Abstract
Acanthamoeba castellanii is a free-living amoebae commonly found in water systems. Free-living amoebae might be pathogenic but are also known to bear phagocytosis-resistant bacteria, protecting these bacteria from water treatments. The mode of action of these treatments is poorly understood, particularly on amoebae. It is important to examine the action of these treatments on amoebae in order to improve them. The cellular response to chlorine, chlorine dioxide, and monochloramine was tested on A. castellanii trophozoites. Doses of disinfectants leading to up to a 3-log reduction were compared by flow cytometry and electron microscopy. Chlorine treatment led to size reduction, permeabilization, and retraction of pseudopods. In addition, treatment with chlorine dioxide led to a vacuolization of the cytoplasm. Monochloramine had a dose-dependent effect. At the highest doses monochloramine treatment resulted in almost no changes in cell size and permeability, as shown by flow cytometry, but the cell surface became smooth and dense, as seen by electron microscopy. We show that these disinfectants globally induced size reduction, membrane permeabilization, and morphological modifications but that they have a different mode of action on A. castellanii.
INTRODUCTION
Free-living amoebae are protozoa commonly found in soils and water (25). Some of them are pathogenic, and interest in these microorganisms has recently increased because they might be reservoirs of pathogenic bacteria in the environment (7). Indeed, some bacteria, collectively referred to as amoeba-resistant bacteria, resist amoeba phagocytosis (11). Some of them, such as Legionella pneumophila, are even able to multiply within amoeba trophozoites (23, 29) and could be found within amoeba cysts (14, 27). Intracellular L. pneumophila is more resistant to biocides (3, 4, 14). Consequently, free-living amoebae, such as Acanthamoeba, have been recently described as important targets in water treatment (19, 30). Common biocides used in water treatment (i.e., chlorine, monochloramine, and chlorine dioxide) were assessed against pathogenic protozoa such as Giardia, Cryptosporidium, and to a lesser extent Entamoeba histolytica (20). However, there are only a few studies on the activity of these biocides against Acanthamoeba (8–10, 17, 22). Moreover, despite the extent use of these oxidants in water treatment, little is known about their mode of action. Chlorine is known to oxidize various molecules, such as thiol groups within proteins and phospholipids (18). Chlorine is more potent at pH under 7.5, where hypochlorous acid is the major form. The mode of action of chlorine and chlorine compounds has been investigated, but researchers concluded that the mode of action is not fully understood (21, 28). We recently published a study on chlorine activity on Acanthamoeba castellanii trophozoites (22), but there is hardly any literature about chlorine dioxide and monochloramine activity on eukaryotic cells.
We sought here to compare the cellular effects of three water disinfectants (chlorine, chlorine dioxide, and monochloramine) on A. castellanii trophozoites. The cells were analyzed by a flow cytometric permeability assay and by both transmission and scanning electron microscopy.
MATERIALS AND METHODS
Acanthamoeba strain and culture.
A. castellanii ATCC 30234 was cultured axenically in peptone-yeast extract-glucose (PYG) medium (26). Cultures were incubated in flask at 25°C for 72 h leading to ca. 90 to 95% confluence.
Disinfectant preparation.
Three different disinfectants that are commonly applied in water treatment were used: chlorine, chlorine dioxide, and monochloramine. All of the solutions were prepared from reagent-grade chemicals and deionized water. Stock solutions were stored at 4°C. All of the glassware was cleaned with chlorine (100 mg/liter) for at least 1 h and carefully rinsed with deionized water. Chlorine solution was freshly prepared by dilution of sodium hypochlorite (13%; Acros Organics). The chlorine concentration was measured by the 4500-Cl G DPD method (2) before and during treatments in order to determine the residual concentration. Stock solution of monochloramine was obtained by adding free chlorine in a solution of ammonium chloride under agitation, with a chlorine/nitrogen molar ratio of 0.5 and at a pH of 8.5. The final concentration of the stock solution of monochloramine was 2 mM (or ∼140 mg of Cl2/liter). The stock solution of chlorine dioxide was prepared by slowly adding sulfuric acid to a sodium chlorite solution and then by collecting the gaseous chlorine dioxide produced in ultrapure water according to the 4500-ClO2 B method (2). The concentration of the stock solution was ∼400 mg/liter. The DPD method was also used to measure the residual concentration of monochloramine and chlorine dioxide.
Disinfection treatment.
All disinfection experiments were performed with 72-h cultures of A. castellanii in PYG. The trophozoites were washed twice (500 × g, 5 min) with phosphate buffer (0.05 M, pH 7). The cell pellet was resuspended in the same buffer and adjusted to 105 to 106 cells/ml. A. castellanii trophozoites were treated with various concentrations of disinfectants for 30 s (or 60 s for monochloramine). After 15 and 30 s (30 and 60 s for monochloramine), residual concentration of disinfectants was measured. The disinfectant exposure was quantified by Ct (concentration × time, in mg·min/liter), which corresponds to the geometric area under the disinfectant decay curve. Treatments with chlorine, monochloramine, and chlorine dioxide were stopped by the addition of sterile sodium thiosulfate 0.1 M in excess.
Cultivability by MPN method.
Cultivable cells were determined by a microtiter plate most-probable-number (MPN) method (6). Each sample of treated or untreated trophozoites was subjected to 10-fold serial dilutions in PYG. Then, aliquots of 200 μl of each dilution were distributed in quintuplicates on 96-well plates and incubated at 25°C for 14 days. Positive growth response was indicated by a visible turbidity in wells. The cultivable trophozoite concentration was estimated by referring to the MPN tables (6) and is expressed as MPN/ml. Microbial inactivation (loss of cultivability) was recorded as function of Ct, to evaluate the effectiveness of the disinfectants.
Flow cytometry analysis.
Flow cytometric measurements were performed at the Image′UP flow cytometry platform (University of Poitiers) on a FACSCanto II flow cytometer (BD Biosciences) with a 488-nm argon excitation laser. The forward scatter (FSC) is reflects the cell size, while the side scatter (SSC) reflects the cell granularity. Cells were stained either with propidium iodide (PI) at 30 μM during 15 min. PI is a viability marker that enters permeable cells. Optical filters were set up so that PI fluorescence was measured with a 670 LP filter. The FSC threshold was adjusted to 30.000 (arbitrary unit) to eliminate noise and debris. Two controls were used to define analysis gates: (i) amoebae treated with a high concentration of chlorine for the permeabilized dead cells (PI-positive) population and (ii) untreated amoebae. A total of 10,000 events were analyzed in treated and untreated samples, using BD FACSDiva 6 software (BD) for data acquisition and analysis. All experiments were repeated at least three times.
Electron microscopic analysis.
For transmission electron microscopy, cell suspensions were centrifuged (500 × g, 10 min), and the pellets were fixed with 3% glutaraldehyde in phosphate buffer (0.1 M; pH 7.4) for 2 h at 4°C. Samples were then washed three times in phosphate buffer, postfixed in phosphate buffer containing 1% osmium tetroxide (1 h at 4°C), processed through a graded acetone series, and embedded in araldite (Fluka, Buchs, Switzerland) polymerized overnight at 60°C. Thin sections (60 nm) were cut with a diamond knife on a Reichert Ultracut S, recovered on Cu grids, and contrasted with uranyl acetate (4%) and lead citrate. Samples were observed under a JEOL 1010 transmission electron microscope (JEOL, Ltd., Tokyo, Japan). For scanning electron microscopy, cell suspensions were centrifuged (500 × g, 10 min), and the pellets were fixed with 3% glutaraldehyde in phosphate buffer (0.1 M; pH 7.4) for 2 h at 4°C. Cells were washed three times in phosphate buffer, fixed on coverslips through a graded acetone series, and dried by critical point drying (BAL-TEC CPD 030) using acetone and liquid carbon dioxide as the transition fluid. The dried specimens were coated with gold (25- to 35-nm thickness) using a sputtering device (BAL-TEC LCD 005). The samples were examined with a JEOL JSM-840 electron microscope.
RESULTS
Inactivation of A. castellanii.
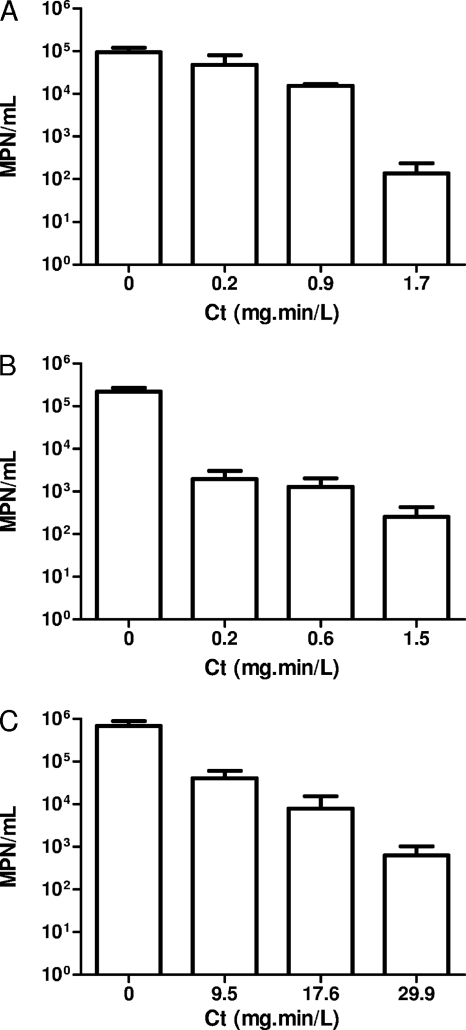
In order to determine the doses inducing a 3-log inactivation and to compare the efficacy of each oxidant on A. castellanii cultivability, increased concentrations were tested against A. castellanii trophozoites. The cultivability was assessed after the treatment and represented in function of Ct for each oxidant. We have verified that the treatments did not result in significant cell lysis by direct microscopic enumeration (data not shown). The results show that chlorine dioxide and chlorine were more effective (Ct99.9% = 1.5 and 1.7 mg·min/liter, respectively) than monochloramine (Ct99.9% = 29.9 mg·min/liter) (Fig. 1).
Fig. 1.
Inactivation of A. castellanii with increasing doses of disinfectants chlorine (A), chlorine dioxide (B), and monochloramine (C). Values are averages calculated from three independent experiments ± the standard deviation. Statistical analysis were performed by using a Student t test (*, P < 0.005).
Treatments induce size modification and membrane permeabilization.
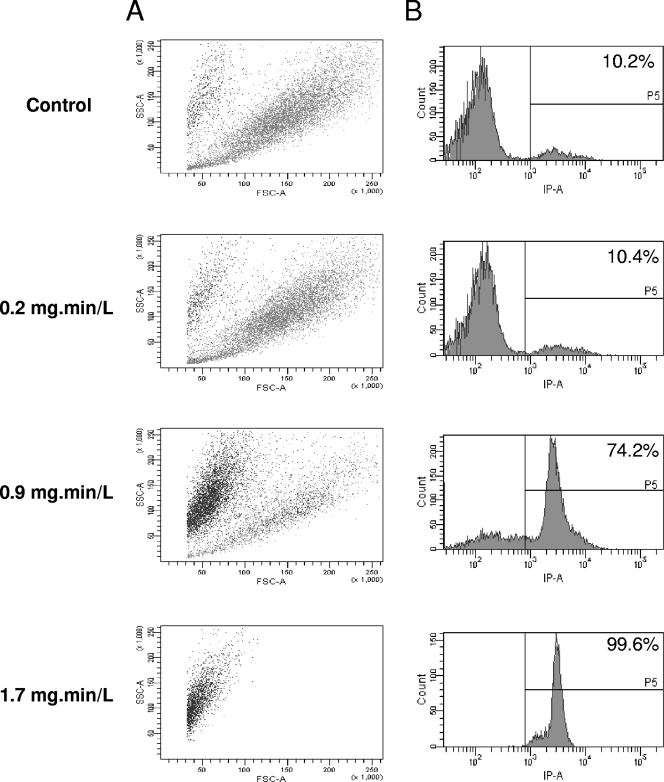
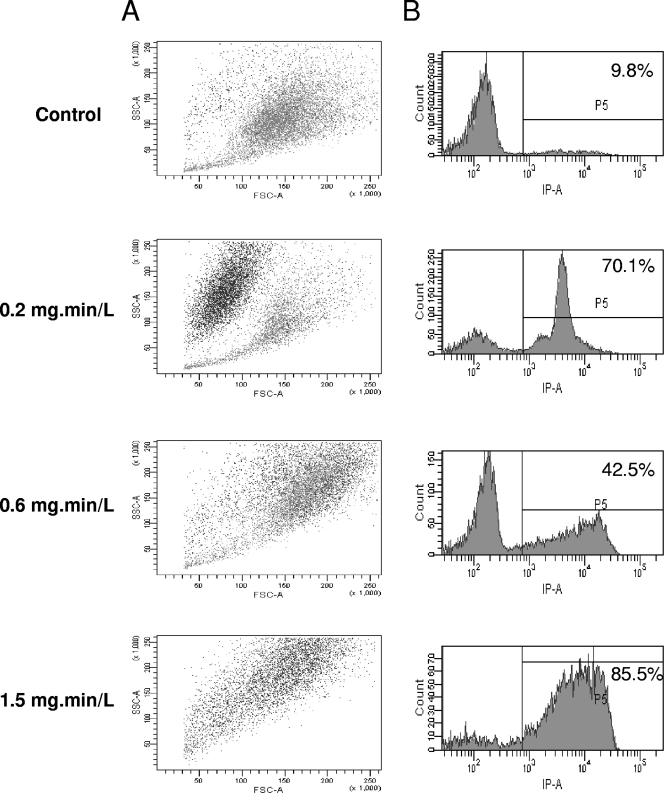
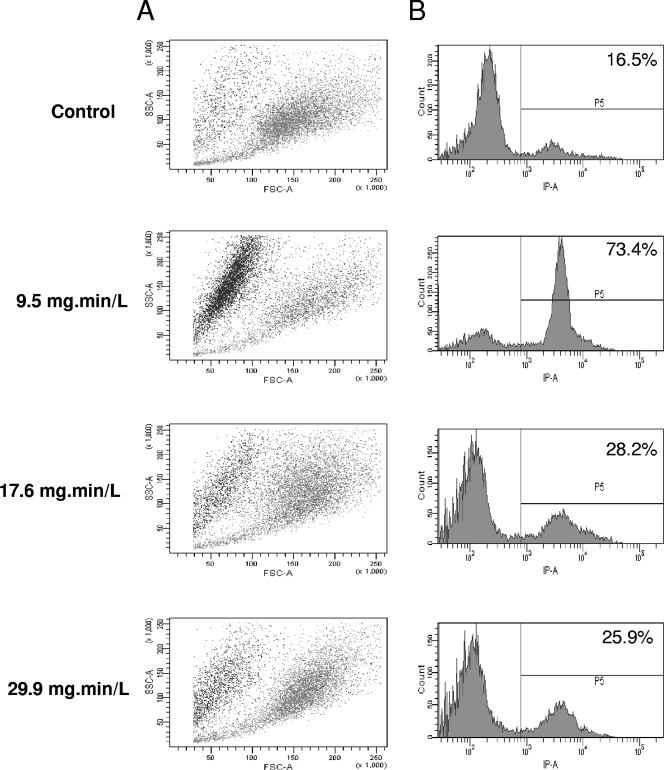
The effect of biocides on various cells is classically assessed by determining cell permeabilization using trypan blue or PI. A. castellanii trophozoites were treated, stained with PI, and analyzed by flow cytometry in order to determine the morphology and the permeability of the cells. With chlorine, the FSC value, related to cell size, decreased, and the ratio of PI-positive cells, related to permeability, increased, along with an increase of chlorine concentration (Fig. 2). In addition, these PI-positive cells corresponded to the small cells (data not shown). We have previously reported both size reduction and permeabilization after chlorine treatment (22). With chlorine dioxide, there was a decrease in the size and an increased in ratio of PI-positive cells at the lowest Ct (0.2 mg·min/liter) (Fig. 3). At the intermediary Ct (0.6 mg·min/liter), the cells became clearly larger (FSC parameter), and there was a decreased in the ratio of PI-positive cells (42.5% at Ct = 0.6 mg·min/liter). At the highest Ct (1.5 mg·min/liter), there was a novel increase of PI-positive count (85.5%) and the peak was much larger reflecting a large range of PI intensity within the cells. With monochloramine, the lowest dose (Ct = 9.5 mg·min/liter) induced a classical response, i.e., reduction of size and increase of PI-positive cells (Fig. 4). Surprisingly, at a higher Ct, there was only a slight modification of these parameters compared to control cells.
Fig. 2.
Chlorine activity on A. castellanii assessed by flow cytometry. (A) SSC is represented in function FSC, where each spot corresponds to PI-positive cells (black) or PI-negative cells (gray). (B) The number of cells is represented in the function of PI fluorescence. The ratio of PI-positive cells (>8 × 102) is given as a percentage.
Fig. 3.
Chlorine dioxide activity on A. castellanii assessed by flow cytometry. (A) SSC is represented in function FSC, where each spot corresponds to PI-positive cells (black) or PI-negative cells (gray). (B) The number of cells is represented in the function of PI fluorescence. The ratio of PI-positive cells (>8 × 102) as given as a percentage.
Fig. 4.
Monochloramine activity on A. castellanii assessed by flow cytometry. (A) SSC is represented in function FSC, where each spot corresponds to PI-positive cells (black) or PI-negative cells (gray). (B) The number of cells is represented in the function of PI fluorescence. The ratio of PI-positive cells (>8 × 102) is given as a percentage.
Treatments induce subcellular changes.
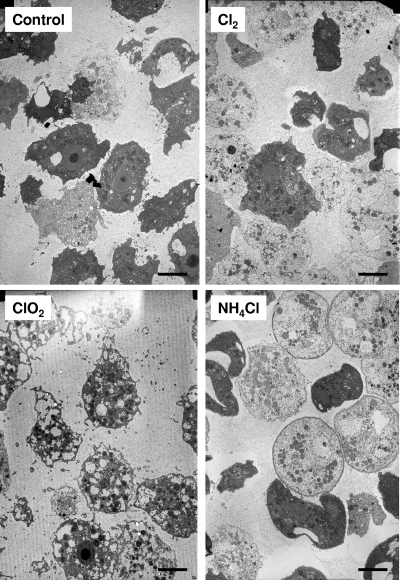
In order to better understand, at the cellular level, the response of A. castellanii to these oxidants, we performed electronic microscopy. Control and treated cells were analyzed by both scanning electron microscopy and transmission electron microscopy (SEM and TEM, respectively). Representative pictures of cells after treatments at highest doses were presented. With TEM, the response of the cells was clearly different between the oxidants (Fig. 5). Chlorine appeared to have slight effect on cell structure, but most of the cells lost electron-dense material and became clear. With chlorine dioxide, the cells became highly vacuolated but cytoplasm remained rather dense. With monochloramine, the cells became clear and spherical, and the membrane became more dense to electrons.
Fig. 5.
Cellular effect of disinfectants at their highest doses (i.e., chlorine at 1.7 mg·min/liter, chlorine dioxide at 1.5 mg·min/liter, and monochloramine at 29.9 mg·min/liter) as assessed by TEM. The control corresponds to untreated cells. Scale bars, 10 μm.
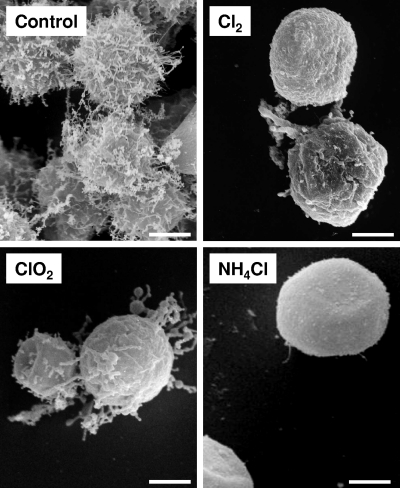
With SEM, we observed that the treatments induced a retraction of the acanthapods (Fig. 6). However, with monochloramine, the cells were spherical, and the surface was very smooth, while with other treatments small acanthapods and ripples could be viewed.
Fig. 6.
Cellular effect of the disinfectants at their highest doses (i.e., chlorine at 1.7 mg·min/liter, chlorine dioxide at 1.5 mg·min/liter, and monochloramine at 29.9 mg·min/liter) as assessed by SEM. The control corresponds to nontreated cells. Scale bars, 5 μm.
DISCUSSION
In this study, we consider the activity, at the cellular level, of chlorine, chlorine dioxide, and monochloramine on A. castellanii trophozoites. We first determined the doses of these disinfectants leading to 3-log reductions (Ct99.9%). The Ct99.9% values with chlorine, chlorine dioxide, and monochloramine were 1.5, 1.7, and 29.9 mg·min/liter, respectively. These doses are on the same order as those already published by our group (10) and used classically for water treatment (15). For example, a residual of 1 mg of chlorine/liter is usually found, suggesting that 2 min of contact should be enough to inactivate A. castellanii. Also, the highest Ct99.9% found with monochloramine is in accordance with most studies, although we have recently shown that this could be dependent of the target strain (10).
Flow cytometry analyses demonstrate that cellular responses were greatly variable between disinfectants. Chlorine induced cell shrinking and permeabilization. The decrease of PI staining after chlorine dioxide treatment at 0.6 mg·min/liter suggested that DNA might be partly destroyed, as shown with chlorine treatment on bacteria (24). Surprisingly, with the highest dose, cells became highly PI positive, but the range of fluorescence level was wider (large peak). Further observation of these cells by fluorescence microscopy revealed that the cytoplasm was stained (data not shown), which was not observed with a lower dose. We can speculate that this observation might be due to the staining of mitochondrial DNA. Finally, monochloramine results were quite surprising since, at the highest dose, there was hardly any effect on cell morphology and permeabilization. Nevertheless, this dose yielded a 3-log reduction in A. castellanii viability. We hypothesized that this behavior could be due to a decrease in cell permeability, preventing PI staining. A previous study also showed that A. castellanii was poorly stained with PI even after treatment with high doses of monochloramine (5). Also, several studies have suggested that chlorine and monochloramine treatments of bacteria could result in viable but nonculturable (VBNC) cells (1, 12); thus, we can speculate that such a VBNC state may exist within amoebae.
Further analysis by electronic microscopy confirmed that the disinfectants have different effects on A. castellanii. Indeed, the cell morphologies after treatments were highly dissimilar. Most importantly, monochloramine led to spherical cells with dense membranes and smooth surfaces. Previously, chlorhexidine diacetate treatment was shown to result in a smooth surface, while polyhexamethylene biguanide induced a rough and granular surface (13). We hypothesize that, with monochloramine, the modification of the membrane could explain why there was almost no change evident by flow cytometry. Such a denser membrane was also observed after treatment of Acanthamoebae with propylene glycol (16). These peculiar cells were named pseudocysts by the authors because the cells resembled cysts but did not evolve into mature cysts. Regarding these results with monochloramine, it might be interesting to assess Acanthamoeba survival after treatments because the observed loss of cultivability might be only temporary and, in certain unknown conditions, Acanthamoeba could recover their ability to be cultivated. Survival could be assessed by testing regrowth under different conditions or, alternatively, by using qRT-PCR to look for de novo RNA synthesis.
In conclusion, comparison of cellular effects of oxidizing disinfectants on A. castellanii has demonstrated that their effect is highly disparate, reflecting different modes of action. In particular, monochloramine led to a unique response, since the highest doses did not result in modifications of size and permeability. Also, monochloramine treatment at the highest doses seemed to greatly modify the cellular membrane morphology. We have previously proposed that monochloramine should have a different mode of action compared to chlorine and chlorine dioxide on the basis of inactivation studies (10). Although further experiments are needed to better understand the mode of action of these oxidants, these results might be helpful to further improve water treatment.
ACKNOWLEDGMENTS
We acknowledge Florence Berne for the technical help in oxidant preparation.
C.B. was supported by a CNRS grant.
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Alleron L., Merlet N., Lacombe C., Frere J. 2008. Long-term survival of Legionella pneumophila in the viable but nonculturable state after monochloramine treatment. Curr. Microbiol. 57:497–502 [DOI] [PubMed] [Google Scholar]
- 2. American Public Health Association 2005. Standard methods for the examination of water and wastewater, 21st ed. American Public Health Association, Washington, DC [Google Scholar]
- 3. Barker J., Brown M. R., Collier P. J., Farrell I., Gilbert P. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 58:2420–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barker J., Scaife H., Brown M. R. 1995. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 39:2684–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berry D., Horn M., Xi C., Raskin L. Mycobacterium avium infections of Acanthamoeba strains: host strain variability, grazing-acquired infections, and altered dynamics of inactivation with monochloramine. Appl. Environ. Microbiol. 76:6685–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown E. J., Braddock J. F. 1990. Sheen screen, a miniaturized most-probable-number method for enumeration of oil-degrading microorganisms. Appl. Environ. Microbiol. 56:3895–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown M. R., Barker J. 1999. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 7:46–50 [DOI] [PubMed] [Google Scholar]
- 8. Critchley M., Bentham R. 2009. The efficacy of biocides and other chemical additives in cooling water systems in the control of amoebae. J. Appl. Microbiol. 106:784–789 [DOI] [PubMed] [Google Scholar]
- 9. Cursons R. T., Brown T. J., Keys E. A. 1980. Effect of disinfectants on pathogenic free-living amoebae: in axenic conditions. Appl. Environ. Microbiol. 40:62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dupuy M., et al. 2011. Efficiency of water disinfectants against Legionella pneumophila and Acanthamoeba. Water Res. 45:1087–1094 [DOI] [PubMed] [Google Scholar]
- 11. Greub G., Raoult D. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard K., Inglis T. J. 2003. The effect of free chlorine on Burkholderia pseudomallei in potable water. Water Res. 37:4425–4432 [DOI] [PubMed] [Google Scholar]
- 13. Khunkitti W., Hann A. C., Lloyd D., Furr J. R., Russell A. D. 1998. Biguanide-induced changes in Acanthamoeba castellanii: an electron microscopic study. J. Appl. Microbiol. 84:53–62 [DOI] [PubMed] [Google Scholar]
- 14. Kilvington S., Price J. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519–525 [DOI] [PubMed] [Google Scholar]
- 15. Kim B. R., Anderson J. E., Mueller S. A., Gaines W. A., Kendall A. M. 2002. Literature review: efficacy of various disinfectants against Legionella in water systems. Water Res. 36:4433–4444 [DOI] [PubMed] [Google Scholar]
- 16. Kliescikova J., Kulda J., Nohynkova E. 2011. Propylene glycol and contact-lens solutions containing this diol induce pseudocyst formation in acanthamoebae. Exp. Parasitol. 127:326–328 [DOI] [PubMed] [Google Scholar]
- 17. Kuchta J. M., et al. 1993. Impact of chlorine and heat on the survival of Hartmannella vermiformis and subsequent growth of Legionella pneumophila. Appl. Environ. Microbiol. 59:4096–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambert P. A. 2004. Mechanisms of action of biocides, p. 139–153 In Fraise A. P., Lambert P. A., Mallard J.-Y. (ed.), Principles and practice of disinfection, preservation, and sterilization. Blackwell Publishing, New York, NY [Google Scholar]
- 19. Loret J. F., Greub G. 2010. Free-living amoebae: biological by-passes in water treatment. Int. J. Hyg. Environ. Health 213:167–175 [DOI] [PubMed] [Google Scholar]
- 20. Maillard J.-Y. 2004. Intestinal protozoa and biocides, p. 258–271 In Fraise A. P., Lambert P. A., Mallard J.-Y. (ed.), Principles and practice of disinfection, preservation, and sterilization. Blackwell Publishing, New York, NY [Google Scholar]
- 21. McDonnell G., Russell A. D. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mogoa E., Bodet C., Legube B., Hechard Y. 2009. Acanthamoeba castellanii: cellular changes induced by chlorination. Exp. Parasitol. 126:97–102 [DOI] [PubMed] [Google Scholar]
- 23. Molmeret M., Horn M., Wagner M., Santic M., Abu Kwaik Y. 2005. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phe M. H., Dossot M., Block J. C. 2004. Chlorination effect on the fluorescence of nucleic acid staining dyes. Water Res. 38:3729–3737 [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez-Zaragoza S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225–241 [DOI] [PubMed] [Google Scholar]
- 26. Schuster F. L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15:342–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skinner A. R., Anand C. M., Malic A., Kurtz J. B. 1983. Acanthamoebae and environmental spread of Legionella pneumophila. Lancet ii:289–290 [DOI] [PubMed] [Google Scholar]
- 28. Stewart M. C., Olson B. H. 1996. Bacterial resistance to potable water disinfectants, p. 140–192 In Hurst C. J. (ed.), Modeling disease transmission and its prevention by disinfection. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 29. Taylor M., Ross K., Bentham R. 2009. Legionella, protozoa, and biofilms: interactions within complex microbial systems. Microb. Ecol. 58:538–547 [DOI] [PubMed] [Google Scholar]
- 30. Thomas V., et al. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97:950–963 [DOI] [PubMed] [Google Scholar]