Abstract
Most known basic-region helix-loop-helix (bHLH) proteins belong to a superfamily of transcription factors often involved in the control of growth and differentiation. Therefore, inappropriate expression of genes encoding bHLH proteins is frequently associated with developmental dysfunction. In our previously reported study, a novel bHLH protein-encoding gene (AO090011000215) of Aspergillus oryzae was identified. The gene-disrupted strain was found to produce dense conidia, but sparse sclerotia, relative to the parent strain. Here, to further analyze its function, we generated an overexpressing strain using the A. oryzae amyB gene promoter. Genetic overexpression led to a large number of initial hyphal aggregations and then the formation of mature sclerotia; it was therefore designated sclR (sclerotium regulator). At the same time, the sclR-overexpressing strain also displayed both delayed and decreased conidiation. Scanning electron microscopy indicated that the aerial hyphae of the sclR-overexpressing strain were extremely branched and intertwined with each other. In the generation of the SclR-enhanced green fluorescent protein (EGFP) expression strain, the SclR-EGFP protein fusion was conditionally detected in the nuclei. In addition, the loss of sclR function led to rapid protein degradation and cell lysis in dextrin-polypeptone-yeast extract liquid medium. Taken together, these observations indicate that SclR plays an important role in hyphal morphology, asexual conidiospore formation, and the promotion of sclerotial production, even retaining normal cell function, at least in submerged liquid culture.
INTRODUCTION
The basic helix-loop-helix (bHLH) proteins belong to a superfamily of transcription factors that have been well characterized, especially in the mammalian system (1, 16, 19). The bHLH domain contains approximately 60 amino acids and is comprised of two functionally distinctive regions: the basic region and the HLH region. The basic region is located at the N terminus of the domain and functions as a DNA-binding motif. It consists of approximately 15 amino acids, which typically include six basic residues (2). The HLH region, which is located at the C terminus of the domain, functions as a dimerization domain and is constituted mainly of hydrophobic residues that form two amphipathic α helices, along with a linking loop of variable length. The amphipathic α-helices of two bHLH proteins (or bHLH and HLH proteins) can interact, allowing the formation of homodimers or heterodimers (8, 28, 29). Some bHLH proteins have been shown to bind to sequences containing a consensus core element called the E box (5′-CANNTG–3′), with the G box (5′-CACGTG-3′) being the most common form. In addition, the nucleotides flanking the core element may also have a role in binding specificity (2, 22, 24, 31).
In the animal system, bHLH proteins have been classified into six main groups (designated group A to F [1]) according to their phylogenetic relationships, DNA-binding motifs, and functional properties. The HLH family transcription factors play an important role in the regulation of neurogenesis, myogenesis, cell proliferation and differentiation, cell lineage determination, sex determination, and other essential processes in organisms ranging from yeasts to mammals (18, 26, 27, 32, 35). A phylogenetic classification of 242 bHLH proteins has been proposed (1, 3).
Compared to animals, only a small number of fungal bHLH proteins have been functionally characterized. In Aspergillus nidulans, three bHLH transcription factors (AnBH1, DevR, and StuA) were previously described. The AnBH1 is involved in the regulation of a penicillin biosynthesis gene and functions as a promoter of penicillin biosynthesis (5). DevR was shown to be a part of the tcsA signal transduction network and is required for both asexual and sexual development of A. nidulans (36). Another A. nidulans protein, StuA, conserved an APSES domain that was demonstrated to be a bHLH-like structure (7). The StuA regulates development and cell cycle progression. StuA has also been shown to act as a transcriptional repressor in A. nidulans (7).
The filamentous fungus A. oryzae, which is known for its capacity to secrete large amounts of hydrolytic enzymes, is widely utilized in the traditional food fermentative industry. On the basis of the available A. oryzae genomic sequence information (15, 21), 13 HLH proteins were predicted. However, none of the A. oryzae HLH transcription factors have been characterized to date.
In our previous study, we identified a novel bHLH protein-encoding gene (sclR; AO090011000215) by systematically deleting large chromosomal segments and further deletion analysis to screen a phenotype of dense conidia (14). A. oryzae SclR shares high similarity with those of putative proteins from genome sequencing projects of A. niger, A. fumigatus, and A. nidulans (14). The gene-disrupted strain was found to produce dense conidia, but sparse sclerotia, relative to the parent strain, suggesting that it possibly plays an important role in morphology and growth. Here, we further report the characterization of the sclR gene, along with analysis of its function. Overexpression of the sclR gene led to abnormal hyphal morphology and sclerotial formation. The loss of function of the sclR gene also led to rapid degradation of protein in liquid medium.
MATERIALS AND METHODS
Strains and media.
The A. oryzae wild-type strain, RIB40, was used as a DNA donor, strain RkuAFN, a niaD derivative from strain RkuptrP2-1ΔAF/P (Table 1), was used for the sclR overexpression in A. oryzae. To construct the RkuAFN strain, two arms were amplified by PCR. The L-arm with the primer pair AoniaDD1 and AoniaDD2 and the R-arm with the primer pair AoniaDD3 and AoniaDD4 were amplified, and then the two arms were fused by Fusion PCR with the primers AoniaDD1 and AoniaDD4. These primers are listed in Table 2. The generated fusion PCR product was introduced into the RkuptrP2-1ΔAF/P strain (Table 1), and the niaD mutants were selected on Czapek Dox (CD) minimal medium supplemented with KClO3, and NaNO2, glutamate, NH4Cl, or hypoxanthine served as the sole source of nitrogen. The exon2 of niaD open reading frame (ORF) was removed because of the introduction of the fusion PCR product. Strain HHDR (Table 1), which can visualize nuclei with the DsRed fluorescence, was used as a host strain for the expression of SclR-EGFP. For the generation of the HHDR strain, the plasmid pgHHDR was created using the Multisite Gateway cloning system (Invitrogen, Carlsbad, CA). First, the histone H2B promoter region was amplified by PCR using the primer pair H2B5 and H2BP3-2. The PCR product was digested with SmaI and then inserted into the SmaI site of the plasmid pg5′Pp (20), and the plasmid pg5′PH-2 was generated. Next, histone H2B ORF was amplified by PCR using the primer pair H2B5-2 and H2B3-2. The PCR product was digested with SmaI and then inserted into the SmaI site of the plasmid pgEHH (20), and the plasmid pgEH2B-3 was constructed. The generated 5′ entry clone and center entry clone plasmids (pg5′PH-2 and pgEH2B-3), 3′ entry clone plasmid pg3′DRM-CF (mdsred) (9), and the destination vector pgDSO containing the A. oryzae sC gene as a selectable marker (9), were used for Multisite Gateway LR reaction. The generated plasmid was named pgHHDR and introduced into the NS4 (niaD− sC−) strain (37). The resulting transformants were designated HHDR. Escherichia coli DH5α was used for DNA manipulation. DPY medium (2% dextrin, 1% polypeptone, 0.5% yeast extract, 0.5% KH2PO4, and 0.05% MgSO4′7H2O) was used for the liquid cultivation of A. oryzae strains. CD minimal agar medium (0.2% NaNO3, 0.05% KCl, 0.05% NaCl, 0.1% KH2PO4, 0.05% MgSO4′7H2O, 0.002% FeSO4′7H2O, 3% glucose [pH 5.5]) was used for the selection of A. oryzae transformants. Malt medium (malt extract, 80 g/liter; CuSO4, 2.0 mg/liter; Na2B4O7, 0.04 mg/liter; FePO4, 0.87 mg/liter; MnSO4, 0.95 mg/liter; Na2MoO4 0.8 mg/liter; ZnSO4, 8.0 mg/liter; agar, 15 g/liter [pH 6.0]) was used as the complete medium.
Table 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| RkuptrP2-1ΔAF | Δku70::ptrA ΔAF ΔpyrG | 34 |
| RkuptrP2-1ΔAF/P | Δku70::ptrA ΔAF pyrG+ | 30 |
| RkuAFN | Δku70::ptrA ΔAF ΔniaD | This study |
| Control-1 | Δku70::ptrA ΔAF ΔniaD pAPTLN[niaD] | This study |
| OE-sclR | Δku70::ptrA ΔAF ΔniaD pamy215[niaD] | This study |
| ΔsclR | Δku70::ptrA ΔsclR::pyrG | 14 |
| HHDR | niaD−sC− pgHHDR[sC] | This study |
| Control-2 | niaD−sC− pgHHDR[sC] pAPTLN[niaD] | This study |
| SclR-EGFP | niaD−sC− pgHHDR[sC] p215GFP[niaD] | This study |
Table 2.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| AoniaDD1 | GCATATCGGCATCTCAATAAAAACC |
| AoniaDD2 | TTAGCTCTTTGCGTCGCTATCTGGAGTTCCTTAATCTACG |
| AoniaDD3 | GGAACTCCAGATAGCGACGCAAAGAGCTAAACAATGTACG |
| AoniaDD4 | CGCCAGGGTGGCCTTCAAGAAATGC |
| H2B5 | AATCCCGGGTCGATTGTACCAGTTGGACG |
| H2BP3-2 | AATCCCGGGTTTAAAAGTTGATAAATTCGAAAGTTGAAT |
| H2B5-2 | AATCCCGGGATGCCTCCCAAAGCTGC |
| H2B3-2 | AATCCCGGGTTTGGCAGATGAGGAATACTTCGT |
| 215F(SpeI) | GGACTAGTATGGCATACACTAGAACTGATCC |
| 215R(SmaI) | TCCCCCGGGTTAAGCGCGAAATGTCTCAGC |
| QRT215-F(187) | GAGAACTTCGCTCTCGATGTGC |
| QRT215-R(311) | CCGAAGTGATAGAACCGGCAT |
| H2B-F(20) | CTGAGAAGAAGCCCAGCACTG |
| H2B-R(270) | GAGTTCAGAATAGACATGGCAC |
| TMtef1FW | AGCCCATGTGTGTGGAGTCTT |
| TMtef1RV | AACCGACTTGATAACTCCGACG |
| 215p-F(−990IF) | AACAGCTATGACCATGCAGGATGATGCCAGTCAACTGTAG |
| 215R(IF) | AGTGGTGGTGATGGTGTTAAGCGCGAAATGTCTCAGC |
| V-F | CACCATCACCACCACTAACCC |
| V-R | CATGGTCATAGCTGTTTCCTGTG |
| amyT-F(IF) | GTAGTCGTACCCGATGATGAAAC |
| 215-R2(IF) | AGCGCGAAATGTCTCAGCCCCT |
| GFPc-F(IF) | TGAGACATTTCGCGCTATGGTGAGCAAGGGCGAGGAG |
| GFPc-R(IF) | CATCGGGTACGACTACGCTTTACTTGTACAGCTCGTC |
| VeA2-TF | GATCAACCAGTACCAGCACGG |
| VeA2-TR | CTGTGCACCCTGGAAACCAT |
| abaA-TF | CCATCCAGGTCGAGTGCTTC |
| abaA-TR | AGGCATTGGGTGAGTTGGGACC |
| TMbrlA-FW | TATGCCCGACTTTCTGTCCG |
| TMbrlA-RV | ATGGGAGGCTGTGTGTTCCA |
| TMwetA-FW | CACAGTGGGCGAAGGATTTC |
| TMwetA-RV | AACAGCAGAGGGATGAACGG |
| qRTsspA-F(191) | AACACGATGCGGATTTCCAA |
| qRTsspA-R(291) | GGTCCCCTCAGAAAGGATCTTG |
Construction of plasmid for sclR overexpression.
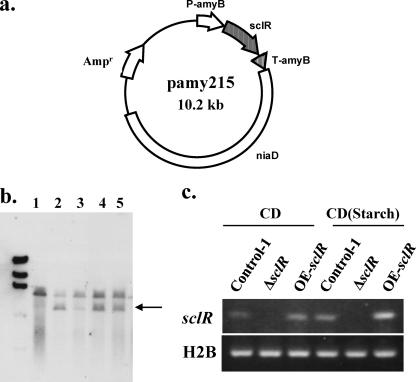
To construct the plasmid for sclR overexpression, the sclR ORF was amplified by PCR using the A. oryzae RIB40 genomic DNA and the primer pair 215F(SpeI) and 215R(SmaI). After digestion with SpeI and SmaI, the fragment was ligated into the SpeI-SmaI site of the multicloning site of the pAPTLN vector (25), which contains the amyB promoter and the niaD gene as a selectable marker. The generated plasmid pamy215 (Fig. 2a) was then used for the transformation.
Fig. 2.
Construction of the sclR overexpression strain. (a) Construction of the plasmid for sclR overexpression. The construction of the plasmid is described in detail in Materials and Methods. The full-length sclR ORF is inserted into the terminus of the amyB promoter. (b) Confirmation of sclR overexpression strain by Southern blot analysis. Genomic DNAs were digested with EcoRI and hybridized with the probe (sclR ORF). The expected DNA sizes are shown by the arrow. Lane 1, parent strain; lanes 2 to 5, transformants with pamy215. (c) Confirmation of sclR overexpression by semiquantitative RT-PCR analysis. Total RNA was isolated from the strains cultured on CD or starch-containing CD media for 2 days. Negative-control PCR analysis using each mRNA as a template showed no amplified fragments (data not shown).
Southern blot analysis.
sclR-overexpressing strains were identified by PCR and Southern blot analysis. Genomic DNA of A. oryzae strains was extracted as previously described (33). After electrophoresis, the digested genomic DNA was transferred onto a Hybond-N+ membrane (Amersham Biosciences, Amersham, United Kingdom). Southern hybridization was performed as described previously (33). A digoxigenin (DIG)-labeled probe was constructed by using a PCR DIG labeling kit (Roche Diagnostics, Mannheim, Germany) with the primer pair 215F(SpeI) and 215R(SmaI). Hybridization and detection of signals with the DIG system were performed according to the manufacturer's instructions (Roche Diagnostics).
RNA isolation and real-time PCR analysis.
Total RNA was isolated from the strains grown under different culture conditions by using Isogen (Wako, Osaka, Japan) and an RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturers' protocols. The total RNA was treated with DNase I (TaKaRa, Japan) to remove the chromosomal DNA. Reverse transcription-PCR (RT-PCR) was performed with 1 μg of total RNA and a SuperScript VILO cDNA synthesis kit (Invitrogen) in accordance with the manufacturer's instructions. Semiquantitative real-time PCR was carried out with cDNA as a template. The primer pair QRT215-F(187) and QRT215-R(311) was used to amplify sclR, and the primer pair H2B-F(20) and H2B-R(270) was used to amplify the histone H2B as a positive control. PCR was performed for 23 cycles using a program of 94°C for 5 s and 60°C for 30 s. PCR products were collected and electrophoresed on a 2% agarose gel and stained with GelRed (Wako). Quantitative real-time PCR was performed for sclR using the same primer pair mentioned above, and TEF1, as a reference gene, was amplified by using the primer pair TMtef1FW and TMtef1RV. In addition, the expression levels of brlA, wetA, abaA, veA, and sspA were tested by using the primer pairs TMbrlA-FW/TMbrlA-RV, TMwetA-FW/TMwetA-RV, abaA-TF/abaA-TR, veA2-TF/veA2-TR, and qRTsspA-F(191)/qRTsspA-R(291), respectively. Gene expression was analyzed with 300 nM primers using the Brilliant II Fast SYBR green QPCR master mix (Stratagene) and the Mx3000P instrument (Stratagene). Cycle conditions were 2 min at 95°C and 40 cycles of 5 s at 95°C and 20 s at 60°C. A standard curve method was used here for the quantification of gene expression. The relative level of expression was determined by the ratio of the target gene to the reference gene after amplification. All reactions were performed in duplicate, and the mixture included a negative no-RT control.
Conidial count and sclerotial count.
Each strain was cultured onto a malt agar plate (diameter, 85 mm) at 30°C for 5 to 10 days. Conidia were suspended in 10 ml of 0.01% Tween 80 and filtered through a 70-μm-pore-size cell strainer (BD Falcon) to remove the mycelial matter. Separated conidia were further diluted and counted with a hemacytometer. Simultaneously, the mycelial matter containing the sclerotia after filtration was resuspended in 20 ml of distilled water and vortexed 10 times. Then, 1 ml of resuspension was extracted, and the black or dark brown sclerotial formations were counted.
Measurement of biomass dry weight.
A total of 106 conidia of each strain were cultivated in 40 ml of DPY liquid medium for 1, 3, and 6 days. Mycelia were collected by filtration through a Miracloth (Calbiochem) and then rapidly frozen in liquid nitrogen for 5 min. The frozen samples were lyophilized for 18 h. The weights of the freeze-dried samples were measured.
Construction of the strain for the production of the SclR-EGFP fusion protein.
The DNA sequence containing the ∼1.0-kb sclR promoter region and full-length ORF was amplified by PCR using the A. oryzae RIB40 genomic DNA and the primer pair 215p-F(−990IF) and 215R(IF). The PCR product was then inserted into a pAPTLN vector that was linearized by PCR with the primers V-F and V-R using an In-Fusion PCR cloning kit (Takara) according to the manufacturer's protocols, because the designed PCR primers of the inserts have 16 bases with homology to the terminal sequences of the linearized pAPTLN vector. Next, the generated plasmid p215 was linearized by PCR with the primer pair amyT-F(IF) and 215-R2(IF). Using the plasmid pNH2BG harboring the h2b-egfp fusion gene (23) as a template, egfp was amplified by PCR with the primer pair GFPc-F(IF) and GFPc-R(IF) and then was further inserted into the C-terminal of the sclR ORF of linearized p215 by use of an In-Fusion cloning kit (Takara). Finally, the plasmid (p215GFP) was generated and introduced into the HHDR strain to obtain the SclR-EGFP fusion protein-expressing strain.
SDS-PAGE, CBB staining, and Western blot analysis.
Conidia were cultured into 40 ml of DPY liquid medium at 30°C for 3 days. Proteins were extracted from the mycelia with radioimmunoprecipitation assay buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 50 mM Tris-HCl [pH 7.4], and a 1% proteinase inhibitor [Wako]). A BCA protein assay kit (Pierce Chemicals) was used to quantitate the protein concentration. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 10 to 20% polyacrylamide gel (Wako). Portions of 10 μl/well (24 μg) of each concentrated sample were loaded. The polyacrylamide gel was stained with a quick-CBB staining kit (Wako) for 30 min. For Western blot analysis, samples separated by electrophoresis on SDS-polyacrylamide gels were transferred onto a polyvinylidene difluoride membrane (GE Healthcare, Amersham, United Kingdom). The membrane was probed with primary antibody to green fluorescent protein (GFP; 1:5,000 dilution, ab290; Abcam, Cambridge, United Kingdom), and horseradish peroxidase-conjugated anti-rabbit antibody (1:10,000; GE Healthcare) was used as the secondary antibody. The peroxidase reaction products were detected using the ECL Plus Western blotting detection reagent (GE Healthcare).
SEM.
Conidia were cultivated on malt agar medium at 30°C for 2 to 5 days. Samples were cut and fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M phosphate-buffered saline (pH 7.4) for 3 h and postfixed with 1% osmium tetroxide in the same buffer for 1.5 h. They were rapidly frozen in liquid nitrogen for 3 to 5 min, and then lyophilization was performed. The specimens were examined by scanning electron microscopy (SEM; TM-1000 Miniscope; Hitachi High Technologies, Tokyo, Japan).
Microscopy and image analysis.
Confocal microscopy was performed with an IX71 inverted microscope (Olympus, Tokyo, Japan) equipped with ×100 and ×40 Neofluor objective lenses (1.30 numerical aperture), 488-nm (Furukawa Electric, Tokyo, Japan) and 561-nm (Melles Griot, California) semiconductor lasers, GFP, DsRed and DualView filters (Nippon Roper, Chiba, Japan), a CSU22 confocal scanning system (Yokogawa Electronics, Tokyo, Japan), and an Andor iXon cooled digital charge-coupled device camera (Andor Technology, PLC, Belfast, United Kingdom). Images were analyzed using Andor iQ software (Andor Technology).
RESULTS
Pattern of sclR gene expression.
In our previous study, we identified a bHLH protein-encoding gene, sclR, the disruption of which led to dense conidia and sparse sclerotia. SclR is predicted to encode an unknown protein of 302 amino acids containing a putative bHLH motif (14).
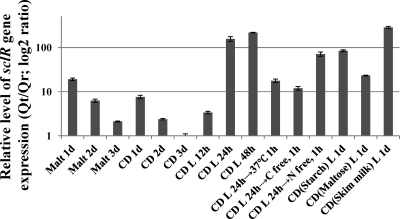
To learn more about the mechanism of sclR gene, the timing of sclR gene expression were examined using quantitative RT-PCR. The wild-type strain A. oryzae RIB40 was grown under different culture conditions, the total RNA was extracted, and a target segment of the sclR gene was amplified from the purified RNA. The relative amounts of sclR message were determined from the ratio of the target to the reference gene after amplification (Qt/Qr). Quantitative RT-PCR (qRT-PCR) data analysis indicated that the sclR gene was expressed at the early stage (on the first day) when cultivated on both malt and CD agar media, and the expression level was found to gradually decrease from 2 days onward (Fig. 1). In addition, it is obvious that the expression level of sclR is higher in a liquid shaking incubator than on the agar medium, and the expression level showed an increasing tendency during 2-day cultivation. sclR also showed higher expression level in skim milk-containing CD liquid medium (Fig. 1).
Fig. 1.
Pattern of sclR gene expression. The relative levels of expression of sclR message were determined by qRT-PCR. The relative amounts of sclR message (target) were determined from the ratio of the target to the reference gene after amplification (Qt/Qr). TEF1 was used as an endogenous reference gene. Malt, malt agar medium; CD, CD agar medium; CD L, CD liquid medium; CD(Starch) L, CD liquid medium contained 3% soluble starch instead of glucose; CD(Maltose) L, CD liquid medium contained 3% maltose instead of glucose; CD(Skim milk) L, CD liquid medium contained 0.2% skim milk instead of NaNO3. The expression value of sclR gene at CD 3d point was used as the baseline.
Construction of A. oryzae sclR overexpression strain.
To construct the sclR-overexpressing strain, RkuAFN was transformed with the generated plasmid, pamy215, as depicted in Fig. 2a. Genomic DNA extracted from transformants was isolated, digested with EcoRI, and then analyzed by Southern blot analysis using the sclR ORF as a probe. A single band was detected in the wild-type strain, while two individual bands were detected in that of the sclR-overexpressing strains. The size of the second band confirmed that the plasmid pamy215 has been inserted into the genomic niaD locus (Fig. 2b). To further verify whether the sclR gene was overexpressed in sclR-overexpressing strains, the transcription level of sclR was examined by semiquantitative RT-PCR analysis. For this analysis, total RNA was isolated from the strain grown under noninducing and inducing conditions. Starch-containing medium was used as an A. oryzae amyB promoter induction medium, since the promoter can be strongly induced by starch. The results of semiquantitative RT-PCR analysis show that sclR was more effectively transcribed in the sclR-overexpressing strain (OE-sclR) than in the wild-type strain when grown under the inducing culture condition. At the same time, no amplified fragment was detected in the sclR-disrupted strain (ΔsclR) (Fig. 2c).
sclR overexpression promotes sclerotium initiation.
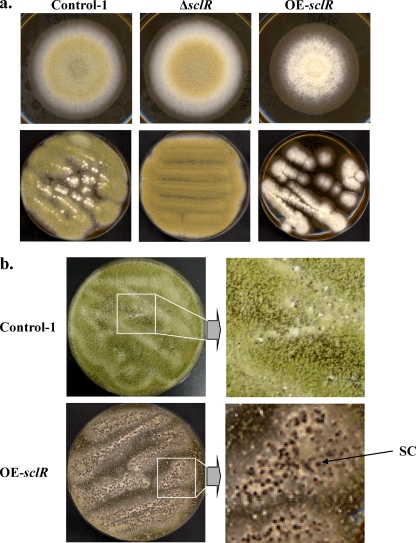
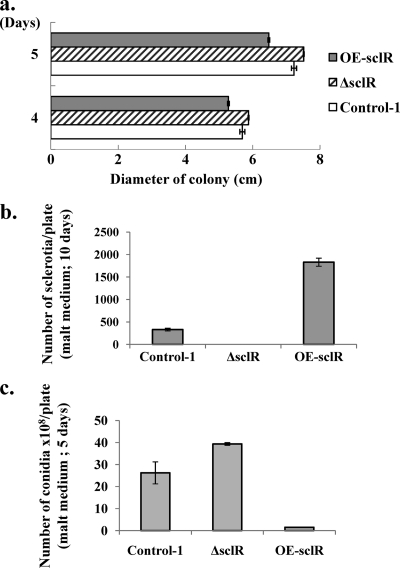
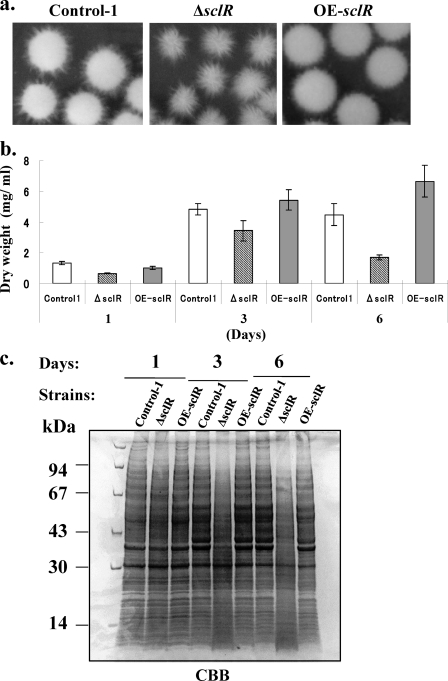
As shown in our previous research, the ΔsclR strain exhibited dense conidiation and sparse sclerotia relative to the control strain (14). Conversely, in the OE-sclR strain, hyphal aggregation was highly promoted, at the same time numerous sclerotial formations were observed (Fig. 3). When grown on the malt agar medium, the OE-sclR strain initially displayed abnormal polar growth and formed cottony and fluffy hyphae (Fig. 3a). Then, the abnormal hyphae gradually aggregated and a number of sclerotium-like structures were observed 7 to 9 days after inoculation (Fig. 3b). Measurements of the diameter of the colonies showed that the OE-sclR strain had a slower growth rate than the control strain, which was the opposite of what we observed with the ΔsclR strain (Fig. 4 a). To verify whether the sclerotia of OE-sclR strain actually exceeded that of the control strain, the sclerotial number was counted. The result indicated that the sclerotial number of the OE-sclR strain had increased >5.5 times compared to the control strain when spot cultured on the malt agar medium (Fig. 4b). Conversely, the ΔsclR strain did not produce sclerotia or produced only a negligible number. At the same time, the asexual development-conidiation of the OE-sclR strain was notably reduced (Fig. 4c).
Fig. 3.
Growth phenotype of the sclR-overexpressing strain and the sclR-disrupted strain. (a) Comparison of growth phenotypes. The Control-1 strain, sclR-disrupted strain (ΔsclR), and sclR-overexpressing strain (OE-sclR) were cultivated on malt agar medium at 30°C using spot and streak culture. (b) sclR overexpression promotes sclerotial production. The OE-sclR strain and the Control-1 strain were cultured on malt agar medium at 30°C for 9 days. The magnification of the panels on the right hand site is the same. SC, sclerotium.
Fig. 4.
Comparison of growth of the sclR-overexpressing strain, the sclR-disrupted strain, and the control strain. The OE-sclR strain, the ΔsclR strain, and the Control-1 strain were spot cultured on malt agar medium at 30°C for several days. The colony diameter (a), sclerotial number (b), and conidial number (c) were measured and compared. Each experiment was performed in duplicate.
Electron microscopy of hyphal morphology.
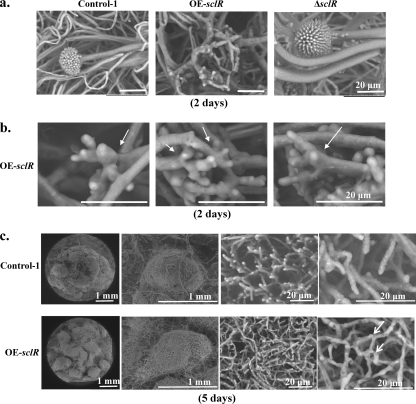
To further observe hyphal morphology, these strains were streak cultured on malt agar medium for 2 days. The marginal regions of the colonies were cut, fixed, and lyophilized. SEM indicated that a large number of the aerial hyphae of the OE-sclR strain were extremely branched. Instead of having the erect structure of conidial head, these branched aerial hyphae looked like a hand trying to grasp the neighboring hyphae and were highly intertwined with each other (Fig. 5 a and b). The aberrant hyphal morphogenesis is a possible reason for the large number of sclerotial formation. In contrast, the sclR-disrupted strain produced almost no abnormal aerial hyphae such as those in the OE-sclR strain. The sclR-overexpressing strain was cultured continuously for 5 days, and many white sclerotia were further observed using SEM. Although the control strain was also seen hyphae connections and therefore produced a small number of the sclerotia, the OE-sclR strain displayed many more and tighter hyphal connections and fusions than did the control strain (Fig. 5c).
Fig. 5.
Electron microscopy. (a) Observation of hyphal morphology. The Control-1 strain, the OE-sclR strain, and the ΔsclR strain were cultivated on malt agar medium at 30°C for 2 days. Scale bar, 20 μm. (b) Further observation of superhyphal branching of the sclR-overexpressing strain. The sclR overexpression strain that was cultivated on malt agar medium for 2 days was further magnified and observed by SEM. The white arrows indicate the hyphal branching sites at the apex. (c) Comparison of sclerotial structure between the OE-sclR strain and the Control-1 strain. The control strain and OE-sclR strain were cultivated on malt agar medium at 30°C for 5 days. Hard white sclerotial structures were observed and compared. The arrows indicate the hyphal fusions and interconnections.
sclR gene-disruption leads to dramatic cell-lysis and protein degradation in DPY liquid medium.
The OE-sclR strain, ΔsclR strain, and control strain were cultivated in DPY liquid medium at 30°C for 1, 3, and 6 days. Their phenotypes were observed and compared. We found that the ΔsclR strain produced an abnormal pellet that was comprised of a hollow structure surrounded by soft and fluffy hyphae (Fig. 6 a). The time course of the change in biomass indicated that sclR gene disruption resulted in an evident decrease in biomass dry weight from the first day after inoculation, and the biomass was reduced to ca. 38% of the control strain on day 6 after inoculation. Conversely, the biomass of the OE-sclR strain increased slightly (Fig. 6b). The protein production pattern was further observed. The protein was extracted from the mycelia, and SDS-PAGE was performed. The protein products of the ΔsclR strain were severely decreased after 3 days (Fig. 6c). The results confirmed that the loss of sclR gene function led to a rapid degradation of the cellular proteins in the sclR-disrupted strain grown under DPY liquid medium conditions, and the sclR-encoding protein may play an important role in retaining normal cell function, at least in a liquid medium.
Fig. 6.
Growth phenotype of the sclR-overexpressing strain and the sclR-disrupted strain in DPY liquid medium. (a) Growth phenotype of the OE-sclR strain and the ΔsclR strain. Each strain was cultivated in DPY liquid medium at 30°C for 3 days. (b) Biomass comparison. A total of 106 conidia of each strain were cultivated in 40 ml of DPY liquid medium for 1, 3, and 6 days. Mycelia were collected by filtering through Miracloth, and then lyophilization was performed. The dry weight of each strain was determined. (c) Protein production pattern in DPY liquid medium. A total of 106 conidia of each strain were cultivated in 40 ml of DPY liquid medium for 1, 3, and 6 days. The protein was extracted from the mycelia, and SDS-PAGE was performed with a 10 to 20% polyacrylamide gel. Then, 10 μl/well (24 μg) of each sample was loaded. The polyacrylamide gel was further stained with quick-CBB.
Localization of SclR-EGFP fusion protein.
Construction of the strain for the expression of SclR-EGFP fusion protein was described in Materials and Methods. An ∼1.0-kb upstream fragment of sclR was used as promoter, and egfp was fused to the C terminus of the full-length sclR ORF (see Fig. S1a in the supplemental material). The generated plasmid was used to transform the HHDR strain, the nuclei of which can be visualized by use of DsRed fluorescent protein fused to a nuclear localized protein, histone H2B (23). Genomic DNA of transformants was digested with EcoRI, and Southern blot hybridization was performed with the sclR probe. The results showed that the strains having the SclR-EGFP fusion gene had been obtained (see Fig. S1b in the supplemental material). Expression of the SclR-EGFP fusion protein was further confirmed by Western blot analysis (see Fig. S1c in the supplemental material). To verify whether the GFP fusion protein is functional, a complementation experiment was performed. The sclR-egfp expression plasmid, in which the niaD marker was replaced by pyrG marker, was used to transform the pyrG-deficient sclR-disrupted strain. The phenotype of sclR-disrupted strain was restored by introducing sclR-egfp expression plasmid (data not shown).
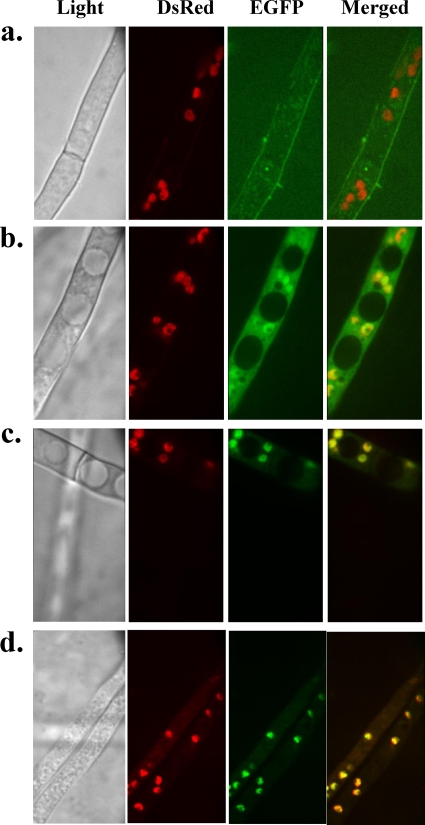
The SclR-EGFP strain was cultured in DPY liquid medium at 30°C. GFP fluorescence was observed under light and fluorescence microscopy fitted with a GFP filter. In some mycelia, the GFP fluorescence was localized in the nucleus, which is in accord with the result observed with DsRed fluorescence, while in others the GFP fluorescence observed in the whole cytoplasm of mycelia on day 3 after the inoculation (Fig. 7 b and c). On the fourth day, in the majority of mycelia, especially in the mycelia that existed in the central region of the pellet, the GFP fluorescence was easily observed to localize mainly in the nuclei (Fig. 7d).
Fig. 7.
Localization of SclR-EGFP fusion protein. SclR-EGFP strains were cultured in DPY liquid medium at 30°C for several days. Light and fluorescence microscopic observations were performed. The fluorescence of the Control-2 strain (a), the SclR-EGFP strain on day 3 after inoculation (b) and (c), and the SclR-EGFP expression strain on day 4 after inoculation (d) was observed with GFP, DsRed, and DualView filters.
qRT-PCR analysis.
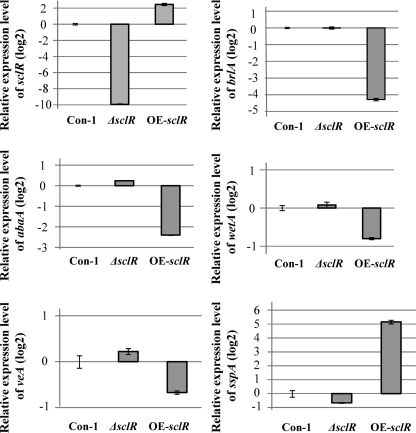
Since the sclR-overexpressing strain displayed significant morphological change, such as a large number of sclerotial formations and decreased conidiation, we assume that the expression of numerous genes involved in sexual/asexual development should have been changed in this strain. A transcription factor cascade (BrlA→AbaA→WetA) that regulates asexual development has been well characterized in A. nidulans and A. oryzae (30). The changes in expression level of these genes in the OE-sclR and ΔsclR strains were tested by qRT-PCR. These strains were cultured onto the malt agar medium for 48 h, and the result indicated that the expression of brlA and abaA, which are associated with asexual development-conidiation, were reduced severely in the OE-sclR strain relative to the control strain (Fig. 8). In contrast to the OE-sclR strain, expression of these genes did not cause significant change in the ΔsclR strain. In addition, sclR overexpression did not result in important change in the expression of veA, which is involved in sexual development in A. nidulans. Besides the above-mentioned findings, the expression of a gene homologous to ssp1, which was previously described as the major protein present in mature sclerotia of Sclerotinia sclerotiorum (17), was drastically increased in the OE-sclR strain (Fig. 8).
Fig. 8.
qRT-PCR analysis. The relative expression levels of genes involved in sexual/asexual development and sclerotial formation were examined and compared by using qRT-PCR. The expression levels of all genes were normalized to the expression level of the endogenous control gene TEF1. The expression value of each gene in the Control-1 strain was used as the baseline. Con-1, Control-1 strain. Each experiment was performed in duplicate.
DISCUSSION
We report here the characteristics of a newly identified bHLH transcription factor, SclR (AO090011000215), in A. oryzae. The overexpression of sclR stimulates sclerotial production. A sclerotium is a compact and hardened mass of fungal mycelium. One important role of sclerotia is to endure extreme environments. In the OE-sclR strain, in addition to the numerous sclerotial formations, the conidia were severely decreased. In other word, instead of the conidia, the sclerotia formed by the sclR-overexpressing strain became the primary survival structure in extreme environments.
VeA is known as a global transcription factor with a role in activating sexual development and inhibiting asexual development in A. nidulans. In A. parasiticus and A. flavus, it has been reported that VeA has an effect on sclerotial formation (4, 6). Sclerotial bodies are similar to cleistothecia but have lost the capacity to produce spores. Therefore, the sclerotium is hypothesized to be a degenerate sexual structure and may represent a vestige of cleistothecium production (10). Deletion of veA results in a complete blockage of sclerotial production in both of the above-mentioned fungi (4, 6). However, in A. oryzae, the expression of veA was not increased, according to the qRT-PCR analysis (Fig. 8), even though the sclerotial formation increased in the sclR-overexpressing strain. Similarly, in the ΔsclR mutant, although the expression level of veA was unchanged, the sclerotial production was still largely blocked (Fig. 4b). We conclude from these results that the promotion of sclerotial formation by sclR overexpression seems to be not mediated via veA overexpression.
To further explore how the sclerotial formation initiates, we examined the hyphal morphology by using SEM. Sclerotial development could be divided into three distinguishable stages: (i) initiation, (ii) white sclerotium (development), and (iii) melanized sclerotium (maturation). In the sclR-overexpressing strain, the three stages were readily observed. At the initial stage of sclerotial development, a large number of branched hyphae were observed (Fig. 5). These aberrant hyphae more easily aggregated, connected, and fused with neighboring hyphae and furthermore formed compact structures. The structures became progressively tighter and gradually developed into the mature sclerotia. Conversely, almost all of the sclR-disrupted strain produced normal hyphae on which conidiophores were formed (Fig. 5a). Phenotypic analysis further confirmed that SclR is a negative regulator of conidiation (asexual development) and, simultaneously, a positive regulator of sclerotial formation.
To further characterize sclR gene function, the phenotypes of the sclR-disrupted strain and sclR-overexpressing strain were examined when the strains were cultivated in DPY liquid medium. The ΔsclR strain produces an abnormal pellet and displays a soft and fluffy hyphal morphology (Fig. 6a). When the time in culture was sufficiently long, extreme cell lysis was observed in the ΔsclR strain. Rapid protein degradation (Fig. 6c) is the most likely reason why abnormal cell lysis happened in the sclR-disrupted strain. It is obvious that the sclR-encoding protein plays an important role in retaining both normal pellet structure and cell function in a liquid medium. The result shown in Fig. 1, where sclR expression shows a marked increase in liquid medium, also indicates that the SclR may serve a more important function in liquid medium, for example, by possibly promoting the connection and fusion of mycelia that is potentially useful for nutrition transportation. The sclR expression pattern observed by qRT-PCR also indicates that the sclR expression was strongly induced when glucose was used as the sole source of carbon or when skim milk was used as the source of nitrogen instead of NaNO3 in liquid medium (Fig. 1).
As a DNA-binding transcription factor, SclR is expected to be localized in the nucleus. Although there is no obvious nuclear localization signal motif in the N-terminal region, the SclR is still predicted to localize to the nucleus with the high probability of 73.9% as determined by the PSORT II prediction method. Interestingly, in the SclR-EGFP strain, GFP fluorescence was observed to localize in the entire cytoplasm in the majority of mycelia during the early stages. After 3 days, two GFP fluorescence distribution states were observed. One was that the GFP fluorescence was localized in the entire cytoplasm, but the fluorescence in the nuclei was a little stronger. The other was that the GFP fluorescence was found to be abundant in the nuclei. On day 4 postincubation, the GFP fluorescence localized mainly in the nuclei. We also found that the nuclear localization was readily observed in the mycelia that exist in the central region of the pellet. Considering this result together with the phenotype of the sclR-disrupted strain, which cannot form normal pellets and lysed mycelia in DPY liquid medium, SclR presumably plays an important role in regulating entwinement with neighboring mycelia and nutrition transportation to the central region of pellets for normal pellet formation in DPY liquid medium. This speculation is also in accord with another result, in which overexpression of the sclR gene promoted hyphal aggregation and sclerotial initiation on malt agar medium. Based on these observations, it is possible that, in a manner resembling the light-dependent nuclear localization of VeA, SclR localizes in the entire cytoplasm under normal conditions, but a specific environmental stimulus allows the SclR to enter the nucleus and function as a transcriptional regulator. Further research will be needed to fully understand this phenomenon.
SclR belongs to the family of bHLH transcription factors. Therefore, it seems very likely that the protein is able to form homodimers or heterodimers with as-yet-unknown partner proteins, which may include other HLH family members. The bHLH proteins bind DNA as dimers and usually recognize a short palindromic sequence, CANNTG, called the E-box element (19). SclR is a member of group B of the six main groups (A to F) of the bHLH family, since SclR contains the amino acids histidine (H), isoleucine (I), and arginine (R) at amino acid positions 5, 8, and 13 of the bHLH motif, respectively. Members of this subclass are suggested to bind the consensus sequence, most commonly either CACGTG or CAGCTG.
In the promoter region of sclR, there are three putative E-boxes in front of the transcriptional start site: E-box 1 (CAACTG) at −974, E-box 2 (CAGTTG) at −528, and E-box 3 (CACTTG) at −319. It is thus conceivable that SclR regulates its own expression. In addition, we investigated the 1.0-kb upstream region of the brlA and sspA ORFs since they both displayed significant changes in expression in the OE-sclR strain (Fig. 8). Within the promoter regions of brlA and sspA, five E-box sites and 11 E-box sites, respectively, were identified. It is likely that the SclR transcription factor also regulates the expression of the two genes through direct binding to the E-box elements. SclR also putatively contains 3 glycosylation sites and 18 phosphorylation sites. SclR may serve as a substrate for phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and casein kinase II, based on ppsearch (prosite search for protein motifs). It is consistent with the previous report that many HLH proteins are targets for phosphorylation, and it has been shown to be essential for functional activity as a transcription factor (11–13).
VeA is known to be involved in regulating conidiation and cleistothecial/sclerotial formation, as mentioned above. However, except for veA, no other gene has been previously identified for sclerotial development in Aspergillus. Thus, although the precise role of sclR remains to be determined, it is clearly required for sclerotial formation. These studies on SclR provide certain important clues to the formation and dynamics of sclerotia in A. oryzae.
Supplementary Material
ACKNOWLEDGMENTS
We thank our coworkers in the laboratory for many valuable discussions.
This study was supported by a program for the promotion of basic research activities for innovative biosciences of the Bio-Oriented Technology Research Advancement Institution.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 6 May 2011.
REFERENCES
- 1. Atchley W. R., Fitch W. M. 1997. A natural classification of the basic helix-loop-helix class of transcription factors. Proc. Natl. Acad. Sci. U. S. A. 94:5172–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atchley W. R., Terhalle W., Dress A. 1999. Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J. Mol. Evol. 48:501–516 [DOI] [PubMed] [Google Scholar]
- 3. Atchley W. R., Wollenberg K. R., Fitch W. M., Terhalle W., Dress A. W. 2000. Correlations among amino acid sites in bHLH protein domains: an information theoretic analysis. Mol. Biol. Evol. 17:164–178 [DOI] [PubMed] [Google Scholar]
- 4. Calvo A. M., Bok J., Brooks W., Keller N. P. 2004. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 70:4733–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caruso M. L., Litzka O., Martic G., Lottspeich F., Brakhage A. A. 2002. Novel basic-region helix-loop-helix transcription factor (AnBH1) of Aspergillus nidulans counteracts the CCAAT-binding complex AnCF in the promoter of a penicillin biosynthesis gene. J. Mol. Biol. 323:425–439 [DOI] [PubMed] [Google Scholar]
- 6. Duran R. M., Cary J. W., Calvo A. M. 2007. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 73:1158–1168 [DOI] [PubMed] [Google Scholar]
- 7. Dutton J. R., Johns S., Miller B. L. 1997. StuAp is a sequence-specific transcription factor that regulates developmental complexity in Aspergillus nidulans. EMBO J. 16:5710–5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellenberger T., Fass D., Arnaud M., Harrison S. C. 1994. Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 8:970–980 [DOI] [PubMed] [Google Scholar]
- 9. Escaño C. S., et al. 2009. Disruption of the Aopex11-1 gene involved in peroxisome proliferation leads to impaired Woronin body formation in Aspergillus oryzae. Eukaryot. Cell 8:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geiser D. M., Timberlake W. E., Arnold M. L. 1996. Loss of meiosis in Aspergillus. Mol. Biol. Evol. 13:809–817 [DOI] [PubMed] [Google Scholar]
- 11. Gupta S., Seth A., Davis R. J. 1993. Transactivation of gene expression by Myc is inhibited by mutation at the phosphorylation sites Thr-58 and Ser-62. Proc. Natl. Acad. Sci. U. S. A. 90:3216–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henriksson M., Bakardjiev A., Klein G., Luscher B. 1993. Phosphorylation sites mapping in the N-terminal domain of c-myc modulate its transforming potential. Oncogene 8:3199–3209 [PubMed] [Google Scholar]
- 13. Iijima S., Teraoka H., Date T., Tsukada K. 1992. DNA-activated protein kinase in Raji Burkitt's lymphoma cells: phosphorylation of c-Myc oncoprotein. Eur. J. Biochem. 206:595–603 [DOI] [PubMed] [Google Scholar]
- 14. Jin F. J., Takahashi T., Machida M., Koyama Y. 2009. Identification of a basic helix-loop-helix-type transcription regulator gene in Aspergillus oryzae by systematically deleting large chromosomal segments. Appl. Environ. Microbiol. 75:5943–5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi T., et al. 2007. Genomics of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 71:646–670 [DOI] [PubMed] [Google Scholar]
- 16. Ledent V., Vervoort M. 2001. The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 11:754–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li M., Rollins J. A. 2009. The development-specific protein (SspI) from Sclerotinia sclerotiorum is encoded by a novel gene expressed exclusively in sclerotium tissues. Mycologia 101:34–43 [DOI] [PubMed] [Google Scholar]
- 18. Li X., et al. 2006. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 141:1167–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Littlewood T., Evan G. I. 1998. Helix-loop-helix transcription factors, 3rd ed. Oxford University Press, New York, NY [Google Scholar]
- 20. Mabashi Y., Kikuma T., Maruyama J., Arioka M., Kitamoto K. 2006. Development of a versatile expression plasmid construction system for Aspergillus oryzae and its application to visualization of mitochondria. Biosci. Biotechnol. Biochem. 70:1882–1889 [DOI] [PubMed] [Google Scholar]
- 21. Machida M., et al. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157–1161 [DOI] [PubMed] [Google Scholar]
- 22. Martinez-Garcia J. F., Huq E., Quail P. H. 2000. Direct targeting of light signals to a promoter element-bound transcription factor. Science 288:859–863 [DOI] [PubMed] [Google Scholar]
- 23. Maruyama J., Nakajima H., Kitamoto K. 2001. Visualization of nuclei in Aspergillus oryzae with EGFP and analysis of the number of nuclei in each conidium by FACS. Biosci. Biotechnol. Biochem. 65:1504–1510 [DOI] [PubMed] [Google Scholar]
- 24. Massari M. E., Murre C. 2000. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20:429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morita H., Hatamoto O., Masuda T., Sato T., Takeuchi M. 2007. Function analysis of steA homolog in Aspergillus oryzae. Fungal Genet. Biol. 44:330–333 [DOI] [PubMed] [Google Scholar]
- 26. Murre C., Baltimore D. 1992. The helix-loop-helix motif: structure and function, vol. 2 Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 27. Murre C., et al. 1994. Structure and function of helix-loop-helix proteins. Biochim. Biophys. Acta 1218:129–135 [DOI] [PubMed] [Google Scholar]
- 28. Murre C., McCaw P. S., Baltimore D. 1989. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56:777–783 [DOI] [PubMed] [Google Scholar]
- 29. Nesi N., et al. 2000. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12:1863–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogawa M., Tokuoka M., Jin F. J., Takahashi T., Koyama Y. 2010. Genetic analysis of conidiation regulatory pathways in koji-mold Aspergillus oryzae. Fungal Genet. Biol. 47:10–18 [DOI] [PubMed] [Google Scholar]
- 31. Robinson K. A., Koepke J. I., Kharodawala M., Lopes J. M. 2000. A network of yeast basic helix-loop-helix interactions. Nucleic Acids Res. 28:4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun X., Baltimore D. 1991. An inhibitory domain of E12 transcription factor prevents DNA binding in E12 homodimers but not in E12 heterodimers. Cell 64:459–467 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi T., Hatamoto O., Koyama Y., Abe K. 2004. Efficient gene disruption in the koji-mold Aspergillus sojae using a novel variation of the positive-negative method. Mol. Genet. Genomics 272:344–352 [DOI] [PubMed] [Google Scholar]
- 34. Takahashi T., Jin F. J., Sunagawa M., Machida M., Koyama Y. 2008. Generation of large chromosomal deletions in koji molds Aspergillus oryzae and Aspergillus sojae via a loop-out recombination. Appl. Environ. Microbiol. 74:7684–7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toledo-Ortiz G., Huq E., Quail P. H. 2003. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15:1749–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tüncher A., Reinke H., Martic G., Caruso M. L., Brakhage A. A. 2004. A basic-region helix-loop-helix protein-encoding gene (devR) involved in the development of Aspergillus nidulans. Mol. Microbiol. 52:227–241 [DOI] [PubMed] [Google Scholar]
- 37. Yamada O., Lee B. R., Gomi K. 1997. Transformation system for Aspergillus oryzae with double auxotrophic mutations, niaD and sC. Biosci. Biotechnol. Biochem. 61:1367–1369 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.