:337–345 [DOI] [PubMed] [Google Scholar]
- 8. Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- 9. Fitter A. H., Moyersoen B. 1996. Evolutionary trends in root microbe symbioses. Philos. Trans. R. Soc. London Biol. 351:1367–1375 [Google Scholar]
- 10. Gehrig H., Schuessler A., Kluge M. 1996. Geosiphon pyriforme, a fungus forming endocytobiosis with Nostoc (cyanobacteria), is an ancestral member of the Glomales: evidence by SSU rRNA analysis. J. Mol. Evol. 43:71–81 [DOI] [PubMed] [Google Scholar]
- 11. Giovannetti M., Mosse B. 1980. Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84:489–500 [Google Scholar]
- 12. Helgason T., Daniell T. J., Husband R., Fitter A. H., Young J. P. W. 1998. Ploughing up the wood-wide web? Nature 394:4319697763 [DOI] [PubMed] [Google Scholar]
- 13. Helgason T., Fitter A. H. 2009. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (phylum Glomeromycota). J. Exp. Bot. 60:2465–2480 [DOI] [PubMed] [Google Scholar]
- 14. Helgason T., Fitter A. H., Young J. P. W. 1999. Molecular diversity of arbuscular mycorrhizal fungi colonising Hyacinthoides non-scripta (bluebell) in a seminatural woodland. Mol. Ecol. 8:659–666 [Google Scholar]
- 15. Helgason T., et al. 2002. Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J. Ecol. 90:371–384 [Google Scholar]
- 16. Helgason T., Merryweather J. W., Young J. P. W., Fitter A. H. 2007. Specificity and resilience in the arbuscular mycorrhizal fungi of a natural woodland community. J. Ecol. 95:623–630 [Google Scholar]
- 17. Johnson D., et al. 2004. Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol. 161:503–515 [DOI] [PubMed] [Google Scholar]
- 18. Lekberg Y., Koide R. T., Rohr J. R., Aldrich-Wolfe L., Morton J. B. 2007. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J. Ecol. 95:95–105 [Google Scholar]
- 19. Liu Y., He L., An L. Z., Helgason T., Feng H. Y. 2009. Arbuscular mycorrhizal dynamics in a chronosequence of Caragana korshinskii plantations. FEMS Microbiol. Ecol. 67:81–92 [DOI] [PubMed] [Google Scholar]
- 20. Lumini E., Orgiazzi A., Borriello R., Bonfante P., Bianciotto V. 2010. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Env. Microbiol. 12(8):2165–2179 [DOI] [PubMed] [Google Scholar]
- 21. Maček I., Pfanz H., Francetič V., Batič F., Vodnik D. 2005. Root respiration response to high CO2 concentrations in plants from natural CO2 springs. Environ. Exp. Bot. 54:90–99 [Google Scholar]
- 22. Maček I., Videmšek U., Kastelec D., Stopar D., Vodnik D. 2009. Geological CO2 affects microbial respiration rates in Stavešinci mofette soils. Acta Biol. Slovenica 42:41–48 [Google Scholar]
- 23. Magurran A. E. 2004. Measuring biological diversity. Blackwell Publishing, Oxford, United Kingdom [Google Scholar]
- 24. Öpik M., Moora M., Liira J., Zobel M. 2006. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 94:778–790 [Google Scholar]
- 25. Öpik M., Metsis M., Daniell T. J., Zobel M., Moora M. 2009. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 184:434–437 [DOI] [PubMed] [Google Scholar]
- 26. Öpik M., et al. 2010. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 188:223–241 [DOI] [PubMed] [Google Scholar]
- 27. Paelotti M., Pfanz H., Raschi A. 2005. Pros and cons of CO2 springs as experimental sites. In Omasa K., Nouchi I., De Kok L. J. (ed.), Plant response to air pollution and global change. Springer-Verlag, Tokyo, Japan [Google Scholar]
- 28. Pfanz H., et al. 2007. Photosynthetic performance (CO2-compensation point, carboxylation efficiency, and net photosynthesis) of timothy grass (Phleum pratense L.) is affected by elevated carbon dioxide in post-volcanic mofette areas. Environ. Exp. Bot. 61:41–48 [Google Scholar]
- 29. Pfanz H., Vodnik D., Wittmann C., Aschan G., Raschi A. 2004. Plants and geothermal CO2 exhalations. Survival and adaptation to a high CO2 environment, p. 499–538 In Esser K., Lüttge U., Kadereit J. W., Beyschlag W. (ed.), Progress in botany, vol. 65 Springer, New York, NY [Google Scholar]
- 30. Raschi A., Miglietta F., Tognetti R., van Gardingen P. R. 1997. Plant responses to elevated CO2. Evidence from natural CO2 springs. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 31. Rillig M. C., Hernandez G. Y., Newton C. D. 2000. Arbuscular mycorrhizae respond to elevated atmospheric CO2 after long-term exposure: evidence from a CO2 spring in New Zealand supports the resource balance model. Ecol. Lett. 3:475–478 [Google Scholar]
- 32. Rosendahl S. 2008. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol. 178:253–266 [DOI] [PubMed] [Google Scholar]
- 33. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 34. Schechter S. P., Bruns T. D. 2008. Serpentine and non-serpentine ecotypes of Collinsia sparsiflora associate with distinct arbuscular mycorrhizal fungal Assemblages. Mol. Ecol. 17:3198–3210 [DOI] [PubMed] [Google Scholar]
- 35. Schreiner R. P., Mihara K. L. 2009. The diversity of arbuscular mycorrhizal fungi amplified from grapevine roots (Vitis vinifera L.) in Oregon vineyards is seasonally stable and influenced by soil and vine age. Mycologia 101:599–611 [DOI] [PubMed] [Google Scholar]
- 36. Schüβler A., Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105:1413–1421 [Google Scholar]
- 37. Simon L., Lalonde M., Bruns T. D. 1992. Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl. Environ. Microbiol. 58:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith S. E., Read D. J. 2008. Mycorrhizal symbiosis, 3rd ed. Academic Press, London, United Kingdom [Google Scholar]
- 39. Sokal R. R., Rohlf F. J. 1995. Biometry. W. H. Freeman and Company, New York, NY [Google Scholar]
- 40. Solow A. R. 1993. A simple test for change in community structure. J. Anim. Ecol. 62:191–193 [Google Scholar]
- 41. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The CLUSTAL-X interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trouvelot A., Kough J. L., Gianinazzi-Pearson V. 1986. Mesure du taux de mycorhization VA d'un système radiculaire. Recherche de méthodes d'estimation ayant une signification fonctionnelle. In Gianinazzi-Pearson V., Gianinazzi S. (ed.), Physiological and genetical aspects of mycorrhizae. INRA Press, Paris, France [Google Scholar]
- 43. Vandenkoornhuyse P., et al. 2002. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol. Ecol. 11:1555–1564 [DOI] [PubMed] [Google Scholar]
- 44. Vandenkoornhuyse P., Ridgway K. P., Watson I. J., Fitter A. H., Young J. P. W. 2003. Co-existing grass species have distinctive arbuscular mycorrhizal communities. Mol. Ecol. 12:3085–3095 [DOI] [PubMed] [Google Scholar]
- 45. van der Heijden M. G. A., et al. 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72 [Google Scholar]
- 46. Videmšek U., et al. 2009. Abundance and diversity of CO2-fixing bacteria in grassland soils close to natural carbon dioxide springs. Microb. Ecol. 58:1–9 [DOI] [PubMed] [Google Scholar]
- 47. Vodnik D., Kastelec D., Pfanz H., Maček I., Turk B. 2006. Small-scale spatial variation in soil CO2 concentration in a natural carbon dioxide spring and some related plant responses. Geoderma 133:309–319 [Google Scholar]
- 48. Vodnik D., et al. 2002. Photosynthetic performance of cockspur (Echinochloa crus-galli (L.) Beauv.) at sites of naturally elevated CO2. Photosynthetica 40:575–579 [Google Scholar]
- 49. Vodnik D., Videmšek U., Pintar M., Maček I., Pfanz H. 2009. The characteristics of soil CO2 fluxes at a site with natural CO2 enrichment. Geoderma 150:32–37 [Google Scholar]
- 50. Wang B., Qiu Y. L. 2006. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363 [DOI] [PubMed] [Google Scholar]
- 51. Wirsel S. G. R. 2004. Homogenous stands of a wetland grass harbour diverse consortia of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 48:129–138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.