Abstract
Trichothecenes are mycotoxins produced by Trichoderma, Fusarium, and at least four other genera in the fungal order Hypocreales. Fusarium has a trichothecene biosynthetic gene (TRI) cluster that encodes transport and regulatory proteins as well as most enzymes required for the formation of the mycotoxins. However, little is known about trichothecene biosynthesis in the other genera. Here, we identify and characterize TRI gene orthologues (tri) in Trichoderma arundinaceum and Trichoderma brevicompactum. Our results indicate that both Trichoderma species have a tri cluster that consists of orthologues of seven genes present in the Fusarium TRI cluster. Organization of genes in the cluster is the same in the two Trichoderma species but differs from the organization in Fusarium. Sequence and functional analysis revealed that the gene (tri5) responsible for the first committed step in trichothecene biosynthesis is located outside the cluster in both Trichoderma species rather than inside the cluster as it is in Fusarium. Heterologous expression analysis revealed that two T. arundinaceum cluster genes (tri4 and tri11) differ in function from their Fusarium orthologues. The Tatri4-encoded enzyme catalyzes only three of the four oxygenation reactions catalyzed by the orthologous enzyme in Fusarium. The Tatri11-encoded enzyme catalyzes a completely different reaction (trichothecene C-4 hydroxylation) than the Fusarium orthologue (trichothecene C-15 hydroxylation). The results of this study indicate that although some characteristics of the tri/TRI cluster have been conserved during evolution of Trichoderma and Fusarium, the cluster has undergone marked changes, including gene loss and/or gain, gene rearrangement, and divergence of gene function.
INTRODUCTION
Trichothecenes are a group of over 200 sesquiterpenoid-derived secondary metabolites that vary by the pattern of oxygenations and esterifications of a core tricyclic structure with an epoxide function. These metabolites are produced by species in at least six genera of the fungal order Hypocreales (class Sordariomycetes): Fusarium, Myrothecium, Spicellum, Stachybotrys, Trichoderma, and Trichothecium. Trichothecenes are considered mycotoxins because they are often found as contaminants in food and animal feed and they can induce vomiting, alimentary hemorrhaging, and dermatitis in humans and livestock. These symptoms most likely result from the ability of trichothecenes to inhibit protein synthesis (40) and/or to induce apoptosis in eukaryotic cells (45). Trichothecenes also can act as immunosuppressors (51) and neurotoxins (36). Trichothecenes are phytotoxic (17). Previously (56), we observed that overproduction of trichodermin in Trichoderma brevicompactum reduced tomato seed germination and plant growth while increasing the size of the lesions produced when the pathogen was artificially inoculated on tomato plants previously colonized by Trichoderma spp.
The biosynthesis of the Fusarium trichothecenes T-2 toxin (T-2), nivalenol (NIV), and deoxynivalenol (DON) has been subjected to extensive biochemical and genetic analyses (reviewed in references 4 and 29). These studies have elucidated a complex biosynthetic pathway that begins with the cyclization of farnesyl pyrophosphate (FPP) to form trichodiene, which then undergoes a series of oxygenation, isomerization, cyclization, and esterification reactions to form T-2 toxin, nivalenol, or deoxynivalenol. Most of the Fusarium genes directly involved in synthesis of the toxins are positioned at a locus designated the core trichothecene biosynthetic gene (TRI) cluster (10). In the two most thoroughly examined species, Fusarium graminearum and Fusarium sporotrichioides, the cluster can include seven genes (TRI8, TRI7, TRI3, TRI4, TRI5, TRI11, TRI13) encoding enzymes that catalyze 10 trichothecene biosynthetic reactions, two genes (TRI6 and TRI10) encoding transcriptional regulators, one gene (TRI12) encoding a transport protein, and two genes (TRI9 and TRI14) with uncertain functions. Some Fusarium spp. have two additional TRI loci located on chromosomes other than the one on which the core cluster is located (15, 33). One locus is a cluster that contains two genes (TRI1 and TRI16), which encode biosynthetic enzymes, and the other locus contains a single gene (TRI101) that also encodes a biosynthetic enzyme. In some Fusarium species, however, TRI1 and TRI101 are located in the core TRI cluster (49). Myrothecium roridum has a 40-kb cluster that includes orthologs of TRI4, TRI5, and TRI6 (57). Whether other TRI orthologs are present in this cluster is not known.
The genus Trichoderma includes species that have been evaluated extensively as biological control agents (21, 41). Strains of Trichoderma can produce extracellular enzymes and antibiotics but also may compete with fungal pathogens for space and nutrients through rhizosphere competence (26). Some species of Trichoderma can produce the trichothecenes trichodermin and harzianum A (HA). These metabolites are similar to the Fusarium trichothecenes T-2 toxin, NIV, and DON in that they have the core tricyclic 12,13-epoxytrichothec-9-ene (EPT) structure. They are also similar to T-2 and NIV in that they have an oxygen atom attached to carbon atom 4 (C-4). Although their structures are well characterized, biosynthesis of HA and trichodermin by Trichoderma has been subjected to little genetic analysis. However, orthologues of TRI5 have been identified in a trichodermin-producing strain (IBT 40841) of Trichoderma brevicompactum (56) and a harzianum A-producing strain (ATCC 90237) of Trichoderma arundinaceum (16, 19). The identification of the TRI5 orthologues in the two Trichoderma species indicates that the first committed reactions in trichothecene biosynthesis in Fusarium and Trichoderma are the same (16). However, the absence of the C-3, C-8, and C-15 oxygen atoms in trichodermin and HA indicates that there are multiple differences in trichothecene biosynthesis in the two genera. These differences probably are reflected in the presence/absence/divergence of functional orthologs of some trichothecene biosynthetic genes in the two genera. For example, in Fusarium, the TRI4-encoded cytochrome P450 monooxygenase catalyzes oxygenation of trichodiene at four carbons, C-2, C-3, C-11, and C-13 (38). In contrast, the Myrothecium TRI4-encoded monooxygenase catalyzes oxygenation of trichodiene at only three carbons, C-2, C-11, and C-13 (39). This difference in patterns of oxygenation is consistent with the presence of a C-3 oxygen in Fusarium trichothecenes and its absence in Myrothecium trichothecenes. The absence of a C-3 oxygen in trichodermin and HA suggests that the Trichoderma Tri4 orthologue is more similar in function to the Myrothecium than the Fusarium orthologue. The absence of C-8 and C-15 oxygen atoms in trichodermin and HA also suggests that trichothecene-producing Trichoderma strains lack orthologues of the Fusarium trichothecene C-8 and C-15 oxygenase genes TRI1 and TRI11, respectively. In contrast, the presence of the C-4 oxygen atom in trichodermin and HA suggests that T. brevicompactum and T. arundinaceum have an orthologue of the Fusarium trichothecene C-4 oxygenase gene TRI13.
The objective of the current study was to identify trichothecene biosynthetic genes in T. arundinaceum and T. brevicompactum and to compare the functions of these genes with those of previously identified orthologs in Fusarium. The results of our analyses revealed that both Trichoderma species have a trichothecene biosynthetic gene cluster with orthologues of seven genes in the Fusarium TRI cluster but that the TRI5 orthologue is not within this core cluster. Functional analyses demonstrate a key difference in trichothecene biosynthesis among different genera, namely, that the same structural modification of trichothecenes (i.e., C-4 oxygenation) is catalyzed by nonorthologous enzymes in Trichoderma and Fusarium.
MATERIALS AND METHODS
Strains, media, and culture conditions.
Strains of filamentous fungi used in this work are listed in Table 1.
Table 1.
Strains of filamentous fungi used in this study
| Species | Strain no.a | Alternate strain no. | Trichothecene productionb | Reference |
|---|---|---|---|---|
| T. arundinaceum | IBT 40837 | Ta37 | Harzianum A | 44 |
| ATCC 90237 | Harzianum A | 14, 16 | ||
| T. brevicompactum | IBT 40841 | Tb41 | Trichodermin | 44 |
| T. harzianum | CECT 2413 | None | 22 | |
| M. roridum | ATCC 52485 | Roridin E | 31 | |
| F. sporotrichioides | ATCC 24630 | NRRL 3510 | T-2 toxin | 58 |
| F. verticillioides | FRC M-3125 | NRRL 20956 | None | 34 |
IBT, IBT Culture Collection of Fungi, Denmark; ATCC, American Type Culture Collection, United States; CECT, Spanish Type Culture Collection, Spain; NRRL, Agricultural Research Service (ARS) Culture Collection (NRRL), United States; FRC, Fusarium Research Center, United States.
The metabolites listed are the predominant trichothecenes produced by each strain.
For HA analysis and RNA isolation, a two-step culture procedure was employed. The media for this procedure were malt broth medium (CM) (5 g/liter malt extract, 5 g/liter yeast extract, 5 g/liter glucose) and modified potato dextrose broth (PDB) (24 g/liter PDB supplemented with 0.1 g/liter ZnSO4·7H2O and 0.05 g/liter CuSO4·5H2O) (Difco Becton Dickinson, Sparks, MD) (44). CM medium was inoculated with T. arundinaceum conidia at 1 × 107 conidia per ml and then incubated at 28°C in the dark with shaking (250 rpm). After 24 h, 10 ml of the resulting CM culture was transferred to 50 ml of modified PDB medium and incubated at 28°C in the dark with shaking (250 rpm) for 24 to 96 h. Finally, the mycelium was recovered by filtration through a 30-μm-pore-diameter nylon filter (Sefar AG, Heiden, Switzerland), and the supernatant was used for HA purification and quantification. The mycelia were then washed with 0.9% NaCl and dried on absorbent filter paper (Albet-Hahnemuehle, Barcelona, Spain) prior to RNA isolation.
Genetic nomenclature.
Trichothecene biosynthetic genes have been characterized most extensively in Fusarium species. In these species, the gene names employ three uppercase letters and a number (e.g., TRI4, TRI5), following conventions of genetic nomenclature developed for Fusarium (61). For consistency with conventions of Trichoderma genetic nomenclature, we designate trichothecene biosynthetic genes in Ta37 and T. brevicompactum with three lowercase letters and a number (tri4, tri5). When it is appropriate to distinguish between orthologues from these two Trichoderma species, the gene names are preceded by Ta (e.g., Tatri4, Tatri5) for T. arundinaceum and Tb (e.g., Tbtri4, Tbtri5) for T. brevicompactum. Protein designations follow a format consistent with the nomenclature for yeast, Aspergillus, and multiple plant pathogenic fungi (e.g., TaTri4, TaTri5) (13).
Nucleic acid manipulation.
A genomic library of T. arundinaceum strain 37 was constructed in the bacteriophage vector lambda DASH II (Stratagene, La Jolla, CA) by following the manufacturer's instructions.
To optimize the conditions for Southern hybridization, genomic DNAs were hybridized in buffers containing 25, 30, or 40% formamide. Twenty-five-percent formamide was optimal for detecting the Tatri4 gene signal from a λ genomic library, and a buffer with 40% formamide was optimal for chromosome walking. Filters with bound genomic or phage DNA were prehybridized and hybridized at 42°C for 2 and 12 h, respectively. The filters were then washed twice with 2× saline sodium citrate buffer (SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) and 0.1% sodium dodecyl sulfate (SDS) for 5 min each at room temperature and twice with 0.5× SSC and 0.1% SDS for 15 min each at 65°C.
Probes used in Southern analysis were labeled by using the digoxigenin (DIG) DNA labeling kit (Roche, Mannheim, Germany) by following the manufacturer's instructions. Labeling, hybridization, and immunological detection were carried out with a nonradioactive labeling and immunological detection kit with CDP-Star as the chemiluminescent substrate (Roche, Mannheim, Germany), as previously described (12).
For the isolation of genomic DNA, 1 × 107 spores/ml of the Ta37 strain were inoculated in 100 ml of CM and incubated for 24 to 48 h at 28°C and 250 rpm. The mycelia were recovered by filtration through a 30-μm-pore-diameter nylon filter and washed with 0.9% NaCl, and the excess liquid was removed with absorbent filter paper. Finally, the mycelia were lyophilized, and the dried mycelia were used for genomic DNA isolation as previously described (11, 53). To isolate total RNA, the Ta37 strain was grown using the two-step procedure in CM followed by PDB as described above. Mycelia were recovered from PDB medium by filtration, washed with 0.9% NaCl, and dried on absorbent filter paper. RNA was extracted with the phenol-SDS method (6) and treated with DNase and RNase protector (Fermentas, Vilnius, Lithuania). One microgram of total RNA was used for cDNA synthesis in the reverse transcription (RT) system (Promega, Madison, WI). For the amplification, cDNAs were synthesized as described above and subcloned in the TOPO cloning system (Invitrogen, Carlsbad, CA) by following the manufacturer's instructions. To distinguish gene amplification products from genomic DNA and cDNA, we used primers (see Table S1a in the supplemental material) that span the putative introns in the genomic DNA and should yield amplification products from the mRNA that are smaller than the corresponding regions amplified from genomic DNA. The sizes of the amplified Tatri cDNA fragments were as follows: Tatri5, 390 bp; Tatri14, 494 bp; Tatri12, 861 bp; Tatri10, 452 bp; Tatri3, 798 bp; Tatri4, 449 bp with oligonucleotides T44int3/T44int5 or 668 bp with oligonucleotides T437CT/T43E4bNt; and Tatri11, 1,426 bp.
For sequence analysis, CLC Protein Workbench 5.4 (CLC bio) and Jalview 2.6.1 (59) programs were used to compare multiple amino acid sequences (Clustal method) and for pairwise protein alignments between two sequences (Lipman-Pearson method). The Lasergene (DNASTAR, Inc., Madison, WI) PROTEAN program was used to analyze structural features of the proteins (Hoop-Woods algorithm) (25). For the assembly of individual sequence fragments into a contig, the Lasergene program SEQMAN was employed, while EDITSEQ was used for basic sequence editing. Comparative analyses involving protein databases were performed with the BLASTX (5) algorithm. Evolutionary distances between the tri/TRI genes of the different fungi were determined by using a pairwise distance analysis as implemented in MEGA 4.
Tatri gene expression in yeast.
cDNAs of Tatri4, Tatri5, and Tatri11 were amplified by RT-PCR (Superscript III one-step RT-PCR with Platinum Taq polymerase; Invitrogen, Carlsbad, CA) using primer pairs 2096/2097, 2114/2115, and 2105/2106, respectively (see Table S1b in the supplemental material). Each amplicon was gel purified with the Ultraclean DNA purification kit (MoBio, Carlsbad, CA) and then subjected to an incubation at 72°C for 10 min with Taq polymerase to introduce 3′ A overhangs at each end of the amplicon to facilitate cloning into the yeast expression vector pYES2.1/V5-His-TOPO (URA3+) (Invitrogen, Carlsbad, CA). Correct orientation of cDNA inserts in the vector was confirmed by PCR. This procedure yielded an expression plasmid for each of the three genes: pYETri4 for Tatri4, pYETri5 for Tatri5, and pYETri11 for Tatri11. Each plasmid was transformed separately into Saccharomyces cerevisiae strain INVSc1 (MATa his3D1 leu2 trp1-289 ura3-52) by following the high-efficiency yeast transformation protocol described by Gietz et al (20). Transformation reaction mixtures were plated onto MD (minimal medium plus dextrose) (8) amended with leucine (1 mg/ml), histidine (0.2 mg/ml), and tryptophan (0.2 mg/ml) (MD+LHT) for selection of transformants carrying pYES2.1 plasmids.
Tatri4 expression in Fusarium verticillioides.
The Ta37 tri4 gene was amplified by PCR using Pfu polymerase (Fermentas, Vilnius, Lithuania) with primers Tri437-5 and Tri437-3b (see Table S1b in the supplementary material), using the plasmid pF42E4 as the template, which included the entire 1,791-bp Tatri4 coding region. The Tatri4 amplicon was treated with T4 polynucleotide kinase and ligated into plasmid pAN52.1 (50) that had been digested with BamHI and then treated with Klenow fragment and calf intestine alkaline phosphatase (CIAP; Fermentas, Vilnius, Lithuania). The resulting plasmid, pSPT4-1, contained a 4,914-bp Tatri4 expression cassette that consisted of the Tatri4 coding region fused to the Aspergillus nidulans gpdA gene promoter region and trpC gene terminator region. The expression cassette was then isolated by digestion of pSPT4-1 with BglII and EheI and treated with the Klenow fragment followed by gel purification. The isolated expression cassette was ligated to pAN7-1 (50) that had been digested with HindIII and treated with Klenow and CIAP. Ten micrograms of the resulting plasmid, pSPT4-4, was then transformed into F. verticillioides via the protoplast method with selection of transformants on regeneration medium containing 300 μg/ml hygromycin B as previously described (48).
Bioassay with Kluyveromyces marxianus GK1005.
Fifty milliliters of malt agar medium (MA) (20 g/liter glucose, 20 g/liter malt extract, and 1 g/liter mycopeptone) was inoculated with one single colony of K. marxianus GK1005, a yeast strain sensitive to trichothecenes (18, 55), and incubated overnight at 30°C and 250 rpm. Cell density was measured as an optical density at 560 nm (an OD560 of 1 corresponds to 1.1 × 109 cells). Bioassay plates were made by adding 2.2 × 108 cells to 100 ml of 1% MA. Sixty microliters of the high-performance liquid chromatography (HPLC) fractions taken each minute was added into holes with a 5.5-mm diameter and 3.5-mm depth that were formed in the bioassay plates. Plates were maintained at 4°C for 5 h to allow the solution to diffuse and then incubated for 14 h at 30°C to visualize the growth inhibition zone.
Purification and analysis of HA.
Two-day-old PDB cultures of the Ta37 strain (1.5 liters) were filtered through a 30-μm-pore-diameter nylon filter, the resulting supernatant was mixed with one volume of acetone, and the solution was evaporated to the original filtrate volume, or less, with a rotary evaporator at room temperature and 40 rpm. The concentrated solution was extracted three times with equal volumes of ethyl acetate. The extracts were pooled, mixed vigorously with anhydrous Na2SO4, and then allowed to stand for 1 h before filtration through filter paper. The filtrate was evaporated to dryness with a rotary evaporator at room temperature, and the residue was resuspended in acetonitrile to a tenth of the original filtrate volume and stored at −30°C until analysis.
A Waters 600 HPLC with a 996 photodiode detector set at 306 nm with an ODS-H80 preparative column (150 mm, 10 mm internal diameter, 4 μm particle size, 8 nm pore diameter; YMC Waters), was used to purify the HA. The method used in this work was based on the one described by Lee et al. (32), with some modifications.
The mobile phase consisted of 60:40 water plus 0.1% trifluoroacetic acid-acetonitrile. Two hundred microliters of sample was applied and eluted with a 3-ml/min flux of mobile phase for 40 min, reaching 100% of acetonitrile in 7 min, held for 10 min, and then returned to the initial conditions (total time of the program, 62 min). Under these conditions, HA was eluted at 34.8 min. HA-containing fractions were combined and completely evaporated on a rotary evaporator at 4°C. Finally, the solid obtained was resuspended in acetonitrile and stored at −30°C. HA was identified by nuclear magnetic resonance (NMR) (27) and quantified by using a standard curve.
For routine analysis, Ta37 cultures were extracted with equal volumes of ethyl acetate, and the upper phase was recovered, evaporated to dryness in a rotary evaporator at room temperature, and resuspended in acetonitrile to a tenth of the initial volume. Twenty microliters of the concentrated extract was applied to a YMC Waters analytical column of 150 by 4.6 mm and eluted with a 1-ml/min flux of mobile phase (as described above) for 30 min, reaching 100% of acetonitrile in 10 min, held for 5 min, and then returned to the initial conditions (total time of the program, 50 min). Using these conditions, HA was eluted at 22.9 min.
Chemical analysis of HA intermediates.
Gas chromatography low-resolution mass spectrometry (GCMS) measurements were made with a Hewlett Packard 6890 gas chromatograph fitted with an HP-5MS column (30-mm by 0.25-mm film thickness) and a 5973 mass detector. The carrier gas was helium with a 20:1 split ratio and a 20-ml/min split flow. Two temperature programs were used: (i) the column was held at 120°C at injection, heated to 210°C at 15°C/min and held for 1 min, and then heated to 260°C at 5°C/min and held for 3 min (total run time of 20 min) or (ii) the column was held at 120°C at injection and heated to 260°C at 25°C/min and held for 4.4 min (total run time of 10 min). The first program was used to detect isotrichodiol, and the second program was used to detect trichodiene and trichodermol. HA intermediates were identified by comparison of retention times and mass spectral fragmentation patterns with standards.
Precursor feeding experiments.
For yeast experiments, cells were grown in MD+LHT medium at 28°C and 200 rpm for 48 h and then transferred to YGal medium (YD plus 2% galactose) supplemented with trichodiene (Tri4 assays) or trichothecene (Tri11 assays) dissolved in acetone to a final concentration of 250 μM. No substrate was added for Tri5 assays. Untransformed yeast cultures were used as negative controls. A 3-ml aliquot was removed from each culture after 3 to 4 days and mixed with 3 ml acetone to precipitate the yeast cells. The supernatant was then extracted with hexane-ethyl acetate (85:15), concentrated under nitrogen, and analyzed by GCMS.
For F. verticillioides experiments, spores were inoculated into 5-GYEP (50 g/liter glucose, 1 g/liter yeast extract, 1 g/liter peptone) medium, and the cultures were incubated at 28°C and 200 rpm. After 24 h, trichodiene dissolved in acetone was added to the cultures to a final concentration of 250 μM. After 4 days, cultures were extracted with ethyl acetate, and the extracts were analyzed by GCMS for trichothecene content. As a negative control, the transformant FvTaTri4#19-2 was grown in 5-GYEP medium without trichodiene.
Nucleotide sequence accession numbers.
Sequences have been deposited in the GenBank database. Accession numbers of the T. arundinaceum tri genes are as follows: FR715494 for Tatri5, FN394491 for Tatri14, FN394492 for Tatri12, FN394493 for Tatri11, FN394494 for Tatri10, FN394495 for Tatri3, FN394496 for Tatri4, and FN394497 for Tatri6. Accession numbers of the T. brevicompactum tri genes sequenced in this work are as follows: FR775447 for Tbtri14, FR839627 for Tbtri12, FR839626 for Tbtri11, FR775446 for Tbtri10, FR775444 for Tbtri3, FR822202 for Tbtri4, and FR775445 for Tbtri6.
RESULTS
Identification of HA in T. arundinaceum culture broth.
HPLC analysis of culture supernatants of the T. arundinaceum Ta37 strain resolved three main peaks at 21.1, 22.9, and 39.0 min. The antifungal activity of HA had been previously reported (14), and our bioassay confirmed this. K. marxianus was sensitive only to the fractions collected around the 22.9-min peak. We confirmed that the bioactive fraction contained HA with 1H NMR and 13C NMR spectroscopy.
Identification of trichothecene biosynthetic loci in T. arundinaceum and T. brevicompactum.
Initially, an internal Tatri5 fragment from genomic DNA of the Ta37 strain was amplified with the previously described tri5 PCR primers Tri5F and Tri5R (see Table S1c in the supplemental material). The resulting amplicon was sequenced to confirm its similarity to previously described TRI5 orthologues from other trichothecene-producing fungi (e.g., Fusarium and Myrothecium) and then used as a hybridization probe to screen the Ta37 lambda library. This screen yielded two overlapping clones containing Tatri5, F72 and F73, which included a 1,226-bp coding region that was 50 to 66% identical to previously characterized TRI5 orthologues from F. graminearum (47), F. sporotrichioides (23), M. roridum (57), Stachybotrys echinata (30), and Trichothecium roseum (30).
Since some of the trichothecene biosynthetic genes are clustered in Fusarium and in Myrothecium, we examined the regions flanking Tatri5 in clones F72 and F73. We were unable to identify any other known TRI orthologs in the 18 kb of DNA of these two clones.
To identify other TRI orthologues in T. arundinaceum, we used PCR-amplified fragments of F. sporotrichioides TRI4 (FsTRI4) and M. roridum TRI4 (MrTRI4) as hybridization probes to screen the lambda library prepared from the Ta37 strain. We selected TRI4 as a hybridization probe because TRI4 is essential for trichothecene production in both Fusarium and Myrothecium. The library screen identified three clones (F41, F42, and F44) that hybridized to the FsTRI4 and MrTRI4 fragments. Based on partial sequence analysis, these clones contain orthologs of the Fusarium TRI4, TRI6, and TRI12 genes. An F44 subclone that included the TRI12 orthologue was used as a hybridization probe to rescreen the Ta37 lambda library to obtain two additional clones (F51 and F52) that overlapped clone F44.
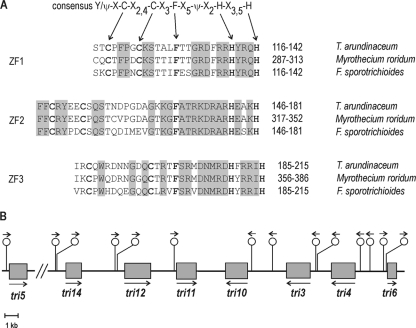
Sequence analysis of 41 kb of DNA spanned by lambda clones F42, F44, F51, and F52 identified seven orthologs of Myrothecium and/or Fusarium TRI genes in the order tri14, tri12, tri11, tri10, tri3, tri4, and tri6 (Fig. 1). There also were two genes, designated orfA and orfB, in the region flanking tri14 and two more genes, orfC and orfD, flanking tri6 (Fig. 1). These tri6- and tri14-flanking genes have no detectable DNA sequence similarity to known TRI genes, but their deduced amino acid sequences exhibit significant levels of similarity (BLAST) to metallo-β-lactamase superfamily proteins (orfA), α-N-acetylglucosaminidases (orfB), proteins of unknown function (orfC), and oligopeptide transporter proteins (orfD). Based on these results, we hypothesize that the region of genomic DNA that spans 24,291 bp from tri14 to tri6 is the core tri cluster in T. arundinaceum.
Fig. 1.
(A) Genomic organization of the Trichoderma arundinaceum IBT 40837 HA core cluster of tri genes. Overlapping phages representing the genomic region comprising the trichothecene biosynthetic gene cluster are also represented. The arrows indicate predicted positions and directions of transcription of individual genes/open reading frames (ORFs). Small rectangles inside each gene indicate the introns of each ORF. The names above each arrow correspond with their Fusarium orthologue designation. The red arrows refer to those structural genes involved in the HA biosynthesis (tri11, tri3, tri4) or in the export of HA outside the cell (tri12), the green arrows refer to those genes with regulatory functions in the trichothecene biosynthesis (tri6 and tri10), the yellow arrow (tri14) has an unknown function, and the blue arrows indicate non-tri genes. The genes tri11 and tri4, whose names are illustrated in green, were those whose role in the biosynthesis have been studied in the present work by expression in yeast and, in the case of tri4, also in F. verticillioides. Note that the tri5 gene has not been illustrated, even when it has been also expressed in yeast, since it is located in another region of the Ta37 genome outside the core cluster of tri genes. (B) Genomic organization of the trichothecene gene cluster of T. arundinaceum in comparison with T. brevicompactum and Fusarium species (10). The arrows indicate predicted positions and directions of transcription of individual genes/ORFs. Numbers indicate gene designations based on their Fusarium orthologues (e.g., 6 refers to tri6 or TRI6). Colored designations illustrate their role in trichothecene biosynthesis, as indicated above. Colored rectangles represent those genes that are grouped in a similar fashion in the different clusters.
Using the sequences of the Tatri orthologues, we designed PCR primers (see Table S1d in the supplemental material) to amplify and sequence the corresponding orthologues in Tb41. The resulting sequence contig spanned 25,498 bp and included orthologues of the seven TRI genes in the same order and orientation as those in the T. arundinaceum core cluster. The deduced amino acid sequences of TaTri and TbTri orthologues exhibited ≥92% identity (Table 2). The most notable difference between the T. arundinaceum and T. brevicompactum clusters is the size of the tri11-tri12 intergenic region, which is 1,793 bp in T. arundinaceum and 3,000 bp in T. brevicompactum. Thus, the core tri cluster in these species contains seven orthologs of trichothecene biosynthetic genes in Fusarium but does not include tri5 (Fig. 1).
Table 2.
Amino acid comparison of TRI orthologues from Trichoderma to other fungi
| T. arundinaceumTRI orthologue | No. of amino acids | Intron length(s) (bp) | % identity toa: |
||
|---|---|---|---|---|---|
| T. brevicompactum | M. roridum | F. sporotrichioides | |||
| tri3 | 520 | 57, 55, 56, 54 | 92 | ND | 48 |
| tri4 | 517 | 70, 58, 56, 53 | 98 | 67 | 73 |
| tri5 | 388 | 59 | 99 | 56 | 57 |
| tri6 | 218 | None | 99 | 51 | 46 |
| tri10 | 422 | 70 | 98 | ND | 58 |
| tri11 | 499 | 80 | 96 | ND | 38 |
| tri12 | 602 | 55, 68 | 92 | ND | 55 |
| tri14 | 363 | 59 | 99 | ND | 64 |
ND, not done.
The numbers and organization of genes in the cluster are the same in both Trichoderma species examined but markedly different from the organization in Fusarium. The Trichoderma cluster has orthologues of only seven of the 10 to 14 genes that occur in the Fusarium cluster. Although the arrangement of genes differs from that in the Fusarium cluster, the Trichoderma tri cluster can be divided into two regions (or blocks) that exhibit conservation of gene content and order relative to blocks within the TRI clusters of F. graminearum and F. sporotrichioides (Fig. 1). A region orthologous to the tri3-tri4-tri6 block in Trichoderma also exists in the F. graminearum and F. sporotrichioides cluster (i.e., TRI3-TRI4-TRI6). Likewise, a region orthologous to the tri14-tri12-tri11-tri10 block in Trichoderma also exists in the F. graminearum and F. sporotrichioides cluster but includes two additional genes, TRI9 and TRI13, which are not in the Trichoderma cluster. Within the two blocks, gene orientation is also largely the same in Trichoderma and Fusarium; only tri3/TRI3 and tri14/TRI14 differ in orientation with respect to other genes in the blocks.
Functional characterization of Tatri genes.
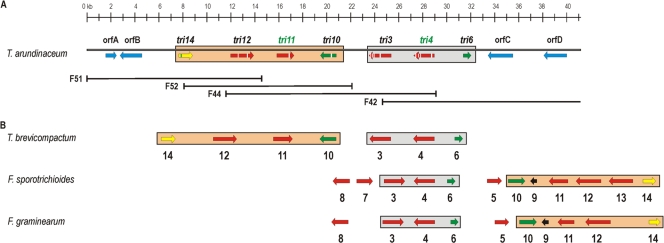
We examined the functions of Tatri4, Tatri5, and Tatri11 by expressing each gene independently in yeast. For Tatri5 expression, transformed yeast cells were analyzed without the addition of a trichothecene biosynthetic intermediate, because the substrate for the Fusarium Tri5 enzyme is farnesyl pyrophosphate, which is an intermediate of primary metabolism in yeast. Extraction and GCMS analysis of pYETri5-transformed yeast cultures following galactose induction revealed the presence of trichodiene (Fig. 2). This is the same product of the reaction catalyzed by the Fusarium Tri5 enzyme and the first intermediate in the trichothecene biosynthetic pathway. The presence of trichodiene was observed in four independent yeast transformants carrying pYETri5. These results indicate that the TaTri5 enzyme catalyzes cyclization of farnesyl pyrophosphate to form trichodiene.
Fig. 2.
Reconstructed ion chromatograms of extracts from 4-day-old Saccharomyces cerevisiae YGal liquid cultures. (A) INVSc; (B) INVSc expressing Tatri5 (transformant INVtri5#4). The arrow indicates trichodiene (TD) at 4.0 min.
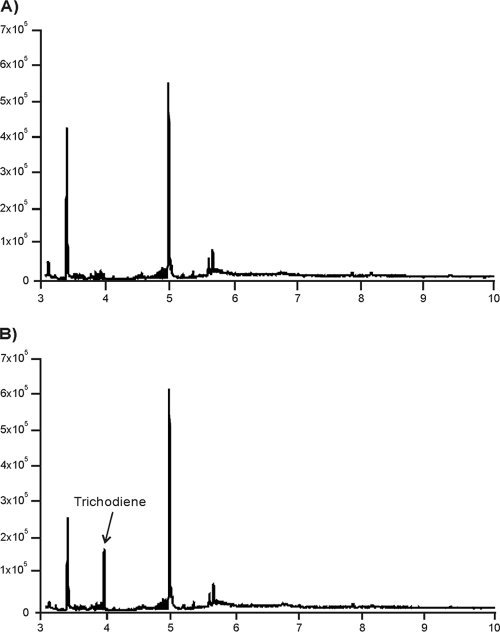
For Tatri4 expression, cultures of pYETri4-transformed yeast were induced with galactose and then fed trichodiene, the substrate of the Tri4 enzymes in Fusarium and Myrothecium. Extraction and GCMS analysis of the resulting cultures revealed the presence of the trichothecene biosynthetic intermediate isotrichodiol (Fig. 3). This result was confirmed in eight yeast transformants. The function of TaTri4 was also assessed by introducing a Tatri4 expression cassette, on plasmid pSPT4-4, into the trichothecene-nonproducing fungus F. verticillioides. The addition of trichodiene to cultures of eight F. verticillioides transformants carrying the expression cassette also resulted in the formation of isotrichodiol (Fig. 3).
Fig. 3.
(1) Reconstructed ion chromatograms of extracts from 3-day-old Saccharomyces cerevisiae YGal liquid cultures after feeding trichodiene: INVSc yeast (A) and INVtri4#3 (B). (2) Reconstructed ion chromatograms of extracts from 4-day-old 5-GYEP liquid cultures of F. verticillioides expressing Tatri4 (transformant FvTaTri4#19-2): control culture with no trichodiene (A) and after feeding with trichodiene (B). The arrows indicate the position of isotrichodiol at 11.1 min.
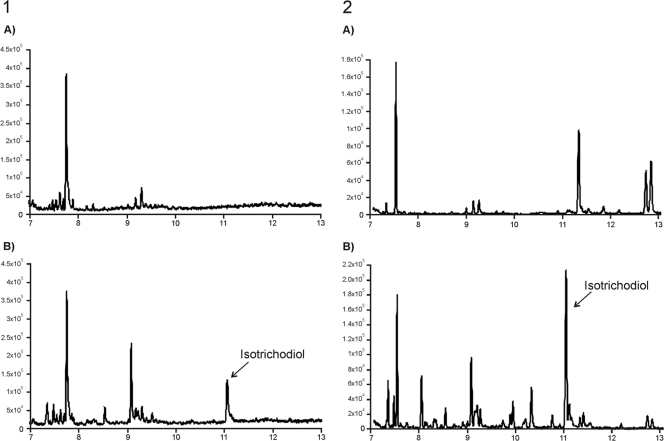
For Tatri11 expression, cultures of pYETri11-transformed yeast cells were induced with galactose and then fed 12,13-epoxytrichothec-9-ene (EPT). Extraction and GCMS analysis of the cultures of five independent transformants revealed the presence of 4-hydroxytrichothecene (trichodermol) (Fig. 4). These results indicate that the TaTri11 enzyme catalyzes hydroxylation of C-4 of the trichothecene skeleton. This activity differs from that of the Fusarium Tri11 enzyme, which catalyzes hydroxylation of C-15 of the trichothecene skeleton.
Fig. 4.
Reconstructed ion chromatogram of extracts from 3-day-old Saccharomyces cerevisiae YGal liquid cultures after feeding 12,13-epoxytrichothec-9-ene (EPT): INVSc (A) and INVSc expressing Tatri11 (transformant INVTri11#3) (B). The arrow indicates the position of trichodermol at 6.0 min.
In silico characterization of TaTri proteins.
Analysis of the deduced protein sequence of TaTri5 showed two conserved metal binding motifs that coordinate Mg2+ ion-bridged binding of the diphosphate moiety of FPP, which is similar to other proteins belonging to class 1 of cis-trans terpene cyclases that catalyze the cyclization of FPP to trichodiene. The first motif is a DDXX consensus sequence, DDSR, in TaTri5. The first D coordinates with the second conserved region, (N/D)XXX(S/T)XXXE, which corresponds to amino acids (aa) 234 to 242 (NDLFSFYKE) of TaTri5.
Both TaTri4 and TaTri11 were identified as cytochrome P450 monooxygenases and contained all the conserved structural features described for this family of proteins. The highest structural conservation occurs in the core of the protein around the heme and reflects a common mechanism of electron and proton transfer and oxygen activation. This region comprises (i) the heme-binding loop, containing the most characteristic P450 consensus sequences (FXXGXRXCXG), FSQGSRQCIG (positions 445 to 454) in TaTri4 and FNVGPRNCVG (positions 435 to 444) in TaTri11, with the absolutely conserved Cys that serves as the fifth ligand to the heme iron, (ii) the absolutely conserved motifs (EXXR), EGLR (positions 369 to 372) in TaTri4 and EAFR (positions 352 to 355) in TaTri11, probably required to stabilize the core structure, and (iii) the central part containing the consensus sequences of the P450 signature [(A/G)GX(D/E)T(T/S)], AGTETT (positions 311 to 316) in TaTri4 and AGSETT (positions 294 to 299) in TaTri11, which corresponds to the protein transfer groove (1, 60). In spite of the high conservation of the consensus motifs for P450 monooxygenases between TaTri11 and TaTri4, they showed a low amino acid sequence similarity of 19% to each other.
TaTri3 most likely belongs to the chloramphenicol acetyl-transferase family (CAT) of acetyltransferases, as it contains the consensus sequence HXXXDG (HLFWDG) (at positions 188 to 193) (43) but not the 3′ consensus DFGWGKP (see reference 3 for acetyltransferase comparisons).
The analysis of the 422-aa TaTri10 protein with BLASTP, CDART, and CDD (Conserved Domain Database) algorithms detected no putative transmembrane conserved domains. However, other algorithms, such as DAS (Dense Alignment Surface), TMpred (TransMembrane Prediction), SMART (Simple Modular Architecture Research Tool), and TMHMM (transmembrane helices), did detect putative transmembrane sites, with aa 196 to 218 receiving the highest score. The last program (TMHMM) gave a lower probability score to the sequence from Tb41 than to Ta37, most likely due to the two amino acid differences at aa 209 and 210 in the two strains. MotifFinder found no conserved domains in either Tri10 protein. Compared to the FsTRI10 sequence, the sequence from the TaTri10 protein has a 58% identity (Table 2).
The analysis of the TaTri6 protein revealed the presence of three regions in the carboxy-terminal end with similarity to zinc finger domains found in a wide range of gene regulatory proteins. These domains are important for DNA binding. Motif ZF1 has a high similarity to the consensus sequence (Ψ/Y) XCX2,4CX3FX5ΨX2HX3,5H (Ψ denotes a hydrophobic amino residue) (7) of zinc finger binding proteins and correlates well with the ZF1 sequence from M. roridum and F. sporotrichioides (Fig. 5A). The sequences of ZF2 and ZF3 of TaTri6, while retaining the CCFHH amino acids of the consensus sequence, have additional amino acid inserts and correlate highly with those regions of Tri6 from M. roridum and F. sporotrichioides (Fig. 5A). When the carboxy-terminal region of the TaTri6 protein was compared with the same region of the Tri6 from M. roridum and F. sporotrichioides, the identities were 84% and 73%, respectively. However, when the entire TaTri6 amino acid sequence was compared to M. roridum and F. sporotrichioides, the amino acid identities were only 51% and 47%, respectively.
Fig. 5.
(A) Sequence alignment between the ZF1, ZF2, and ZF3 domains, located at the carboxy-terminal end of the predicted Tri6 protein sequences from Ta37, M. roridum, and F. sporotrichioides. The Cys and His residues within the proposed zinc fingers, as well as other residues conserved with respect to the consensus sequence proposed for these zinc finger domains, are shown in bold (7). Those residues conserved within each zinc finger domain between the four analyzed Tri6 sequences are shaded. (B) Localization of the putative binding sequences for the transcription factor Tri6 localized in the promoter region of the Tatri genes.
In Fusarium, the Tri6 protein induces expression of TRI cluster genes, in part by binding to the DNA sequence YNAGGCC that is present in the promoter regions of the genes (24). This same sequence motif was observed in the promoter regions of all the Tatri genes (Fig. 5B). Thus, Trichoderma Tri6 likely binds to the promoter regions of Trichoderma tri cluster genes and thereby regulates their expression as described for Fusarium (24).
The analysis of the TaTri12 sequence identified the protein as having a relatively high predicted amino acid sequence similarity with Fusarium Tri12 (Table 2), which is known to serve as a trichothecene efflux pump (2). The high sequence similarity with proteins in the MFS (major facilitator superfamily) indicates that TaTri12 should facilitate transport, presumably of HA, across the cytoplasmic or other intracellular membranes. The PROTEAN program (25) predicts that TaTri12 contains 14 transmembrane alpha helix domains (TM) connected by hydrophilic groups. This structure is consistent with the presence of 272 hydrophobic amino acids, 45% of the total 602 amino acids of the protein, a percentage similar to that of the Tri12 protein from F. sporotrichioides (44%) and higher than that detected in any of the other Tri proteins.
The analysis of the deduced TaTri14 protein sequence found no conserved domains compared against the CDD. Thus, TaTri14 function remains unknown.
DISCUSSION
Within the fungal order Hypocreales, trichothecene biosynthesis and the TRI cluster have been examined extensively in selected species of Fusarium, and there is evidence for a TRI cluster in Myrothecium. In the current study, we describe trichothecene biosynthesis and a tri cluster in a third trichothecene-producing genus, Trichoderma. The presence of a tri/TRI cluster in species of Trichoderma, Myrothecium, and Fusarium could result if an ancient hypocrealean fungus, a common ancestor of all three genera, produced trichothecenes and had a tri/TRI cluster. Although the Trichoderma cluster (seven genes) differs markedly in gene content and organization from the larger Fusarium cluster (10 to 14 genes), the Trichoderma cluster includes genes encoding a range of functions similar to those encoded by the Fusarium cluster. These functions include biosynthetic enzymes (Tri3, Tri4, Tri11), regulatory proteins (Tri6 and Tri10), and a transport protein (Tri12).
The difference in gene content of the clusters indicates that genes have been gained and/or lost during evolution of Trichoderma and Fusarium. Perhaps the most notable difference is the absence of a tri5/TRI5 orthologue in the Trichoderma cluster. Whether the Trichoderma tri5 and tri clusters are physically linked on the same chromosome or located on different chromosomes has not been determined. Results from the current study and those from previous studies (23) indicate that the Trichoderma and Fusarium tri/TRI5 orthologues encode trichodiene synthase, the enzyme that catalyzes the first committed step in trichothecene biosynthesis. Thus, tri5/TRI5 is essential for trichothecene production in both fungi. The location of the tri5/TRI5 orthologue outside the tri cluster in Trichoderma but inside the cluster in Fusarium and Myrothecium (57; R. H. Proctor, unpublished data) suggests that the ancestral tri/TRI cluster included a tri5/TRI5 gene but that the gene moved out of the cluster to another genomic location during evolution of Trichoderma. One hypothesis for the function of secondary metabolite biosynthetic gene clusters is that they facilitate vertical and horizontal inheritance of all genes directly involved in the biosynthesis of a secondary metabolite (28). A second hypothesis for the function of secondary metabolite biosynthetic gene clusters is that they facilitate coordinated expression of all genes directly involved in biosynthesis of a metabolite (28). The location of tri5 outside the Trichoderma tri cluster runs counter to both hypotheses in that tri5 would not necessarily be inherited with the rest of the cluster and its expression is correlated with other tri genes and with trichothecene production by Trichoderma (S. Gutiérrez, unpublished data).
Multiple sesquiterpene-derived metabolite biosynthetic gene clusters have been characterized in filamentous fungi. These include the abscisic acid and botrydial clusters in Botrytis cinerea (46, 52), the Ao cluster in Aspergillus oryzae (42), and the gibberellin cluster in Fusarium fujikuroi (35). Of these clusters, only the B. cinerea abscisic acid cluster lacks a sesquiterpene cyclase gene that encodes the enzyme that catalyzes conversion of farnesyl pyrophosphate to the first committed intermediate in synthesis of the metabolite.
In this study, we demonstrated that the Tatri11-encoded cytochrome P450 monooxygenase catalyzes trichothecene C-4 hydroxylation. This finding was unexpected, because C-4 hydroxylation is catalyzed by a different monooxygenase, encoded by the TRI13 gene, in Fusarium. Thus, although Trichoderma and Fusarium employ the same enzymatic mechanism (i.e., cytochrome P450 monooxygenase) for trichothecene C-4 hydroxylation, the two genera do not employ orthologous enzymes to catalyze the reaction. This pattern indicates that the enzymes responsible for trichothecene C-4 hydroxylation may have evolved independently in Trichoderma and Fusarium. As far as we are aware, this is the first report of nonorthologous enzymes catalyzing the same structural modification during synthesis of structurally related secondary metabolites in different fungal genera. Although the structural modifications catalyzed by TaTri11 and FsTri13 are the same, the substrates and products of the reactions are not. The substrate for TaTri11 is the trichothecene skeleton, EPT, whereas the substrate for FsTri13 is calonectrin, i.e., a trichothecene that has been oxygenated and acetylated at C-3 and C-15 (9).
The role of TaTri11 in trichothecene C-4 hydroxylation differs from the role of the Fusarium Tri11 enzyme in trichothecene C-15 hydroxylation. Despite this difference in function, we used the tri11 designation for the Trichoderma orthologue, because in BLASTX analyses, Tatri11 exhibited significant levels of identity (score = 382, E value of 1e−88) to Fusarium TRI11 orthologues but did not exhibit significant levels of identity to Fusarium TRI13 orthologues. However, of the eight Tatri genes identified in this study, Tatri11 exhibits the lowest level of sequence identity to the corresponding Fusarium orthologues; the deduced Tatri11 sequence is 38% identical to the FsTRI11 sequence, whereas other Tatri genes exhibit 46 to 73% identity to their corresponding F. sporotrichioides orthologues (Table 2). In addition, the number and pattern of introns in Tatri11 and FsTRI11 are markedly different from those of other Trichoderma and Fusarium tri/TRI orthologues; Tatri11 has one intron near the 3′ end of the coding region, whereas FsTRI11 has four introns that are dispersed across the coding region. The deduced TaTri11 sequence also exhibits higher levels of sequence identity to proteins from more distantly related fungi than it does to Fusarium Tri11 orthologues. For example, the deduced amino acid sequence for TaTri11 exhibits 58% and 42% identity to protein ATEG_08832 from Aspergillus terreus (class Eurotiomycetes) and SS1G_13923 from Sclerotinia sclerotiorum (class Leotiomycetes), respectively. These data suggest that although Tatri11 and FsTRI11 share an ancestor, the most recent common ancestor was likely not a trichothecene biosynthetic gene and could have occurred in a fungus that was distantly related to the Hypocreales. One possible scenario is that the ancestral trichothecene cluster lacked a tri11/TRI11 homologue and that acquisition of the homologues by the ancestral Trichoderma and Fusarium clusters occurred independently and from different sources. Another possible scenario is that the ancestral trichothecene cluster included a tri11/TRI11 homologue but that the gene was lost from either the ancestral Trichoderma cluster or the ancestral Fusarium cluster, and subsequently the cluster that lost tri11/TRI11 acquired a more distantly related homologue. Despite the likely distant evolutionary origins of Tatri11 and FsTRI11, the position of these homologues relative to other tri/TRI genes is conserved in the core clusters in Trichoderma and Fusarium. Whether this position within the cluster is fortuitous or has a functional significance remains to be determined.
In this study, functional analysis also indicated that the T. arundinaceum tri4-encoded enzyme (TaTri4) catalyzes oxygenation of trichodiene at C-2, C-11, and C-13. These are the same three oxygenations catalyzed by the M. roridum Tri4 enzyme (MrTri4) (39). In contrast, F. graminearum Tri4 (FgTri4) catalyzes trichodiene oxygenation at C-3 as well as C-2, C-11, and C-13 (38). The inability of TaTri4 and MrTri4 to catalyze C-3 oxygenation is consistent with the absence of a C-3 oxygen in trichothecenes produced by Trichoderma and Myrothecium (39, 44). Although TaTri4 and MrTri4 have the same activity, the identity (67%) of their predicted amino acid sequences is lower than the identity (73%) of TaTri4 and FsTri4 (Table 2). Thus, identity of Tri4 orthologues is not correlated with differences in their functions among the three genera examined. This finding raises the question of how differences in Tri4 function arise among trichothecene-producing genera. Did the ancestral Tri4 catalyze four oxygenation reactions and then lose the ability to catalyze the C-3 oxygenation during evolution of Trichoderma and Myrothecium, or did the ancestral Tri4 catalyze only three reactions and then gain the ability to catalyze the C-3 oxygenation during the evolution of Fusarium? Functional and phylogenetic comparisons of orthologues from additional trichothecene-producing genera (e.g., Stachybotrys and Trichothecium) with those of Trichoderma, Myrothecium, and Fusarium should provide further insight into the evolution of Tri4.
Although we did not characterize the function of the TaTri3, its similarity to the corresponding orthologues (Tri3) in Fusarium suggests a function. The numbers and positions of introns are the same in Tatri3 and FsTRI3, and their deduced amino acid sequences exhibit a higher level of identity (48%) than those of TaTri11 and FsTri11. Together, these data indicate that Tatri3 and FsTRI3 are more closely related orthologues than Tatri11 and FsTRI11 and more likely to be descended directly from the same ancestral trichothecene biosynthetic gene. In Fusarium, the TRI3-encoded enzyme (Tri3) is an acetyltransferase; it catalyzes acetylation of the oxygen at C-15 of the trichothecene skeleton (37). However, Trichoderma trichothecenes (e.g., HA and trichodermin) lack an oxygen at C-15 and, therefore, are not acetylated at this position. The functional analyses of TaTri4 and TaTri11 demonstrate that Tri orthologues can have different functions in Trichoderma and Fusarium. Thus, Trichoderma Tri3 likely functions as an acetyltransferase because of (i) the relatively high level of amino acid sequence identity between TaTri3 and FsTri3 and (ii) Trichoderma Tri3 has the amino acid sequence motif, HXXXDG, that is indicative of acetyl-transferases (43). Given these data, a possible function of the Trichoderma Tri3 is acetylation of the oxygen at C-4 of the trichothecene skeleton. This putative function is consistent with the presence of an acetyl moiety at the C-4 oxygen in trichodermin, the predominant trichothecene produced by T. brevicompactum strain IBT 40841. Some acetyltransferases utilize acyl groups other than acetyl as substrates (54). If Trichoderma Tri3 utilizes such a substrate, then the enzyme might also be able to catalyze esterification of octatriendioic acid to the C-4 oxygen to yield HA in T. arundinaceum. This hypothesis can be tested by gene inactivation, i.e., if Tri3 is responsible for the esterification of an octatriendioyl or acetyl moiety to the C-4 oxygen in T. arundinaceum or T. brevicompactum, respectively, inactivation of tri3 should result in production of trichodermol (i.e., C-4-hydroxylated EPT) in both species.
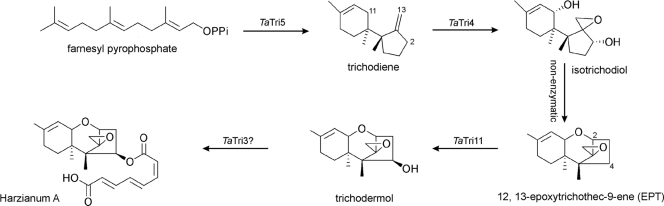
The Trichoderma trichothecenes trichodermin and HA have simpler structures than the Fusarium trichothecenes DON, NIV, and T-2, suggesting that fewer genes are involved. The results of molecular and functional characterization of tri genes in Trichoderma are consistent with this relative structural simplicity and suggest a biosynthetic pathway for HA that requires as few as five biosynthetic enzymes: Tri5 for formation of trichodiene; Tri4 for oxygenation of trichodiene, which leads to the formation of EPT; Tri11 for the oxygenation of EPT at C-4; and the acylation of the C-4 oxygen, possibly by Tri3 (Fig. 6). Formation of HA would most likely require another enzyme, possibly a polyketide synthase (PKS), for synthesis of the octatriendioyl moiety that is esterified to the C-4 oxygen (Fig. 6). The absence of a PKS gene within or adjacent to the Trichoderma tri5 or tri cluster suggests that there could be a third tri locus in T. arundinaceum with a PKS gene responsible for synthesis of octatriendioic acid. Production of trichodermin would not require any other enzymes besides Tri5, and those encoded by the tri cluster since a cellular pool of acetyl coenzyme A (acetyl-CoA) would be available for Tri3 acetylation of the C-4 oxygen.
Fig. 6.
Hypothetical HA biosynthetic pathway deduced by heterologous expression of the Tatri5, Tatri4, and Tatri11 genes in S. cerevisiae and F. verticillioides and the identification of the products of the feeding experiments with the corresponding substrate for each enzyme.
The results of this study have provided insight into the trichothecene toxin biosynthetic pathway in Trichoderma and into complexities of the evolutionary history of the tri/TRI cluster in the fungal order Hypocreales. These complexities include gene loss and/or gain as well as divergence and convergence of gene function. The relocation of tri5 outside the Trichoderma tri cluster contrasts the situation in some Fusarium species, where genes (e.g., TRI1 and TRI101) have relocated into the core cluster from smaller TRI loci (49). This contrast suggests that different forces have acted to affect movement of genes into and out of the tri/TRI cluster during evolution of trichothecene-producing genera. Thus, elucidation of the function of trichothecenes in the ecology of the fungi that produce them may provide insight into factors that affect assembly, growth, and disassembly of secondary metabolite biosynthetic gene clusters.
In conclusion, eight tri genes involved in the trichothecene biosynthesis by T. arundinaceum and T. brevicompactum have been cloned. TaTri5 produce trichodiene from FPP in a fashion similar to that of Tri5 from other trichothecene producers. TaTri4 carried out three consecutive oxygenations at C-2, C-13, and C-11 to give isotrichodiol, similar to Myrothecium and Trichothecium species but different from Fusarium Tri4, that oxygenates trichodiene at four different positions to produce isotrichotriol. Finally TaTri11 carried out a hydroxylation at the C-4 position, which differs substantially from Fusarium Tri11 that hydroxylates at C-15. All these results, together with the possible role of TaTri3 in the trichothecene biosynthesis, allowed us to propose a hypothetical biosynthetic pathway of HA and trichodermin, two mycotoxins with antibiotic and antitumor activities that may also be important agricultural contaminants.
Supplementary Material
ACKNOWLEDGMENTS
Research project funding was from Junta de Castilla y León (GR67) and the Spanish Ministry of Science and Innovation (AGL2006-05660, AGL2009-13431-C01 and AGL2009-13431-C02). M. Gómez was awarded an FPU fellowship by the Spanish Ministry of Science and Innovation (AP2007-02835).
We thank Ulf Thrane for providing the strains T. brevicompactum IBT 40841 and T. arundinaceum IBT 40837 and J. Álvarez and J. Teresi for technical assistance.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Alexander N. J., Hohn T. H., McCormick S. P. 1998. The TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl. Environ. Microbiol. 64:221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander N. J., McCormick S. P., Hohn T. M. 1999. TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: gene isolation and expression in yeast. Mol. Gen. Genet. 26:977–984 [DOI] [PubMed] [Google Scholar]
- 3. Alexander N. J., McCormick S. P., Hohn T. M. 2002. The identification of the Saccharomyces cerevisiae gene AYT1(ORF-YLL063c) encoding an acetyltransferase. Yeast 19:1425–1430 [DOI] [PubMed] [Google Scholar]
- 4. Alexander N. J., Proctor R. H., McCormick S. P. 2009. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 28:198–215 [Google Scholar]
- 5. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 6. Ausubel F. M., et al. 1987. Current protocols in molecular biology. Wiley, New York, NY [Google Scholar]
- 7. Berg J. M., Godwin H. A. 1997. Lessons from zinc-binding peptides. Annu. Rev. Biophys. Biomol. Struct. 26:357–371 [DOI] [PubMed] [Google Scholar]
- 8. Birky C. W. 1975. Effects of glucose repression on the transmission and recombination of mitochondrial gene in yeast (Saccharomyces cerevisiae). Genetics 80:695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown D. W., McCormick S. P., Alexander N. J., Proctor R. H., Desjardins A. E. 2002. Inactivation of a cytochrome P-450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet. Biol. 36:224–233 [DOI] [PubMed] [Google Scholar]
- 10. Brown D. W., Dyer R. B., McCormick S. P., Kendra D. F., Plattner R. D. 2004. Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet. Biol. 41:454–462 [DOI] [PubMed] [Google Scholar]
- 11. Cardoza R. E., et al. 2006. Cloning and characterization of the erg1 gene of Trichoderma harzianum: effect of the erg1 silencing on ergosterol biosynthesis and resistance to terbinafine. Fungal Genet. Biol. 43:164–178 [DOI] [PubMed] [Google Scholar]
- 12. Cardoza R. E., et al. 2007. Partial silencing of a hydroxy-methylglutaryl-CoA reductase encoding gene in Trichoderma harzianum CECT 2413 results in a lower level of resistance to lovastatin and a lower antifungal activity. Fungal Genet. Biol. 44:269–283 [DOI] [PubMed] [Google Scholar]
- 13. Clutterbuck A. J., Arst H. 1995. Genetic nomenclature guide. Aspergillus nidulans. Trends Genet. 11:13–24 [PubMed] [Google Scholar]
- 14. Corley D. G., Miller-Wideman M., Durley R. C. 1994. Isolation and structure of harzianum A: a new trichothecene from Trichoderma harzianum. J. Nat. Prod. 57:422–425 [DOI] [PubMed] [Google Scholar]
- 15. Cuomo C. A., et al. 2007. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317:1400–1402 [DOI] [PubMed] [Google Scholar]
- 16. Degenkolb T., et al. 2008. The Trichoderma brevicompactum clade: a separate lineage with new species, new peptabiotics, and mycotoxins. Mycol. Prog. 7:177–219 [Google Scholar]
- 17. Desjardins A. E. 2006. Fusarium mycotoxins: chemistry, genetics and biology, p. 260 The American Phytopathological Society, St. Paul, MN [Google Scholar]
- 18. Engler K. H., Coker R., Evans I. H. 1999. A novel colorimetric yeast bioassay for detecting trichothecene mycotoxins. J. Microbiol. Methods 35:207–218 [DOI] [PubMed] [Google Scholar]
- 19. Gallo A., Mulè G., Favilla M., Altomare C. 2004. Isolation and characterisation of a trichodiene synthase homologous gene in Trichoderma harzianum. Physiol. Mol. Plant Pathol. 65:11–20 [Google Scholar]
- 20. Gietz R. D., St. Jean A., Woods R. A., Schiestl R. H. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harman G. E. 2000. Myths and dogmas of biocontrol. Plant Dis. 84:377–393 [DOI] [PubMed] [Google Scholar]
- 22. Hermosa M. R., et al. 2000. Molecular characterization and identification of biocontrol isolates of Trichoderma spp. Appl. Environ. Microbiol. 66:1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hohn T. M., Beremand P. 1989. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene 79:131–138 [DOI] [PubMed] [Google Scholar]
- 24. Hohn T. M., Krishna R., Proctor R. H. 1999. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet. Biol. 26:224–235 [DOI] [PubMed] [Google Scholar]
- 25. Hopp T. P., Woods K. R. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. U. S. A. 78:3824–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Howell C. R. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87:4–10 [DOI] [PubMed] [Google Scholar]
- 27. Jin H. Z., et al. 2007. Harzianums A and B produced by a fungal strain, Hypocrea sp. F000527, and their cytotoxicity against tumor cell lines. J. Asian Nat. Prod. Res. 9:203–207 [DOI] [PubMed] [Google Scholar]
- 28. Keller N. P., Turner G., Bennett J. W. 2005. Fungal secondary metabolism from biochemistry to genomics. Nat. Rev. Microbiol. 3:937–947 [DOI] [PubMed] [Google Scholar]
- 29. Kimura M., Tokai T., Takahashi-Ando N., Ohsato S., Fujimura M. 2007. Molecular and genetic studies of Fusarium trichothecene biosynthesis: pathways, genes and evolution. Biosci. Biotechnol. Biochem. 71:2105–2123 [DOI] [PubMed] [Google Scholar]
- 30. Koster B., Wong B., Straus N., Malloch D. 2009. A multi-gene phylogeny for Stachybotrys evidences lack of trichodiene synthase (tri5) gene for isolates of one of three intrageneric lineages. Mycol. Res. 113:877–886 [DOI] [PubMed] [Google Scholar]
- 31. Kuti J. O., Bean G. A., Mackay W. A., Ng T. J. 1989. Influence of muskmelon cell wall polysaccharides on roridin E production by a pathogenic strain of Myrothecium roridum. Mycopathologia 108:139–144 [Google Scholar]
- 32. Lee H. B., et al. 2005. A new Hypocrea strain producing harzianum A cytotoxic to tumour cell lines. Lett. Appl. Microbiol. 40:497–503 [DOI] [PubMed] [Google Scholar]
- 33. Lee J., Juergenson J. E., Leslie J. F., Bowden R. L. 2008. Alignment of genetic and physical maps of Gibberella zeae. Appl. Environ. Microbiol. 74:2349–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leslie J. F., Plattner R. D., Desjardins A. E., Klittich C. J. R. 1992. Fumonisin B1 production by strains from different mating populations of Gibberella fujikoroi (Fusarium section Liseola). Mycotoxicology 82:341–345 [Google Scholar]
- 35. Malonek D., et al. 2005. Functional characterization of two cytochrome P450 monooxygenase genes, P450-1 and P450-4, of the gibberellic acid gene cluster in Fusarium proliferatum (Giberrella fujikuroi MP-D). Appl. Environ. Microbiol. 71:1462–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin L. J., Doebler J. A., Anthony A. 1986. Scanning cytophotometric analysis of brain neuronal nuclear chromatin changes in acute T-2 toxin-treated rats. Toxicol. Appl. Pharmacol. 85:207–214 [DOI] [PubMed] [Google Scholar]
- 37. McCormick S. P., Hohn T. M., Desjardins A. E. 1996. Isolation and characterization of tri3, a gene encoding 15-O-acetyltransferase from Fusarium sporotrichioides. Appl. Environ. Microbiol. 62:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCormick S. P., Alexander N. J., Proctor R. H. 2006. Fusarium tri4 encodes a multifunctional oxygenase required for trichothecene biosynthesis. Can. J. Microbiol. 52:636–642 [DOI] [PubMed] [Google Scholar]
- 39. McCormick S. P., Alexander N. J. 2007. Myrothecium roridum tri4 encodes a multifunctional oxygenase required for three oxygenation steps. Can. J. Microbiol. 53:572–579 [DOI] [PubMed] [Google Scholar]
- 40. McLaughlin C. S., et al. 1977. Inhibition of protein synthesis by trichothecenes, p. 263–273 In Rodricks J. V., Hesseltine C. W., Mehlman M. A. (ed.), Mycotoxins in human and animal health. Patholox Publishers, College Park, MD [Google Scholar]
- 41. Monte E. 2001. Understanding Trichoderma: between agricultural biotechnology and microbial ecology. Int. Microbiol. 4:1–4 [DOI] [PubMed] [Google Scholar]
- 42. Mukherjee M., Horwitz B. A., Sherkhane P. R., Hadar R., Mukherjee P. K. 2006. A secondary metabolite biosynthesis cluster in Trichoderma virens: evidence from analysis of genes underexpressed in a mutant defective in morphogenesis and antibiotic production. Curr. Genet. 50:193–202 [DOI] [PubMed] [Google Scholar]
- 43. Murray I. A., Shaw W. V. 1997. O-acetyltransferases for chloramphenicol and other natural products. Antimicrob. Agents Chemother. 41:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielsen K. F., Gräfenhan T., Zafari D., Thrane U. 2005. Trichothecene production by Trichoderma brevicompactum. J. Agric. Food Chem. 53:8190–8196 [DOI] [PubMed] [Google Scholar]
- 45. Okumwai H., et al. 1999. Trichothecenes as potent inducers of apoptosis, p. 221–231 In Johanning E. (ed.), Bioaerosols, fungi and mycotoxins: health effects, assessment, prevention and control. Boyd Printing Co., Inc., Albany, NY [Google Scholar]
- 46. Pinedo C., et al. 2008. Sesquiterpene synthase from the botrydial biosynthetic gene cluster of the phytopathogen Botrytis cinerea. ACS Chem. Biol. 3:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Proctor R. H., Hohn T. M., McCormick S. P. 1995. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 8:593–601 [DOI] [PubMed] [Google Scholar]
- 48. Proctor R. H., Desjardins A. E., Plattner R. D., Hohn T. M. 1999. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genet. Biol. 27:100–112 [DOI] [PubMed] [Google Scholar]
- 49. Proctor R. H., McCormick S. P., Alexander N. J., Desjardins A. E. 2009. Evidence that a secondary metabolic biosynthetic gene cluster has grown by gene relocation during evolution of the filamentous fungus Fusarium. Mol. Microbiol. 74:1128–1142 [DOI] [PubMed] [Google Scholar]
- 50. Punt P. J., Oliver R. P., Dingemanse M. A., Pouwels P. H., Van den Hondel C. A. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124 [DOI] [PubMed] [Google Scholar]
- 51. Rotter B. A., Prelusky D. B., Pestka J. J. 1996. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 48:1–34 [DOI] [PubMed] [Google Scholar]
- 52. Siewers V., Kokkelink L., Smedsgaard J., Tudzynski P. 2006. Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Appl. Environ. Microbiol. 72:4619–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Specht C. A., DiRusso C. C., Novotny C. P., Ullrich R. C. 1982. A method for extracting high-molecular-weight DNA from fungi. Anal. Biochem. 119:158–163 [DOI] [PubMed] [Google Scholar]
- 54. St. Pierre B., De Luca V. 2000. Evolution of acyltransferase genes: origin and diversification of the GAHP superfamily of acyltransferases involved in secondary metabolism, p. 285–315 In Romeo J. T., Ibrahim R., Varin L., DeLuca V. (ed.), Recent advances in phytochemistry, volume 34, evolution of metabolic pathways. Pergamon, Amsterdam, Netherlands [Google Scholar]
- 55. Sukroongreung S., Schappert K. T., Khachatourians G. G. 1984. Survey of sensitivity of twelve yeast genera toward T-2 toxin. Appl. Environ. Microbiol. 48:416–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tijerino A., et al. 2011. Overexpression of trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet. Biol. 48:285–296 [DOI] [PubMed] [Google Scholar]
- 57. Trapp S. C., Hohn T. M., McCormick S. P., Jarvis B. B. 1998. Characterization of the gene cluster for biosynthesis of macrocyclic trichothecenes in Myrothecium roridum. Mol. Gen. Genet. 257:421–432 [DOI] [PubMed] [Google Scholar]
- 58. Ueno Y., Sakai K., Sato N., Ishii K., Enomoto M. 1972. Toxicological approaches to the metabolites of fusaria. V. Neosolaniol, T-2 toxin, and butenolide, toxic metabolites of Fusarium sporotrichioides NRRL 3510 and Fusarium poae 3287. Jpn. J. Exp. Med. 42:461–472 [PubMed] [Google Scholar]
- 59. Waterhouse A. M., Proctor J. B., Martin D. M. A., Clamp M., Barton G. J. 2009. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Werck-Reichhart D., Feyereisen R. 2000. Cytochromes P450: a success story. Genome Biol. 1:3003.1–3003.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yoder O. C., Valent B., Chumley F. 1986. Genetic nomenclature and practice for plant pathogenic fungi. Phytopathology 76:383–385 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.