Abstract
Dynamic processes during wet-heat treatment of individual spores of Bacillus cereus, Bacillus megaterium, and Bacillus subtilis at 80 to 90°C were investigated using dual-trap Raman spectroscopy, differential interference contrast (DIC) microscopy, and nucleic acid stain (SYTO 16) fluorescence microscopy. During spore wet-heat treatment, while the spores' 1:1 chelate of Ca2+ with dipicolinic acid (CaDPA) was released rapidly at a highly variable time Tlag, the levels of spore nucleic acids remained nearly unchanged, and the Tlag times for individual spores from the same preparation were increased somewhat as spore levels of CaDPA increased. The brightness of the spores' DIC image decreased by ∼50% in parallel with CaDPA release, and there was no spore cortex hydrolysis observed. The lateral diameters of the spores' DIC image and SYTO 16 fluorescence image also decreased in parallel with CaDPA release. The SYTO 16 fluorescence intensity began to increase during wet-heat treatment at a time before Tlag and reached maximum at a time slightly later than Trelease. However, the fluorescence intensities of wet-heat-inactivated spores were ∼15-fold lower than those of nutrient-germinated spores, and this low SYTO 16 fluorescence intensity may be due in part to the low permeability of the dormant spores' inner membranes to SYTO 16 and in part to nucleic acid denaturation during the wet-heat treatment.
INTRODUCTION
Spores of Bacillus species are formed under starvation conditions and are metabolically dormant and extremely resistant to a variety of physical and chemical agents, including heat, radiation, and many chemical disinfectants (35). Since spores of many of these species can cause food spoilage and food-borne diseases (37), efforts to eliminate spores from foods are routinely made. High-temperature treatment of hydrated spores is very effective in spore killing and is the most common method used by the food industry for spore inactivation, even though hydrated dormant spores invariably resist much higher temperatures than do the growing cells of the same strain (40). Factors that contribute to spore wet-heat resistance include (i) the spore core's low water content (11, 20, 34), (ii) the core's high level of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) plus its chelated divalent cations, predominantly Ca2+ (CaDPA), and (iii) the protection of spore DNA against depurination by its saturation with a group of α/β-type small, acid-soluble spore proteins (SASP) (10, 30). In contrast to a good understanding of the mechanisms of spore resistance to wet heat, much less is known about the mechanism of spore killing by wet heat, although it is not by DNA damage or oxidative damage (32, 35). The wet-heat killing of spores is accompanied by significant protein denaturation, as much spore protein changes from an α-helical structure to one that is irregular and likely denatured (6, 7, 46). Wet-heat treatment of spores can cause the rapid release of the spore's CaDPA depot, and results with spores of Bacillus subtilis, Bacillus cereus, and Bacillus megaterium suggest that some protein denaturation precedes CaDPA release (6, 7, 46). However, it is not clear how CaDPA leaves the spore core during wet-heat treatment and what factors determine the highly variable lag time prior to CaDPA release during wet-heat treatment of individual spores. It seems likely that rapid CaDPA release at high temperatures is due to failure of the spore's inner membrane, although DPA-specific channels in this membrane are believed to be the pathway for CaDPA release during spore germination (25). Recent work has shown that SYTO 16, a membrane-permeant nucleic acid dye that exhibits a large fluorescence enhancement upon binding to nucleic acids (both DNA and RNA), can pass through the inner membrane only after spores are fully germinated (16). Consequently, it would be of interest to examine the spore inner membrane permeability to this dye during wet-heat treatment.
Raman spectroscopy is capable of providing information on molecular structures, cellular composition, and physiological states and has been widely used for biomedical analysis (18, 29). In combination with an optical trap, Raman spectroscopy (Raman tweezers) allows the nondestructive, noninvasive analysis of single cells in liquid media and has been used to identify single bacterial cells (42) and to monitor the kinetics of germination and wet-heat inactivation of individual Bacillus spores (4, 16, 26, 33, 46). Raman tweezers with two closely separated traps (dual-trap Raman tweezers) have also allowed studies of the dynamic responses of two bacterial spores in nearly identical microenvironments to wet heat, thereby helping identify reasons for heterogeneity in wet-heat inactivation of spores (46). Differential interference contrast (DIC) microscopy is also used frequently for the observation of transparent cells. Recently, a combination of DIC microscopy and Raman spectroscopy revealed a precise correspondence between the rapid drop in spore refractility and the release of the great majority of a spore's CaDPA during germination, and this correspondence was utilized in simultaneously monitoring the kinetics of germination of hundreds to thousands of individual spores (45). The latter work also revealed a further slow drop in spore refractility following CaDPA release that is most likely associated with the degradation of the spore's peptidoglycan cortex (45).
In the present work, we utilized Raman tweezers in combination with DIC and fluorescence microscopy to monitor the kinetics of wet-heat inactivation of single spores of three different Bacillus species and some B. subtilis strains. Raman tweezers were used to measure CaDPA release, protein denaturation, and nucleic acid levels; DIC microscopy was used to follow changes in the spore's refractility, especially after CaDPA release, to examine whether there is cortex degradation during wet-heat treatment; and fluorescence microscopy was used to observe the inner spore membrane's permeability to SYTO 16. The integration of these three analytical techniques has given us further understanding of the process of spore wet-heat inactivation.
MATERIALS AND METHODS
Bacillus strains and spore preparation.
The Bacillus species and strains used in this work were B. cereus T (originally obtained from H. O. Halvorson), B. megaterium QM B1551 (originally obtained from H. S. Levinson), and B. subtilis strain PS832 (wild type, originally obtained from D. J. Tipper) and its isogenic derivatives, including the following: (i) PS578 (termed α− β−), which carries plasmid pUB110 encoding resistance to kanamycin (10 μg/ml) and also lacks the genes encoding the spore's two major α/β-type SASP, such that the α/β-type SASP level in PS578 spores is only ∼25% of that in wild-type spores (31); (ii) FB111 (cwlJ), which lacks the cortex-lytic enzyme (CLE) CwlJ (24); (iii) FB112 (sleB), which lacks the CLE SleB (24); (iv) FB113 (cwlJ sleB), which lacks both CwlJ and SleB (24); (v) PS2307, which lacks the muramic acid-δ-lactam residues in the spore cortical peptidoglycan due to a deletion of the cwlD gene and its replacement with a chloramphenicol resistance (5 μg/ml) cassette, so that spores of this strain are unable to degrade the cortex during germination (27); and (vi) PS4204 (α− β− cwlD), spores of which lack most α/β-type SASP and cwlD and also carry plasmid pUB110. Strain PS4204 was generated by transforming strain PS578 with chromosomal DNA from strain PS2307 (31) and selecting for resistance to chloramphenicol.
B. cereus spores were prepared at 30°C in defined liquid medium (5), B. megaterium spores were prepared at 30°C in liquid-supplemented nutrient broth (12), and spores of B. subtilis strains were prepared on 2×SG medium agar plates at 37°C without antibiotics (23). After harvesting, the spores were suspended in deionized water, purified by repeated centrifugation (12,000 × g for 20 min at 4°C in an SS-34 rotor in a Dupont RC5-B centrifuge) and washing with water, and stored at 4°C in water protected from light (22). All spore preparations were free (>98%) of growing or sporulating cells and germinated spores as determined by phase-contrast microscopy.
Wet-heat inactivation and germination of bacterial spores.
Spore wet-heat treatment was in distilled water at 80°C (B. cereus and B. megaterium) or 90°C (B. subtilis). In some experiments spore viability was measured during wet-heat treatment as described previously (6, 7). For observation of the kinetics of wet-heat inactivation of single spores, an aliquot of spores (∼2 μl of 4°C water with ∼2 × 106 spores/ml) was injected into a homemade microscope sample holder (a copper plate of 50 mm by 30 mm by 3.8 mm with a hole of diameter 6 mm in the center and a quartz coverslip [diameter 10 mm by 0.1 mm; UPG Optics]) adhered to the bottom of the hole) that was filled with distilled water (∼600 μl) and held at 80°C or 90°C using a temperature-stabilized controller (Thorlabs, Newton, NJ), and single suspended spores were chosen randomly to be investigated.
Unless otherwise noted, spores were heat activated prior to germination by incubation of spores in water for 30 min at 70°C (B. subtilis), 20 min at 65°C (B. cereus), or 15 min at 60°C (B. megaterium) and then put on ice for ≥15 min. Spores of B. subtilis and B. cereus were germinated at 37°C with 10 mM l-alanine in 25 mM Tris-HCl buffer (pH 8.3), and B. megaterium spores were germinated at 30°C with 10 mM glucose in 25 mM KPO4 buffer (pH 7.4). For observation of the kinetics of germination of single spores, a drop of heat-activated (B. megaterium and B. subtilis) or unactivated (B. cereus) spores at ∼2 × 106 spores/ml was injected into the microscope sample holder, which was filled with germinants and held at 37 or 30°C, and single suspended spores were chosen randomly to be investigated.
Combination of dual-trap Raman tweezers and DIC and fluorescence microscopy.
The combination of dual-trap Raman tweezers and DIC and fluorescence microscopy was carried out using a modification of a system described in detail recently (45). A diode laser at 780 nm served for both optical trapping and the Raman excitation source. To improve the experimental efficiency, the laser beam was divided into two orthogonally polarized beams by a polarized beam splitter (PBS) to form two optical traps separated by ∼3 μm (46). The Raman scattering light from trapped spores was recorded by a Jobin Yvon Triax 320 spectrograph (Horiba Jobin Yvon Inc., Edison, NJ) equipped with a charge-coupled device (CCD) detector (PIXIS 100; Princeton Instruments, Trenton, NJ). The Raman spectra were recorded from 600 to 1,800 cm−1, with a resolution of ∼6 cm−1. An inverted microscope (TE2000-S; Nikon Instruments, Melville, NY) equipped with a high-numerical-aperture (NA) objective (100×, NA, 1.3; Nikon, Lewisville, TX) and a set of DIC accessories was used for DIC observation. The halogen lamp was replaced with a high-power light-emitting diode (LED) (DigiKey, Thief River Falls, MN) at 470 nm as both the illumination source for DIC imaging and the excitation source for fluorescence microscopy. The LED was powered by a diode driver (Thorlabs, Newton, NJ), and the light intensity at the specimen was ∼4.0 mW/cm2. After being separated from the Raman scattering light using a dichroic mirror, the illumination light and the fluorescence light were delivered to the two observation ports of the microscope by a beam splitter inserted between the analyzer and the dichroic mirror. The beam splitter had a reflectivity of 80%, and the transmitted light passed through the analyzer and was imaged onto a 12-bit digital CCD camera (CCE-B013-U; Mightex Systems, Toronto, Canada) to generate DIC images. The reflected light passed through a band-pass filter (530 nm) and was imaged onto another 16-bit digital CCD camera (QSI 520; Quantum Scientific Imaging, Poplarville, MS) to generate fluorescence images.
Monitoring of dynamics of individual spores during wet-heat treatment or germination.
After addition of spore samples to the sample chamber filled with a mixture of 0.5 μM SYTO 16 (Invitrogen, Carlsbad, CA) and either distilled water (wet-heat inactivation) or germinants (germination), a pair of spores was captured by the Raman tweezers within 2 to 3 min (defined as T0) and the dynamics during wet-heat treatment or germination were monitored by Raman spectroscopy and DIC and fluorescence microscopy. The laser power used for the measurements was 5 mW for each trap, and the integration times for Raman spectrum acquisition and DIC and fluorescence imaging were 20, 2, and 10 s, respectively. To synchronize the acquisition of the three different types of data, the software for fluorescence imaging was programmed to output two synchronized pulse signals at an acquisition interval of 20 s. The two pulse signals were then used to trigger the spectrograph and the DIC-imaging CCD camera. The dynamic wet-heat inactivation of spores was monitored by dual-trap Raman tweezers and DIC and fluorescence microscopy for 30 min, and the dynamics during spore germination were monitored for 30 min (B. megaterium) or up to 1 h (B. cereus and B. subtilis strains). For each measurement, background spectra and images were recorded under the same conditions without spores in the traps and subtracted from the spectra and images of individual spores prior to data analysis.
Measurement of SYTO 16-DNA fluorescence at various temperatures.
Pure double-stranded lambda phage DNA (Ward's Natural Science, Rochester, NY) (10 μg in 80 μl of 10 mM Tris-HCl buffer [pH 7.5] plus 1 mM EDTA [TE buffer]) was mixed with 2 μM SYTO 16 dye at a ratio of 1:1, heat treated at various temperatures in a water bath for ∼30 min, and then loaded into the sample chamber described above held at the corresponding temperatures. The fluorescence of SYTO 16-DNA was collected by an air gap objective (100×, NA 0.9; Nikon, Lewisville, TX) and recorded by the CCD camera. The DNA was also (i) heat treated at 90°C in a water bath for 30 min, cooled on ice for ∼15 min, mixed 1:1 with 2 μM SYTO 16, and fluorescence measured at 23°C or (ii) mixed 1:1 with 2 μM SYTO 16 prior to heat treatment at 90°C for 30 min, cooled on ice for 15 min, and fluorescence measured at 23°C. In addition, double-stranded DNA (dsDNA) (0.5 μg/μl in TE buffer) and single-stranded DNA (ssDNA) (1 μg/μl in TE buffer) of M13 phage mp18 (Bayou Biolabs, Metairie, LA) were diluted in deionized water to 0.125 μg/μl, mixed 1:1 with 2 μM SYTO 16, and fluorescence measured at 23°C. Background images taken at the same temperatures but with the SYTO 16-DNA mixture replaced by 1 μM SYTO 16 were also recorded and subtracted from the fluorescence images of SYTO 16 with DNA prior to data analysis.
Measurement of SYTO 16 fluorescence of dormant, wet-heat-killed, boiled, and germinated spores at room temperature.
The fluorescence of various spore populations in 0.5 μM SYTO 16 was also measured at 23°C in the microscope sample chamber (∼600 μl of 0.5 μM SYTO 16) to avoid any temperature-dependent effects on SYTO 16 fluorescence intensity, as detailed below. After injection of 2 μl of spores into the sample chamber as described above, individual spores were captured by laser tweezers and their fluorescence images were recorded. CaDPA and nucleic acid levels of individual spores were also determined by Raman spectroscopy (2, 3, 14, 19). For measurements with populations of wet-heat-treated spores, ∼2 × 106 spores/ml were treated at wet-heat inactivation temperatures with or without 0.5 μM SYTO 16 for 40 min and cooled on ice for ∼15 min. The spores that were wet-heat treated with SYTO 16 were loaded into the sample chamber for fluorescence measurements; spores heat treated without SYTO 16 were mixed 1:1 with 1 μM SYTO 16 after cooling and then injected into the sample chamber. Fluorescence of spores that retained or had released their CaDPA was then measured.
For measurements with populations of germinated spores, heat-activated spores (∼107 spores/ml) were incubated at appropriate temperatures with germinants for 30 min (B. cereus and B. megaterium), 40 min (B. subtilis strains PS832 and PS578), or 1 h (B. subtilis strains FB111, FB112, FB113, PS2307, and PS4204) and diluted in distilled water to ∼2 × 106 spores/ml prior to injection into the sample chamber for fluorescence measurements. Spores of B. subtilis strains PS832, PS2307, PS4204, FB111, FB112, and FB113 were also first germinated in l-alanine for 1 h, and the germinated spores were heated at 90°C for 40 min and cooled on ice for ∼15 min prior to injection into the sample chamber for fluorescence measurements. Dormant spores of B. subtilis strain PS832 (∼2 × 106 spores/ml) were treated in water at 100°C for 20 min and cooled on ice for ∼15 min, and then SYTO 16 was added to 0.5 μM and spore fluorescence measured.
Data analysis.
The CaDPA level in spores was determined from the intensity of the Raman band at 1,017 cm−1 (averaged over 7 adjacent spectral data points) (14, 46). The time-lapse Raman spectral intensities were fit well by Boltzmann functions f(t) = A/{1 + exp[(t − tc)/τ]}, with A the initial value, tc the time of half height, and τ the time constant (46). The times at which the spores' CaDPA level fell by 8% and 92% (with the difference corresponding to 5-fold the time constant τ) were defined as the lag time (Tlag) between the injection of spores into the sample chamber and the initiation of most CaDPA release and as the time at which CaDPA release was completed (Trelease), respectively (46). The time ΔTrelease for release of the majority of the spores' CaDPA was calculated as ΔTrelease = Trelease − Tlag. The denaturation of spore protein was monitored by the Raman scattering intensity at 1,655 cm−1 (averaged over 11 adjacent spectral data points) (6, 7, 46), and the time at which the intensity of this band began to drop was defined as Td. The level of nucleic acids in spores was monitored by the intensity of the Raman band at 783 cm−1 (averaged over 7 adjacent spectral data points) (2, 3, 19). The mean intensity of the DIC image of a single spore was calculated by averaging the counts of pixels over a region of 60 by 60 pixels (corresponding to 4 by 4 μm in the microscope specimen) that encompassed the whole spore (diameter of 1 to 2 μm) and was used to observe rapid CaDPA release and any hydrolysis of the spore's cortex. For both wet-heat-killed and fully germinated spores, the final DIC image intensity was that after which no further change was observed after 15 min. All DIC image intensities were calculated as the ratio of the particular DIC image intensity to the image intensity at T0 for both wet-heat-treated and fully germinated spores. For each wet-heat-inactivated spore, its phase image in which the intensity was linearly proportional to its optical phase was also retrieved from its original DIC image as described previously (45), and its edges were found at the pixels where the phase gradient was maximum. The edges were then fitted to a circle due to the vertical orientation of spores in optical tweezers, giving the lateral diameter Dl of the spore (41). The outer edge of the spore on the DIC image is undoubtedly the edge of the outer spore coat layer and for B. cereus spores would not include the exosporium because its refractive index is approximately that of water.
The uptake and binding of SYTO 16 to nucleic acids during wet-heat treatment or germination were monitored by the intensity of the fluorescence image, which was calculated by averaging the counts of pixels over a region of 20 by 20 pixels (corresponding to 1.5 by 1.5 μm in the microscope specimen). The times at which a spore's fluorescence intensity increased by 8% and 92% of its maximum fluorescence intensity Imax were defined as T1 and T2, respectively, to keep the point-in-time definitions consistent with those of Tlag and Trelease. The lateral diameter Df of the fluorescence image was also determined using the fitting method as described above. The fluorescence intensities of SYTO 16 with lambda DNA and with dsDNA and ssDNA of M13 phage mp18 at various temperatures were calculated by averaging the counts of pixels over a region of 400 by 300 pixels, which was large enough to achieve a high signal-to-noise ratio.
RESULTS
CaDPA levels and DIC and fluorescence images of individual B. cereus spores during wet-heat treatment.
It is well known that wet-heat treatment can kill spores of Bacillus species. Unfortunately, when examining individual spores by Raman spectroscopy and DIC and fluorescence microscopy during wet-heat treatment of a spore population, it is impossible to know exactly when any individual spore has died. However, there are several types of information that help pinpoint the time of death of at least the majority of spores examined, including the following (6, 7, 38): (i) wet-heat treatment of B. cereus or B. megaterium spores at 80°C or of B. subtilis spores at 90°C gave 90% killing of these spores in 6 to 10 min (data not shown), as expected; (ii) wet-heat killing of spores of these three species at 80 to 90°C precedes CaDPA release, and all spores that have lost CaDPA are dead; and (iii) wet-heat-treated spores of these species that retain CaDPA can be alive or dead, but the great, great majority of these spores in populations that exhibit ≥20% CaDPA release are dead. It is therefore most likely that an individual spore that retains CaDPA in wet-heat-treated populations of spores of these three Bacillus species will be dead if >20% of the spores in this population have lost CaDPA. Consequently, the wet-heat treatment times used in this work were chosen to yield spore populations with ≥97% dead spores of which ≥30% had lost CaDPA.
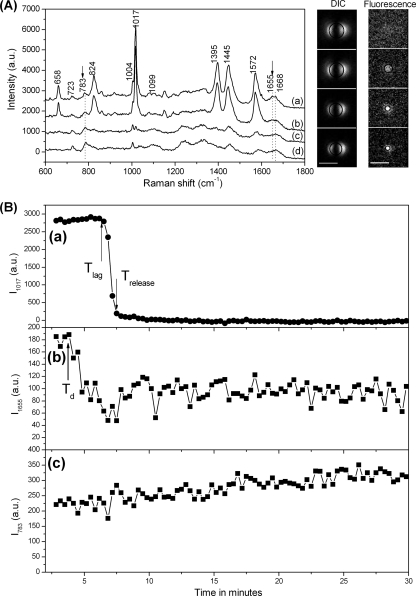
Figure 1A shows the Raman spectra and DIC and fluorescence images of a typical B. cereus spore at several times during wet-heat treatment at 80°C. Likely assignments of the various major Raman bands seen with dormant spores are listed in Table 1. Comparison of Raman spectra taken after 2.8 min (curve a) and 6.5 min (curve b) indicated that although the heights of all CaDPA-specific bands were unchanged after 6.5 min, the protein-specific bands at 1,655 and 1,668 cm−1 had become flattened, suggestive of some spore protein denaturation. This observation is consistent with previous work indicating that some protein denaturation precedes CaDPA release during spore wet-heat treatment (6, 7, 46). The spore's DIC image intensity also exhibited no change at 6.5 min, although there was a notable increase in the fluorescence intensity due to SYTO 16 staining at this time. However, by 7.8 min (curve c), all CaDPA-related bands had disappeared, there was a further decrease in the 1,655-cm−1 band, and the peak at 1,668 cm−1 had become more prominent, suggesting that spore proteins had largely changed from an α-helical structure to one that is most likely denatured. At this time, the spore's DIC image also appeared smaller and darker, and its fluorescence intensity had increased. Over the next ∼20 min, there were no further changes in the Raman spectrum and the DIC image intensity and only a slight reduction in the intensity and width of the spore's fluorescence image. Throughout the wet-heat inactivation process, the nucleic acid-specific Raman bands at 723 and 783 cm−1 were nearly unchanged (Fig. 1A).
Fig. 1.
Analysis of Raman spectra and DIC and SYTO 16 fluorescence images of a B. cereus spore during wet-heat treatment. (A) Raman spectra and DIC and fluorescence images of a B. cereus spore during wet-heat treatment. The B. cereus spore was incubated in water at 80°C as described in Materials and Methods, and the Raman spectra (curves a to d) and images (from top to bottom) were recorded at 2.8 min (T0), 6.5 min (Tlag), 7.8 min (Trelease), and 30 min, respectively, after initiation of wet-heat treatment. The baselines of the Raman spectra were shifted for display. The arrows with the dotted vertical lines below them mark the positions of Raman bands at 783 and 1,655 cm−1, and the dotted line at 1,668 cm−1 marks the Raman band characteristic of denatured protein. The scale bars in the images are 3 μm, and all images are at the same scales. Circles on the images are the fitting of the edges of DIC images and fluorescence images, as described in Materials and Methods. (B) Raman scattering intensities of the spore analyzed in panel A. The Raman scattering intensities at CaDPA-, protein-, and nucleic acid-specific bands, in arbitrary units (a.u.), were determined at various times and plotted as a function of incubation time at 80°C. Plots a to c are for the bands at 1,017, 1,655, and 783 cm−1, respectively. In plot a the upward and downward arrows mark the positions of Tlag and Trelease, respectively. In plot b the upward arrow marks the position of Td.
Table 1.
Assignment of the Raman bands in spectra of individual Bacillus spores
| Band (cm−1) | Assignment | Reference(s) |
|---|---|---|
| 658 | CaDPA | 14 |
| 723 | Adenine | 2, 3, 19, 28 |
| 783 | Guanine, uracil, cytosine | 2, 3, 19 |
| 824 | CaDPA | 14 |
| 1,004 | Phenylalanine | 19, 28 |
| 1,017 | CaDPA | 14 |
| 1,099 | DNA, O-P-O | 28 |
| 1,395 | CaDPA | 14 |
| 1,445 | CaDPA, C-H2 deformation | 14, 19 |
| 1,572 | CaDPA | 14 |
| 1,655 | Amide I (α-helical) | 15 |
| 1,668 | Amide I (nonregular) | 15 |
The changes in CaDPA, protein, and nucleic acid in this spore were described quantitatively by the heights of the corresponding Raman bands, and these values were plotted as a function of heating time (Fig. 1B). As seen previously (46), the CaDPA level remained nearly unchanged until Tlag and then dropped rapidly in ∼1 min to zero at Trelease. The intensity of the protein-specific band at 1,655 cm−1 also decreased rapidly beginning at Td, which was well before Tlag, suggesting that significant protein denaturation took place prior to CaDPA release. The lag observed here between Td and Tlag was more obvious than seen in previous work (46), most likely due to the improved sensitivity and signal-to-noise ratio of the spectral acquisition CCD in the instrument system used in the current work. Throughout the wet-heat inactivation, the height of the nucleic acid-specific band exhibited no major changes except for a slight increase (see Discussion), suggesting that nucleic acids remained in the spore after heat killing, although treatment at such high temperatures might cause the spore DNA to denature (8, 9, 43, 44). Unfortunately, Raman spectroscopy failed to confirm that DNA in wet-heat-treated spores had become denatured, because the two prominent nucleic acid Raman bands at 723 and 783 cm−1 were from both DNA and RNA, and spores contain 3- to 4-fold more RNA than DNA (21).
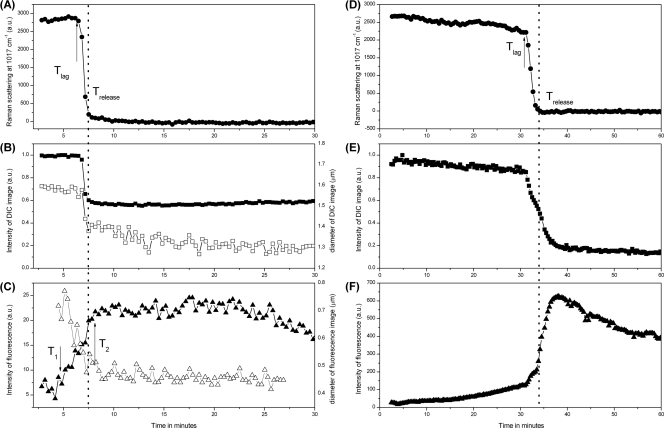
The spore's CaDPA level as well as the intensities of the DIC and fluorescence images of the single B. cereus spore incubated at 80°C were also calculated as a function of heating time (Fig. 2A to C), with the DIC image intensity normalized to the value at T0. The changes in the DIC and SYTO 16 image intensities of spores during germination have been well characterized (16, 45). For comparative purposes, the germination kinetics of an individual B. cereus spore from the same spore preparation were also investigated (Fig. 2D to F). This comparison can help to explain the low SYTO 16 fluorescence intensity and higher brightness of a spore's DIC image after the CaDPA release (see below). During heat killing, the intensity of the DIC image changed in parallel with the spore's CaDPA level, as both remained unchanged until Tlag and then the DIC image intensity dropped rapidly by ∼40% at Trelease, while during spore germination, there was a slow initial release of a small amount of CaDPA and a small decrease in DIC image intensity prior to Tlag. After CaDPA release during wet-heat treatment, there was no further decrease in the intensity of DIC images after Trelease. In contrast, during spore germination there was a further decrease in DIC image intensity following CaDPA release, and a final total decrease of ∼80% of the initial DIC image intensity was achieved after spores were fully germinated (Fig. 2E) (45) (see below). The slow loss in spore refractility following CaDPA release during germination is believed to be associated with the degradation of the spore's peptidoglycan cortex and the attendant swelling of the core due to significant water uptake (13, 16, 45). During wet-heat treatment, the SYTO 16 fluorescence began to increase at a time T1 of ∼5 min which was ∼2 min before Tlag, reached a maximum at T2 which was close to but slightly later than Trelease, and then exhibited a stable phase followed by a slow decrease (Fig. 2C). However, during spore germination, the SYTO 16 fluorescence exhibited a very different pattern: a minimal and slow increase prior to Trelease and then a rapid increase beginning at Trelease, followed by a slow decrease after cortex degradation (Fig. 2F). In addition, the maximum SYTO 16 fluorescence intensity, Imax, during wet-heat killing was much lower than that seen during spore germination (compare Fig. 2C and F). The edges of the spore's DIC and fluorescence images during wet-heat treatment were also found and fitted by circles, allowing determination of the lateral diameters Dl and Df of the spore's DIC and fluorescence images, respectively (Fig. 2B and C). During wet-heat treatment, the lateral diameter of the spore's DIC image decreased in parallel with its intensity and therefore also in parallel with the decrease in the spore's CaDPA level; the lateral diameter Df of the spore's fluorescence image due to SYTO 16 staining also decreased slightly, beginning close to Tlag. In addition, the diameter of the spore's fluorescence image was significantly smaller than that of the spore's DIC image. The diameters Dl and Df of germinated spores were also determined, and the diameters Df of germinated spores were much larger than those of wet-heat-inactivated spores (data not shown), as expected (34).
Fig. 2.
CaDPA levels and DIC and SYTO 16 fluorescence intensities of a single B. cereus spore during wet-heat treatment or germination. Single B. cereus spores were either wet-heat treated (A to C) or germinated (D to F), the CaDPA level in the spores was determined from the Raman band at 1,017 cm−1 (A and D), and the normalized DIC image intensities (B and E, ▪) and SYTO 16 fluorescence intensities (C and F, ▴), all in arbitrary units (a.u.), were determined as described in Materials and Methods. The diameter Dl of the DIC image and the distribution width of the fluorescence image during wet-heat treatment were also determined as described in Materials and Methods and are shown in panels B (□) and C (▵), respectively. The upward arrow in panels A and D marks the position of Tlag; the downward and upward arrows in panel C mark the positions of T1 and T2, respectively; and the vertical dotted line in all panels marks the position of Trelease.
Analysis of multiple individual spores of B. cereus, B. megaterium, and B. subtilis during wet-heat treatment.
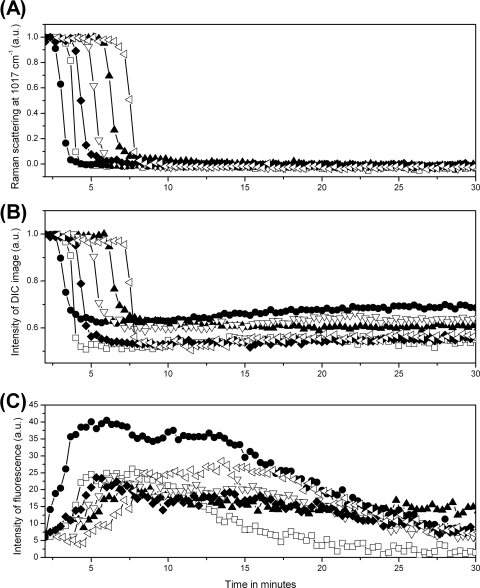
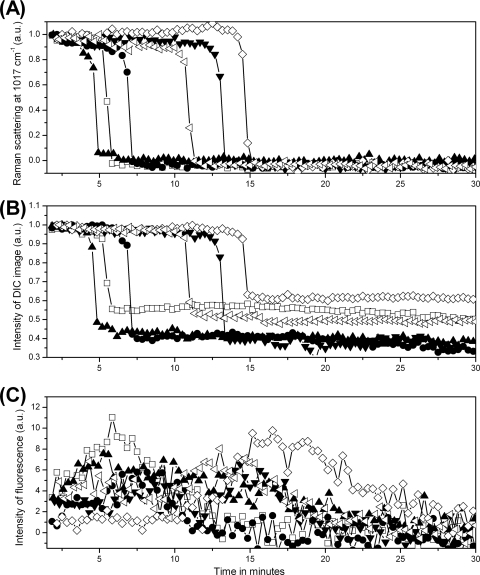
To examine the heterogeneity between spores during wet-heat treatment, the measurements made by Raman spectroscopy and DIC and fluorescence microscopy were repeated on 20 individual B. cereus spores (results for six of them are shown in Fig. 3), and mean values and standard deviations of inactivation parameters for the 20 spores were determined (Table 2). Although values of Tlag and Trelease varied significantly from spore to spore, the time ΔTrelease required for release of the majority of spores' CaDPA was nearly the same at ∼1 min for all individual spores. The time at which the rapid drop in the spores' DIC image intensity began corresponded to the time of initiation of CaDPA release, and both the time at which the rapid drop in the spores' DIC image intensity ended and the time at which the SYTO 16 fluorescence intensity reached its maximum were almost identical to Trelease. Initiation of significant protein denaturation also invariably preceded Tlag, and the time T1 for initiation of SYTO 16 uptake was also invariably 1 to 2 min prior to Tlag and was close to Td (Table 2 and data not shown). The lateral diameters Dl of the spores' DIC images at Trelease and Df of the spores' fluorescence images at T1 relative to the diameters at Tlag also decreased ∼15% and ∼20%, respectively, during wet-heat treatment (Table 2).
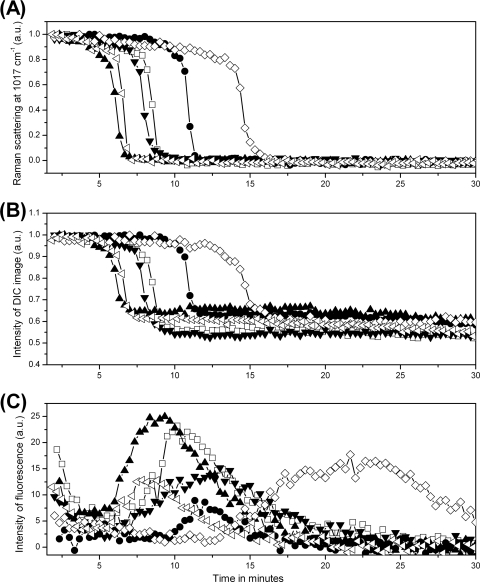
Fig. 3.
CaDPA levels and DIC and fluorescence image intensities for six individual B. cereus spores during wet-heat treatment. Six individual B. cereus spores were wet-heat treated with SYTO 16 present, and intensities of the CaDPA-specific Raman band at 1,017 cm−1 (A), DIC image intensities (B), and fluorescence intensities (C) were determined as described in Materials and Methods. Each symbol represents the wet-heat inactivation of one individual spore. The DIC intensity values were normalized to values at T0, and all intensities are given in arbitrary units (a.u.).
Table 2.
Mean values and standard deviations of parameters of wet heat inactivation of individual spores of various Bacillus species and strainsa
| Species or strain | Mean ± SDa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tlag (min) | Trelease (min) | ΔTrelease (min) | Td (min) | T1 (min) | T2 (min) | Imax (a.u.c) | Decrease (%) ind: |
||
| Dl | Df | ||||||||
| B. cereus | 4.3 ± 1.5 A | 5.5 ± 1.5 A | 1.2 ± 0.2 A | 2.7 ± 1.0 A | 3.1 ± 1.1 A | 5.8 ± 1.4 A | 28.3 ± 7.6 A | 14.9 ± 2.3 A | 20.0 ± 8.9 |
| B. megaterium | 8.7 ± 3.8 B | 10.7 ± 3.8 B | 1.9 ± 0.6 B | 7.8 ± 4.5 B | 8.3 ± 3.5 B | 11.2 ± 4.0 B | 17.2 ± 4.9 B | 13.3 ± 2.5 B | NDe |
| B. subtilis | |||||||||
| PS832 | 8.2 ± 4.6 B | 9.2 ± 4.7 B | 1.0 ± 0.3 C | 7.5 ± 5.5 B | 6.3 ± 5.4 B | 9.6 ± 5.0 B | 8.7 ± 2.2 C | 12.8 ± 4.6 B | ND |
| PS578 (α− β−) | 3.6 ± 1.1 A | 4.4 ± 1.2 C | 0.8 ± 0.2 D | ND | 2.6 ± 1.5 A | 4.3 ± 1.2 C | 12.4 ± 3.9 D | 18.1 ± 2.7 C | ND |
Spores of various Bacillus species or strains were incubated at high temperature in water with SYTO 16, and average parameters and their standard deviations from 20 individual spores each were determined as described in Materials and Methods.
In each column, means with different letters are significantly different (Tukey's HSD test, P < 0.05).
a.u., arbitrary units.
The decreases in Dl and Df were determined at Trelease and T1, respectively, relative to the diameters at Tlag.
ND, not determined.
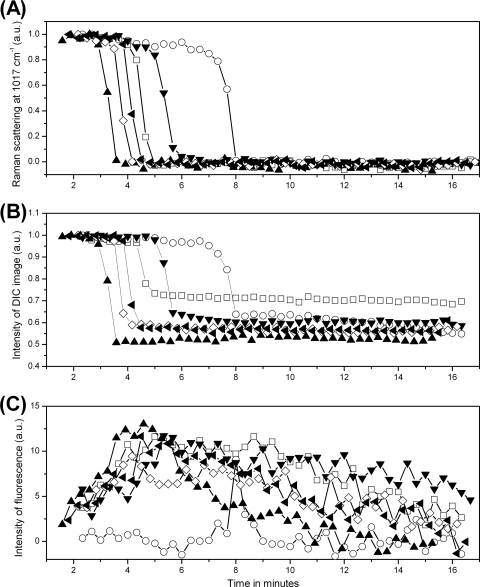
The dynamic wet-heat killing of 20 individual spores each of B. megaterium and B. subtilis strain PS832 was also investigated by Raman spectroscopy and DIC and fluorescence microscopy (Table 2; results for six individual spores of each species are shown in Fig. 4 and 5). As seen with B. cereus spores, protein denaturation always began prior to Tlag with B. megaterium and B. subtilis spores, although the delay between the two events was not as obvious as that seen with B. cereus spores. Correlations between the changes in CaDPA levels, DIC image intensities, and SYTO 16 fluorescence intensities during wet-heat treatment were also largely similar to those seen with B. cereus spores. The time T1 for initiation of SYTO 16 uptake was also ∼ 0.5 and 2 min prior to Tlag with >90% individual spores of B. megaterium and B. subtilis, respectively, and Dl values for B. megaterium and B. subtilis spores' DIC image also decreased by ∼13% after CaDPA release. The changes in Df values of B. megaterium and B. subtilis spores' fluorescence image during wet-heat treatment were not determined due to the weak fluorescence image intensities at Tlag (Fig. 4 and 5). An interesting result found uniquely in B. megaterium spores was that some of these spores exhibited notable fluorescence even at T0, and this initial fluorescence intensity began to decrease slowly to a very low level after the spores were exposed to high temperature and well before Tlag. A major difference between B. megaterium spores and spores of the other two species is the significant level of carotenoids in a B. megaterium spore's inner membrane, and previous work indicated that carotenoid-specific Raman band intensities decreased rapidly beginning as soon as these spores were exposed to elevated temperatures (46). However, B. megaterium spores in water but without SYTO 16 exhibited no fluorescence either before or after CaDPA release (data not shown), suggesting that the fluorescence at T0 and the reduction of its intensity after exposure to high temperature were not due to carotenoids. In addition, wet-heat-treated B. subtilis spores exhibited the weakest SYTO 16 fluorescence among the wet-heat-treated spores of the three species examined (compare panels C in Fig. 3, 4, and 5; Table 2). Perhaps this is due to the higher temperature used to treat the B. subtilis spores.
Fig. 4.
CaDPA levels and DIC and SYTO 16 fluorescence image intensities for six individual B. megaterium spores during wet-heat treatment. Six individual B. megaterium spores were wet-heat treated with SYTO 16 present, and intensities of the CaDPA-specific Raman band at 1,017 cm−1 (A), DIC image intensities (B), and SYTO 16 fluorescence intensities (C) were determined as described in Materials and Methods. Each symbol represents the wet-heat inactivation of one individual spore. The DIC intensity values were normalized to values at T0, and all intensities are given in arbitrary units (a.u.).
Fig. 5.
CaDPA levels and DIC and SYTO 16 fluorescence image intensities for six individual B. subtilis PS832 (wild-type) spores during wet-heat treatment. Six individual PS832 spores were wet-heat treated with SYTO 16 present, and intensities of the CaDPA-specific Raman band at 1,017 cm−1 (A), DIC image intensities (B), and SYTO 16 fluorescence intensities (C) were determined as described in Materials and Methods. Each symbol represents the wet-heat inactivation of one individual spore. The DIC intensity values were normalized to values at T0, and all intensities are given in arbitrary units (a.u.).
Previous work has indicated that the saturation of spores' DNA with α/β-type SASP prevents the binding of SYTO 16 to DNA, and germinated spores that lack the germination protease (GPR), which initiates α/β-type SASP degradation, exhibit <10% of the SYTO 16 fluorescence that germinated wild-type spores do (16). These results suggested that the low SYTO 16 fluorescence intensity seen during spore inactivation by wet heat compared to that seen during spore germination might be because α/β-type SASP are not degraded during wet-heat inactivation. To address this concern, the dynamic wet-heat killing of 20 individual spores of the α− β− B. subtilis strain PS578 was also monitored (Table 2; results for six of the spores are shown in Fig. 6). Surprisingly, during wet-heat inactivation, the α− β− spores exhibited only slightly higher SYTO 16 fluorescence than did the wild-type spores. The α− β− spores likely also had slightly lower wet-heat resistance than the wild-type spores, as suggested by the lower mean values of Tlag and Trelease (Table 2).
Fig. 6.
CaDPA levels and DIC and SYTO 16 fluorescence image intensities for six individual B. subtilis PS578 spores during wet-heat treatment. Six individual PS578 (α− β−) spores were wet-heat treated with SYTO 16 present, and intensities of the CaDPA-specific Raman band at 1,017 cm−1 (A), DIC image intensities (B), and SYTO 16 fluorescence intensities (C) were determined as described in Materials and Methods. Each symbol represents the wet-heat inactivation of one individual spore. The DIC intensity values were normalized to values at T0, and all intensities are given in arbitrary units (a.u.).
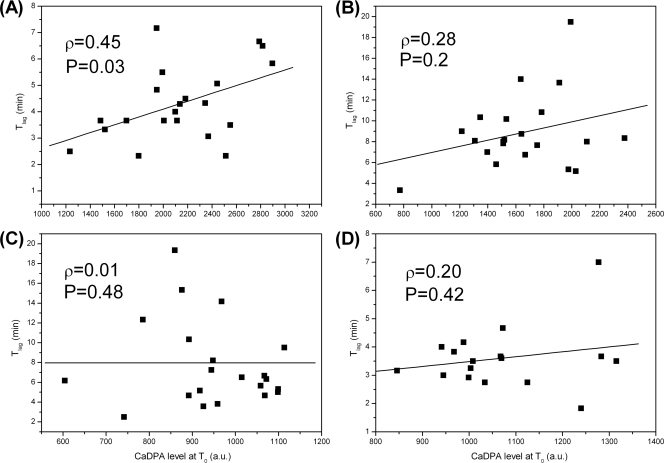
The spore core's high level of CaDPA is a major factor contributing to spore wet-heat resistance, since spores with higher CaDPA levels generally have lower core water content, and spore wet-heat resistance increases at lower core water contents (11, 20, 35). However, this conclusion has been drawn only from results with spore populations. To examine this relationship with individual spores from the same preparation, Tlag values for 20 individual spores each of B. cereus, B. megaterium, and B. subtilis strains PS832 and PS578 were compared as a function of their CaDPA levels at T0, and the Pearson's correlation coefficients ρ between Tlag and a spore's CaDPA level at T0 and their P values were calculated (Fig. 7). Notable positive correlations between Tlag and a spore's CaDPA level were observed with spores of B. cereus, although only a small or no correlation was observed with B. megaterium and B. subtilis spores.
Fig. 7.
Tlag values of multiple individual spores of Bacillus species as a function of the spores' CaDPA level. Tlag values of 20 individual spores each of B. cereus (A), B. megaterium (B), and B. subtilis strains PS832 (wild type) (C) and PS578 (α− β−) (D) were plotted as a function of the spores' CaDPA levels at T0. The solid curves are fitted lines. The Pearson correlation coefficients ρ between values of Tlag and the CaDPA levels as well as their P values are also shown. The CaDPA levels were determined from spores' Raman spectra and are given in arbitrary units (a.u.).
Fluorescence of SYTO 16 plus DNA at various temperatures.
SYTO 16 binds to both DNA and RNA; however, the binding of SYTO 16 to RNA contributes very little to spores' SYTO 16 fluorescence, at least in germinating wild-type B. subtilis spores (16). As noted above, the maximum SYTO 16 fluorescence intensities achieved during spore wet-heat treatment were much lower than those seen during spore germination, even though levels of nucleic acids in spores were unchanged during wet-heat treatment. One possible reason for the low SYTO 16 fluorescence in wet-heat-treated spores at high temperatures is a low fluorescence of the SYTO 16-DNA complex due either to (i) DNA denaturation (8, 9, 43, 44), (ii) SYTO 16 dissociation from DNA, or (iii) a low quantum efficiency of SYTO 16-DNA fluorescence. To obtain evidence to allow a choice between these possibilities, we measured the fluorescence intensities of double-stranded lambda phage DNA plus SYTO 16 at several temperatures. The results indicated that the SYTO 16-DNA fluorescence intensity decreased dramatically at temperatures of ≥65°C and dropped 7- to 20-fold at 80 to 90°C (data not shown), temperatures that would most likely result in significant DNA denaturation (43). If a dsDNA-SYTO 16 mixture was treated at 90°C and then cooled or if dsDNA alone was treated at 90°C and SYTO 16 added after cooling, the SYTO 16 fluorescence of both mixtures at 23°C was 70 to 75% of the maximum value (data not shown). In addition, the measurements with dsDNA and ssDNA of the M13 phage mp18 indicated that the fluorescence of SYTO 16 with ssDNA was ∼3-fold lower than that with dsDNA (data not shown). Together these observations suggest that DNA denaturation and the lower affinity of SYTO 16 for ssDNA than for dsDNA may constitute a significant factor that contributes to the low SYTO 16 fluorescence in wet-heat-treated spores. However, it was also possible that at high temperatures SYTO 16 (i) dissociates from DNA or (ii) has low fluorescence quantum efficiency when bound to DNA.
SYTO 16 fluorescence of wet-heat-killed spores measured at 23°C.
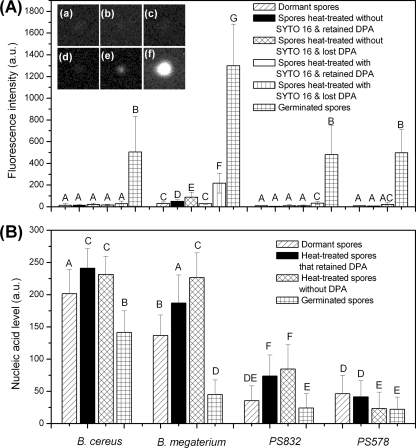
The elevated SYTO 16-DNA fluorescence at 23°C when SYTO-16 was added to cooled wet-heat-treated DNA or a cooled wet-heat-treated DNA-SYTO 16 mixture suggested that SYTO 16 fluorescence intensities during spore wet-heat treatment might be higher if fluorescence was measured at 23°C following heating. Consequently, spore populations of B. cereus, B. megaterium, and B. subtilis strains PS832 and PS578 were wet-heat treated and cooled, SYTO 16 was added, and fluorescence intensities and nucleic acid levels of multiple individual spores were measured at 23°C. For each species or strain, multiple individual spores that had released or retained their CaDPA were analyzed by fluorescence microscopy and Raman spectroscopy, and the results were averaged (Table 3; Fig. 8A and B). For comparative purposes, the SYTO 16 fluorescence of multiple individual dormant spores and fully germinated spores of each species or strain were also measured. Unexpectedly, when measured at 23°C, the average SYTO 16 fluorescence intensities of wet-heat-killed spores of the different species or strains that had released their CaDPA were still >15-fold lower than those of the corresponding germinated spores, although they were higher than those of wet-heat-treated spores that retained CaDPA and of dormant spores. It should be noted that the nucleic acid levels in wet-heat-inactivated spores were not lower, and even appeared higher, than those in dormant and fully germinated spores (Fig. 8B) (see Discussion). Additionally, spores of B. subtilis strains PS832 and PS578 exhibited nearly the same SYTO 16 fluorescence at 23°C whether they were dormant, wet-heat treated, or germinated (Table 3). Even multiple individual PS832 spores killed by boiling exhibited minimal SYTO 16 fluorescence even though these spores had the same nucleic acid levels as the untreated dormant spores (Table 3 and data not shown).
Table 3.
Average fluorescence intensities due to SYTO 16 interaction with spore nucleic acids after various treatmentsa
| Species or strain | Fluorescence intensity measured at 23°C (mean a.u. ± SD) after the following treatmentb: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dormant | Wet-heat treated without SYTO 16 |
Wet-heat treated with SYTO 16 |
Germinated | Wet-heat treated after germination | Boiled | |||
| Retained CaDPA | Lost CaDPA | Retained CaDPA | Lost CaDPA | |||||
| B. cereus | 15.3 ± 19.0 A | 15.0 ± 5.5 A | 21.3 ± 12.0 A | NDc | ND | 504.8 ± 328.9 G | ND | ND |
| B. megaterium | 30.9 ± 13.9 B | 49.4 ± 15.5 C | 88.9 ± 46.3 D | 28.2 ± 4.0 B | 217.7 ± 90.5 E | 1299.5 ± 383.7 H | ND | ND |
| B. subtilis | ||||||||
| PS832 | 9.4 ± 1.9 A | 5.8 ± 2.3 A | 10.7 ± 11.0 A | 10.8 ± 5.1 A | 33.3 ± 13.8 BF | 481.2 ± 268.8 G | 120.5 ± 48.6 I | 5.3 ± 1.7 A |
| FB111 (cwlJ) | ND | ND | ND | ND | ND | 68.6 ± 108.1 BCD | 61.6 ± 36.6 C | ND |
| FB112 (sleB) | ND | ND | ND | ND | ND | 217.8 ± 190.9 E | 110.8 ± 67.2 DI | ND |
| FB113 (cwlJ sleB) | ND | ND | ND | ND | ND | 7.2 ± 1.4 A | 9.4 ± 2.2 A | ND |
| PS578 (α− β−) | 8.5 ± 2.8 A | 6.4 ± 2.2 A | 20.7 ± 20.2 AB | ND | ND | 497.8 ± 214.2 G | ND | ND |
| PS2307 (cwlD) | 8.0 ± 2.0 A | ND | ND | ND | ND | 19.1 ± 45.6 AF | 19.4 ± 13.9 A | ND |
| PS4204 (α− β−cwlD) | 11.4 ± 3.8 A | ND | ND | ND | ND | 15.1 ± 13.3 A | 20.9 ± 20.7 A | ND |
Spores of various Bacillus species or strains were incubated at high temperature in water for 40 min with or without SYTO 16, cooled, and had SYTO 16 added if not present during heating, and the mean fluorescence intensities and standard deviations of 30 individual spores each that were not wet-heat treated, were wet-heat treated and had lost CaDPA, or were wet-heat treated but retained CaDPA were determined at 23°C as described in Materials and Methods. Note that ≥90% of the spores that were wet-heat treated but retained CaDPA were most likely dead as estimated from the data in previous work (6, 7) and data not shown. Spores of various species and strains were also germinated with nutrients for 30 min (B. cereus and B. megaterium), 40 min (B. subtilis strains PS832 and PS578), or 1 h (B. subtilis strains FB111, FB112, FB113, PS2307, and PS4204) and then mixed with SYTO 16, and the average fluorescence intensities and standard deviations of 30 individual spores each were determined at 23°C as described in Materials and Methods. Spores of PS832, PS2307, PS4204, FB111, FB112, and FB113 were also incubated at 90°C for 40 min after 1 h of germination in l-alanine and then mixed with SYTO 16, and the average fluorescence intensities and standard deviations of 30 individual spores were determined at 23°C as described in Materials and Methods.
a.u., arbitrary units. Means with different letters are significantly different (Tukey's HSD test, P < 0.05).
ND, not determined.
Fig. 8.
Average values and standard deviations of SYTO 16 fluorescence, nucleic acid levels, and DIC image intensities of differently treated spores of Bacillus species. (A) Average values and standard deviations of the SYTO 16 fluorescence intensities, in arbitrary units (a.u.) and measured at 23°C, of 30 individual spores each of B. cereus, B. megaterium, and B. subtilis strains PS832 (wild type) and PS578 (α− β−) that were dormant or wet-heat treated for 40 min with or without SYTO 16, cooled, and had SYTO 16 added if not present during heating; 30 spores each that retained or had lost CaDPA were examined as described in Materials and Methods. Spores of these species and strains were also germinated and SYTO 16 added as described in Materials and Methods. The insets (a to f) are typical fluorescence images of a B. subtilis PS832 spore that was as follows: (a) dormant plus SYTO 16; (b) wet-heat treated without SYTO 16 present but retained CaDPA, with SYTO 16 added after spores were cooled; (c) wet-heat treated without SYTO 16 present and had lost CaDPA, with SYTO 16 added after spores were cooled; (d) wet-heat treated with SYTO 16 present but retained CaDPA; (e) wet-heat treated with SYTO 16 present and had lost CaDPA; and (f) fully germinated and then SYTO 16 added. Note that ∼90% of the spores that were wet-heat treated but retained CaDPA were most likely dead (references 6 and 7 and data not shown). (B) Average values and standard deviations of nucleic acid levels, in arbitrary units (a.u.), in 30 individual spores each of B. cereus, B. megaterium, and B. subtilis strains PS832 and PS578 that were dormant, wet-heat treated as described above and without CaDPA, wet-heat treated but retained CaDPA, and fully germinated as described in Materials and Methods. Means with different letters are significantly different (Tukey's honestly significant difference [HSD] test, P < 0.05). Note that the nucleic acid levels determined by Raman tweezers may be not comparable among spores of different species due to the different spore core sizes and therefore the different nucleic acid concentrations.
Given that SYTO 16 was routinely added to the wet-heat-treated spores only after cooling, it seemed possible that the pores or channels allowing SYTO 16 entry into spores at 80°C or 90°C might close once the heat-treated spores were cooled, thus restricting SYTO 16 uptake. To test this possibility, spores of B. megaterium and B. subtilis strain PS832 were wet-heat treated with 0.5 μM SYTO 16 and then cooled, and fluorescence intensities were measured at 23°C (Table 3; Fig. 8A). Strikingly, spores that were wet-heat treated with SYTO 16 and had lost their CaDPA exhibited ∼3-fold-higher fluorescence at 23°C than did spores that were wet-heat treated without SYTO 16 and with the dye added only after cooling, although spores after the same treatment that retained CaDPA exhibited fluorescence comparable to that of spores heat treated without SYTO 16 (Table 3; Fig. 8A). However, the fluorescence of spores that were wet-heat treated with SYTO 16 were still 6- to 15-fold lower than those of germinated spores, while the fluorescence intensity at 23°C of a DNA-SYTO 16 mixture that was heated and then cooled was nearly the same as that of the unheated DNA-SYTO 16 mixture. The SYTO 16 fluorescence intensities of multiple individual PS832 spores that were wet-heat treated after germination and then cooled and had SYTO 16 added were also low but not as low as those of wet-heat-treated spores that had lost CaDPA (Table 3), indicating that the low SYTO 16 fluorescence with the heat-treated spores measured at 23°C was not primarily because denatured DNA inside the spores was not renatured after cooling.
Status of the spore cortex during wet-heat treatment.
The possible low permeability of SYTO 16 across the inner membrane even in wet-heat-treated spores suggests that unlike in spore germination, the spore cortex is not hydrolyzed during wet-heat treatment, since the presence of an intact cortex may interfere with spores' uptake of SYTO 16 either directly or indirectly by contributing somehow to the normal impermeability of the dormant spore's inner membrane (16). An observation consistent with the lack of cortex hydrolysis during spore wet-heat treatment is that there was no further decrease in spores' DIC image intensities after rapid CaDPA release was complete, unlike the situation during spore nutrient germination. To quantitatively describe differences in changes in DIC image intensity during spore germination and wet-heat inactivation, the kinetics of multiple individual spores of all three Bacillus species during wet-heat treatment and germination were monitored, and mean values and standard deviations of the relative final DIC image intensities were determined (Table 4). Strikingly, for spores of all species the relative final DIC image intensity of the wet-heat-killed spores was significantly higher than that of germinated wild-type spores, just as was that of the germinated spores that lacked both CwlJ and SleB (Table 4). This, plus the absence of any notable decrease in spores' DIC image intensities after Trelease during wet-heat treatment, suggests that there is minimal if any degradation of the spore cortex during wet-heat inactivation.
Table 4.
Average relative final DIC image intensities of germinated spores and wet-heat-treated dormant spores of Bacillus speciesa
| Species or strain | Final DIC image intensity (mean ± SD)b |
|
|---|---|---|
| Wet-heat-treated spores | Fully germinated spores | |
| B. cereus | 0.59 ± 0.06 A | 0.31 ± 0.07 C |
| B. megaterium | 0.55 ± 0.07 A | 0.12 ± 0.05 D |
| B. subtilis | ||
| PS832 | 0.42 ± 0.11 B | 0.31 ± 0.05 C |
| FB113 (cwlJ sleB) | NDc | 0.63 ± 0.03 A |
The average final relative DIC image intensities and standard deviations of various Bacillus species relative to intensities at T0 were determined for 20 individual spores following wet-heat treatment of dormant spores or for 10 individual spores during germination as described in Materials and Methods. The dynamics during spore wet-heat treatment were monitored for 30 min, and the dynamics during spore germination were monitored for 30 min (B. megaterium) or 1 h (B. cereus and B. subtilis strains PS832 and FB113).
Means with different letters are significantly different (Tukey's HSD test, P < 0.05).
ND, not determined.
The fluorescence intensities of multiple individual spores lacking one or two CLEs and CwlD mutant spores that were fully germinated and then wet-heat treated after germination were also measured (Table 3). Spores that either lacked both CLEs, CwlJ, and SleB or lacked the CwlD protein needed for the cortex modification allowing cortex degradation during germination exhibited no significant SYTO 16 fluorescence after either germination or subsequent wet-heat treatment, even though all CaDPA was released during the initial germination as expected (reference 24 and data not shown). Previous work with the germination of wild-type spores indicated that there is significant α/β-type SASP degradation during the process of cortex hydrolysis (16); thus, a possible reason for the lack of SYTO 16 fluorescence from germinated cwlJ sleB spores and cwlD spores is that α/β-type SASP are not degraded during the germination of these spores. Consequently, the fluorescence intensities of multiple individual spores lacking both α/β-type SASP and CwlD that had released their CaDPA during germination and then been wet-heat treated were measured (Table 3). Strikingly, the α− β− cwlD spores also exhibited no significant SYTO 16 fluorescence after either germination or subsequent wet-heat treatment. These results together suggest that the low SYTO 16 fluorescence of wet-heat-treated spores is not due to continued binding of α/β-type SASP to spore DNA but rather is due to (i) a low ability of SYTO 16 to permeate across the inner membranes of wet-heat-treated spores, even those that have lost CaDPA, and in particular at room temperature, and (ii) DNA denaturation and a lower affinity of SYTO 16 for ssDNA than for dsDNA. It also is possible that at high temperatures, the SYTO 16 complex has low fluorescence quantum efficiency or readily dissociates.
In contrast to the results with B. subtilis spores lacking both CwlJ and SleB, germinated spores lacking either CwlJ or SleB did exhibit significant SYTO 16 fluorescence, although the maximum SYTO 16 fluorescence intensities of germinated cwlJ spores were lower than those of germinated sleB and wild-type spores, as seen previously (16). Again, wet-heat treatment following spore germination did not increase these spores' SYTO 16 fluorescence (Table 3).
DISCUSSION
The dynamics of the wet-heat inactivation of individual bacterial spores in water have been studied previously by use of dual-trap Raman tweezers and elastic light scattering (46), and a few notable findings were made, including (i) release of the great majority of spores' CaDPA within 1 to 2 min, (ii) significant spore protein denaturation accompanying and perhaps slightly preceding CaDPA release, and (iii) significant heterogeneity between individual spores in the time, Tlag, for initiation of rapid CaDPA release. In the current work, further insight into the wet-heat inactivation of individual bacterial spores has been obtained using a combination of dual-trap Raman tweezers, DIC microscopy, and fluorescence microscopy with the nucleic acid dye SYTO 16 to examine changes during wet-heat treatment of individual spores of a number of Bacillus species and strains. These insights have led to a number of new conclusions and have strongly reinforced one conclusion made previously.
The previous conclusion that was reinforced by the current work is that during wet-heat treatment, some spore proteins are denatured or damaged, as significant amounts of the spore protein's amide I Raman band changes from an α-helical structure to one that is likely denatured. More importantly, this protein denaturation began before the initiation of the rapid CaDPA release at Tlag. Due to the improvement in the sensitivity and signal-to-noise ratio of the spectral acquisition CCD used in the current work, the onset of protein denaturation seen in this work was more obvious than that seen previously, when it was not completely clear that changes in protein structure did not just accompany the rapid CaDPA release during spore wet-heat treatment (46). These results suggest that protein denaturation may be an early event during wet-heat inactivation of spores, and this is consistent with protein inactivation being the major mechanism of spore killing by wet heat. However, specific proteins whose inactivation results in spore killing have not been identified, nor is it clear how inactivation of any protein triggers subsequent events during wet-heat treatment, such as CaDPA release. During spore germination, CaDPA is also almost completely released in 1 to 2 min (4, 26, 45), most likely via specific channels in the spores' inner membrane. It is not clear whether these CaDPA-specific channels are also used for the release of CaDPA during wet-heat treatment. However, this seems unlikely, because wet-heat treatment of spores results in the release of small molecules in addition to CaDPA, in particular molecules such as free nucleotides that are not released during germination (21, 34, 36).
The first new conclusion is that throughout wet-heat treatment, the levels of nucleic acids inside the spores do not go down and even appear to go up slightly, even while the great majority of the spore's CaDPA is released. Although the mechanism for this rapid CaDPA release is not clear, it seems likely to be due to a breakdown in the impermeability of the spore's inner membrane during wet-heat treatment that allows CaDPA and other small molecules to escape, although not allowing the loss of large molecules such as nucleic acids. The reason for the slight apparent increase in the nucleic acid level in wet-heat-treated spores is not completely clear, although it may be due to the significant decrease in spore core size and therefore an increase in effective nucleic acid concentration in the core as suggested by the reduced size of the spores' Df value during wet-heat treatment with SYTO 16. Since the laser focus of the laser tweezers-Raman spectroscopy (LTRS) system is comparable to the diameter of spores in the optical trap, the nucleic acid level determined by Raman spectroscopy may only appear to go up during wet-heat inactivation. The reverse takes place in germinated spores, in which the nucleic acid level appears to go down, most likely due to core expansion (34). Indeed, germinated spores' fluorescence images are much larger than those of wet-heat-inactivated spores (compare the insets in Fig. 8).
The second new conclusion is that for B. cereus spores, the time Tlag at which the release of the spore core's large depot of CaDPA begins is somehow dependent on the CaDPA level in that spore, although this dependence is not obvious with B. megaterium and B. subtilis spores. During wet-heat treatment, the values of Tlag varied significantly from spore to spore even though the spores were from the same preparation, as seen previously (46). This heterogeneity is most likely because of differences between individual spores in the levels of various components important in spore wet-heat resistance, such as core water, divalent cations, and CaDPA, with these differences perhaps due to some heterogeneity when the spores are formed during sporulation. The observation that at least B. cereus spores with higher CaDPA levels have higher Tlag values is consistent with these spores having a lower water content (35), and a lower core water content is known to correlate well with higher spore wet-heat resistance (11, 20, 35).
The third new conclusion is that during wet-heat treatment, the beginning and the end of the rapid drop in an individual spore's DIC image intensity precisely corresponded to the Tlag and Trelease times in CaDPA release from that spore. Establishment of this correspondence will now make it possible to simultaneously monitor the dynamic wet-heat inactivation of hundreds of individual spores adhered on a microscope slide by DIC microscopy, just as in using DIC microscopy to monitor the germination of multiple individual spores (45).
The fourth new conclusion is that the spores' cortical peptidoglycan is not degraded appreciably during wet-heat treatment, unlike what happens in spore germination. This seems predictable, since the CLEs would be expected to be heat labile since they are outside the protective environment of the spore core (33), and this heat lability has been shown directly for CwlJ in B. subtilis spores (1). The lack of cortex degradation during wet-heat treatment is also suggested by the absence of any further decrease in spores' DIC image intensity following CaDPA release, and during spore germination such a decrease of 30 to 35% in spores' DIC image intensity is associated with the degradation of the spore's peptidoglycan cortex and the attendant core swelling due to significant water uptake (13, 16, 45). In addition, the final DIC image intensities of heat-treated spores that have lost their CaDPA are significantly higher than those of the fully germinated spores, and the likely core volume decreases upon wet-heat inactivation rather than increasing as would be expected if the cortex were degraded. Presumably the failure to degrade the spore cortex during wet-heat treatment is due to the inactivation of both CwlJ and SleB. Interestingly, in the absence of CwlJ, CaDPA is released only very slowly between Tlag and Trelease during spore germination for reasons that are as yet not known (26, 33). However, values for ΔTrelease during spore wet-heat treatment are not affected by damage to CwlJ and/or SleB.
The fifth new conclusion is that the lateral diameter Dl of a spore's DIC image decreased in parallel with the change in the CaDPA level in that spore, as did the diameter Df of the fluorescence image of B. cereus spores. The diameter of the DIC image reflects the size of the spore, although the measured size probably differs slightly from the actual size (41). The diameter of the spores' fluorescence image was significantly smaller than that of the whole spore, as expected, and is most likely the spore core, the site of spores' nucleic acid. It is notable that the Df also decreased significantly by Trelease, with this decrease also in parallel with CaDPA release. The parallel decreases in Dl, Df, and CaDPA level further suggest that these events are all correlated and perhaps are also causally connected. While we do not know how CaDPA release from the spore core would result in a decrease in core diameter, one possible scenario is that the release of CaDPA and other small molecules from the spore core, which comprise >25% of core dry weight, would result in a large decrease in the osmotic pressure exerted by the core. This in turn might result in compression of the core due to contraction of the spore cortex that is not degraded during wet-heat treatment as described above; indeed, cortical peptidoglycan was suggested long ago to have contractile properties (17). Contraction of the cortex might in turn lead to contraction of spore coat layers, and these coat layers have been shown to be significantly flexible and to swell or shrink depending on the degree of spore hydration (39, 41).
The sixth new conclusion is that during wet-heat treatment SYTO 16 starts to enter the spore core and bind with spore's nucleic acids at a time that is earlier than Tlag but close to the time Td of initiation of protein denaturation. This suggests that there are significant changes in spore inner membrane permeability during wet-heat treatment before CaDPA release begins. However, at present we do not know the reasons for the impermeability of the spore's inner membrane (16, 35, 37), so it is difficult to know how this permeability barrier is actually breached. However, the breakdown in this permeability barrier seems unlikely to be simply a change in the inner membrane's passive permeability at elevated temperatures, because the time for initiation of SYTO 16 uptake is invariably 0.5 to 2 min prior to Tlag, even though there is significant heterogeneity in Tlag values. The SYTO 16 fluorescence intensity also reached Imax at a time that was close to but slightly later than Trelease. In addition, the fluorescence at 23°C of spores that were heat treated with SYTO 16 was much lower in the treated spores that retained CaDPA than in those that had lost it. Consequently, it appears likely that at least some SYTO 16 uptake takes place in parallel with CaDPA release and is presumably due to the same inner membrane permeability change at high temperature that allows CaDPA release. However, more evidence is required to support this presumption, since other explanations cannot be excluded. The lower SYTO 16 fluorescence of spores that are wet-heat treated without SYTO 16 present, measured at 23°C, compared to that of spores heat treated with SYTO 16 present suggests that this permeability change largely is reversed when wet-heat-treated spores are cooled. However, as noted above, the precise cause of this permeability change is not known.
The final conclusion is that the maximum SYTO 16 fluorescence intensity in wet-heat-killed spores is much lower than that seen during spore germination. One possible reason for this result is that SYTO 16 may penetrate only poorly into the cores of wet-heat-killed spores, since the spore's peptidoglycan cortex is most likely not degraded during wet-heat-treatment. Indeed, spores that lacked both CwlJ and SleB or CwlD alone also exhibited no SYTO 16 fluorescence during germination even though these spores released all spore small molecules. The lack of fluorescence from cwlJ sleB spores and cwlD spores was also not due to the lack of α/β-type SASP degradation during the germination of these spores, since spores that lacked both α/β-type SASP and CwlD also exhibited almost no SYTO 16 fluorescence during germination. Indeed, the fluorescence, measured at 23°C, of spores heat treated with SYTO 16 and that had lost their CaDPA was still much lower than that of germinated spores, although it was higher than that of spores that were heat treated with SYTO 16 present but retained CaDPA or were heat treated without SYTO 16 and had SYTO 16 added only after the spores were cooled. However, the fluorescence intensity of a DNA-SYTO 16 mixture decreased only ∼30% following a 30-min treatment at 90°C and subsequent cooling. Since almost all SYTO 16 fluorescence in spores is due to interaction with DNA (16), possible reasons for the low SYTO 16 fluorescence of wet-heat-treated spores also include (i) DNA denaturation at heat inactivation temperatures (8, 9, 43, 44) and a low affinity of SYTO 16 dye molecules for single-stranded DNA, (ii) SYTO 16 dissociation from DNA at high temperatures, (iii) a low quantum efficiency of SYTO 16-DNA fluorescence at high temperatures, or (iv) some combination of all of these factors, since the experiments with lambda DNA found only low fluorescence of SYTO 16 with DNA at temperatures of ≥65°C.
ACKNOWLEDGMENTS
This work was supported by a grant from the Army Research Office (Y.-Q.L. and P.S.) and by a Multidisciplinary University Research Initiative (MURI) award from the Department of Defense (P.S. and Y.-Q.L.).
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Atrih A., Foster S. J. 2001. In vivo roles of the germination-specific lytic enzymes of Bacillus subtilis 168. Microbiology 147:2925–2932 [DOI] [PubMed] [Google Scholar]
- 2. Benevides J. M., Thomas G. J., Jr 1983. Characterization of DNA structures by Raman spectroscopy: high-salt and low-salt forms of double helical poly(dG-dC) in H2O and D2O solutions and application to B, Z and A-DNA. Nucleic Acids Res. 11:5747–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benevides M. J., Tsuboi M., Bamford J. K. H., Thomas G. J., Jr 1997. Polarized Raman spectroscopy of double-stranded RNA from bacteriophage φ6: local Raman tensors of base and backbone vibrations. Biophys. J. 72:2748–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen D., Huang S. S., Li Y. Q. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936–6941 [DOI] [PubMed] [Google Scholar]
- 5. Clements M. O., Moir A. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman W. H., Chen D., Li Y.-Q., Cowan A. E., Setlow P. 2007. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 189:8458–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman W. H., Zhang P., Li Y. Q., Setlow P. 2010. Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett. Appl. Microbiol. 50:507–514 [DOI] [PubMed] [Google Scholar]
- 8. Depew R. E., Wang J. C. 1975. Conformational fluctuations of DNA helix. Proc. Natl. Acad. Sci. U. S. A. 72:4275–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duguet M. 1993. The helical repeat of DNA at high temperature. Nucleic Acids Res. 21:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fairhead H., Setlow B., Setlow P. 1993. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J. Bacteriol. 175:1367–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerhardt P., Marquis R. E. 1989. Spore thermoresistance mechanisms, p. 43–64 In Smith I., Slepecky R. A., Setlow P. (ed.), Regulation of procaryotic development: structural and functional analysis of bacterial sporulation and germination. American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Goldrick S., Setlow P. 1983. Expression of a Bacillus megaterium sporulation-specific gene in Bacillus subtilis. J. Bacteriol. 155:1459–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashimoto T., Frieben W. R., Conti S. F. 1969. Germination of single bacterial spores. J. Bacteriol. 98:1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang S. S., et al. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 189:4681–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitagawa T., Hirota S. 2002. Raman spectroscopy of proteins, p. 3426–3446 In Chalmers J. M., Griffiths P. R. (ed.), Handbook of vibrational spectroscopy, vol. 5 John Wiley, Hoboken, NJ [Google Scholar]
- 16. Kong L., Zhang P., Yu J., Setlow P., Li Y.-Q. 2010. Monitoring the kinetics of uptake of a nucleic acid dye during the germination of single spores of Bacillus species. Anal. Chem. 82:8717–8724 [DOI] [PubMed] [Google Scholar]
- 17. Lewis J. C., Snell N. S., Burr H. K. 1960. Water permeability of bacterial spores and the concept of a contractile cortex. Science 132:544–545 [DOI] [PubMed] [Google Scholar]
- 18. Mahadevan-Jansen A. R., Richards-Kortum R. 1996. Raman spectroscopy for the detection of cancers and precancers. J. Biomed. Opt. 1:31–70 [DOI] [PubMed] [Google Scholar]
- 19. Maquelin K., et al. 2002. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 51:255–271 [DOI] [PubMed] [Google Scholar]
- 20. Melly E., et al. 2002. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 92:1105–1115 [DOI] [PubMed] [Google Scholar]
- 21. Nelson D. L., Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J. Biol. Chem. 245:1137–1145 [PubMed] [Google Scholar]
- 22. Nicholson W. L., Setlow P. 1990. Sporulation, germination and outgrowth, p. 391–450 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 23. Paidhungat M., Setlow B., Driks A., Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paidhungat M., Ragkousi K., Setlow P. P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paredes-Sabja D., Setlow P., Sarker M. R. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94 [DOI] [PubMed] [Google Scholar]
- 26. Peng L., Chen D., Setlow P., Li Y. Q. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows the monitoring of spore germination dynamics. Anal. Chem. 81:4035–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Popham D. L., Helin J., Costello C. E., Setlow P. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. U. S. A. 93:15405–15410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puppels G. J., et al. 1990. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature 347:301–303 [DOI] [PubMed] [Google Scholar]
- 29. Puppels G. J., Garritsen H. S. P., Segersnolten G. M. J., Demul F. F. M., Greve J. 1991. Raman microspectroscopic approach to the study of human granulocytes. Biophys. J. 60:1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Setlow B., Setlow P. 1994. Heat inactivation of Bacillus subtilis spores lacking small, acid-soluble spore proteins is accompanied by generation of abasic sites in spore DNA. J. Bacteriol. 176:2111–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Setlow B., Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Setlow B., Setlow P. 1998. Heat killing of Bacillus subtilis spores in water is not due to oxidative damage. Appl. Environ. Microbiol. 64:4109–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Setlow B., et al. 2009. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J. Appl. Microbiol. 107:318–328 [DOI] [PubMed] [Google Scholar]
- 34. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 35. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 36. Setlow P., Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J. Biol. Chem. 245:3637–3644 [PubMed] [Google Scholar]
- 37. Setlow P., Johnson E. A. 2007. Spores and their significance, p. 35–67 In Doyle M. P., Beuchat L. R., Montville T. J. (ed.), Food microbiology: fundamentals and frontiers, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 38. Wang G., Zhang P., Setlow P., Li Y. Q. 2011. Kinetics of germination of wet heat-treated individual spores of Bacillus subtilis as followed by Raman spectroscopy and differential interference contrast microscopy. Appl. Environ. Microbiol. 77:3368–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang R., et al. 2007. Fingerprinting species and strains of Bacilli spores by distinctive coat surface morphology. Langmuir 23:10230–10234 [DOI] [PubMed] [Google Scholar]
- 40. Warth A. D. 1978. Relationship between the heat resistance of spores and the optimum temperature and maximum growth temperatures of Bacillus species. J. Bacteriol. 134:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Westphal A. J., Price P. B., Leighton T. J., Wheeler K. E. 2003. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. U. S. A. 100:3461–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie C., et al. 2005. Identification of single bacterial cells in aqueous solution using confocal laser tweezers Raman spectroscopy. Anal. Chem. 77:4390–4397 [DOI] [PubMed] [Google Scholar]
- 43. Yabuki S., Motohiro F., Wada A. 1971. The fine structures in melting curves of deoxyribonucleic acids of bacteriophage lambda. Int. J. Biochem. 69:191–207 [DOI] [PubMed] [Google Scholar]
- 44. Yabuki S., Gotoh O., Wada A. 1975. Fine structures in denaturation curves of bacteriophage lamda DNA. Biochim. Biophys. Acta 395:258–273 [DOI] [PubMed] [Google Scholar]
- 45. Zhang P., Kong L., Wang G., Setlow P., Li Y. Q. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J. Biomed. Opt. 15:056010-1-9 [DOI] [PubMed] [Google Scholar]
- 46. Zhang P., Kong L., Setlow P., Li Y. Q. 2010. Characterization of wet-heat inactivation of single spores of Bacillus species by dual-trap Raman spectroscopy and elastic light scattering. Appl. Environ. Microbiol. 76:1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]