Abstract
Ypd1 is a key phosphorelay protein that controls eukaryotic two-component systems, but its function in Cryptococcus neoformans is not known. Here, we report that Ypd1 is required for the viability of C. neoformans via the Hog1 mitogen-activated protein kinase (MAPK) pathway but plays multiple cellular roles in both a Hog1-dependent and -independent manner.
TEXT
The histidine-to-aspartate (His-Asp) phosphorelay system (referred to as the “two-component system”) is widely employed as a key signaling modulator in a variety of cellular processes, including chemotaxis, stress response, and differentiation, in bacteria, plants, and fungi but not in mammals (9, 14, 15, 17). The His-Asp phosphorelay system consists of either two or multiple signaling components. The two-component phosphorelay system, mostly found in bacteria, comprises a sensor histidine kinase and a response regulator. In contrast, the multicomponent phosphorelay system, mainly observed in plants and fungi, is composed of a hybrid sensor kinase where the histidine kinase and Asp-containing response regulator domains coexist, a His-containing phosphotransfer (HPt) protein and response regulators (2, 14, 15). In the bacterial two-component system, the response regulator plays a pivotal role as an effector protein with a DNA-binding domain to modulate the expression levels of target genes, whereas the multicomponent phosphorelay system is often connected to an additional signaling cascade, such as the mitogen-activated protein kinase (MAPK) pathway, downstream from a response regulator (2).
HPt proteins are key phosphorelay mediators which connect hybrid sensor kinases to response regulators, both often found with several homologs in a single organism. HPt proteins were first discovered in Bacillus subtilis, such as the Spo0B phosphotransferase involved in sporulation induction (7). In fungi, Ypd1 was discovered as the first HPt protein in Saccharomyces cerevisiae and serves as an intermediate phosphorelay protein in the Sln1-Ypd1-Ssk1 system that is critical in the osmosensing mechanism (22). These prokaryotic and eukaryotic HPt proteins exhibit low sequence homology in general, yet the region surrounding the phospho-accepting His residue is evolutionarily conserved (24). Yeast Ypd1 contains a bundle of four helices, αA, αB, αC, and αD, as a core structure, with helix αC harboring the phospho-accepting His residue in position 64 (H64) (Fig. 1 A) (23, 24). Besides H64, several residues were found to be critical for Ypd1 functionality or binding to the Sln1 sensor kinase and response regulators Ssk1 and Skn7 (Fig. 1A) (20, 21). Ypd1 is required for the viability of S. cerevisiae via the Hog1 MAPK signaling pathway (22). To relay signals from the Sln1 sensor kinase, Ypd1 dynamically shuttles between the cytoplasm and nucleus, where it activates the Ssk1 (a cytoplasmic protein) and Skn7 (a nuclear protein) response regulators, respectively (16). Unlike S. cerevisiae Ypd1, however, a Schizosaccharomyces pombe Ypd1 homolog, Mpr1 (also known as Spy1), is dispensable for viability and also regulates the Mcs4 response regulator both positively during the stress response and negatively during the control of the mitotic cell cycle (1, 18). Therefore, the functions of Ypd1 appear to be divergent between fungi.
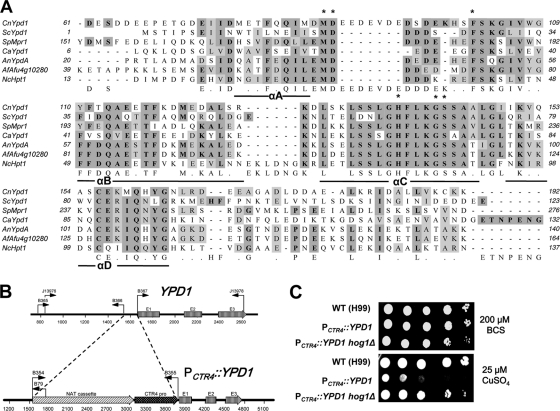
Fig. 1.
Identification of the Ypd1 phosphotransfer protein and its role in the viability of C. neoformans. (A) Multiple sequence alignments of histidine-containing phosphotransfer (HPt) proteins from C. neoformans (CnYpd1), Candida albicans (CaYpd1, GenBank accession number AF213247), Aspergillus nidulans (AnYpdA, ANID_02005.1), Aspergillus fumigatus (AfAfu4g10280, XM_746705), Neurospora crassa (NcHpt1, NCU01489), S. pombe (SpMpr1, NM_001021393), and S. cerevisiae (ScYpd1, U62016) were performed with ClustalW and illustrated with MacVector 7.2.3 (Accelrys). Asterisks indicate the evolutionarily conserved residues in HPt proteins. The boldface letters in the dark shading indicate identical amino acid residues, and the lightface letters in the light shading indicate similar amino acid residues. (B) The strategy for construction of the PCTR4::YPD1 promoter replacement strain. E1, E2, and E3 indicate three exons in the YPD1 gene, and bent arrows indicate primers for overlap PCR and diagnostic PCR for generating the PCTR4::YPD1 promoter replacement cassette and for screening positive strains, respectively. The NAT cassette consists of the ACT1 promoter, NAT (nourseothricin acetyltransferase) gene, and TRP1 terminator, and the dark arrow box illustrates the CTR4 promoter as previously described (19). (C) Growth of the PCTR4::YPD1 strain is tightly controlled by copper levels in a Hog1-dependent manner. The WT H99, PCTR4::YPD1 (YSB859), and PCTR4::YPD1 hog1Δ (YSB1370) strains were grown overnight at 30°C in liquid yeast extract-peptone-dextrose medium, 10-fold serially diluted (1 to 104 dilutions), and spotted (3 μl) on yeast nitrogen base agar medium containing 200 μM BCS and 25 μM CuSO4. Cells were incubated at 30°C for 2 days and then photographed.
In human pathogenic fungi, the phosphorelay system governs diverse stress responses and the production of virulence factors which are essential for colonization and proliferation during host infection (6, 14). In the basidiomycetous human pathogen Cryptococcus neoformans, which causes fatal fungal meningoencephalitis in both immunocompromised and immunocompetent individuals if left untreated, the phosphorelay system consists of seven sensor histidine kinases (Tco1 to -7), two response regulators (Ssk1 and Skn7), and a putative HPt protein (Ypd1). This phosphorelay system is functionally connected to the Ssk2-Pbs2-Hog1 MAPK pathway and controls diverse stress responses, sexual differentiation, the production of two major virulence factors—melanin and capsule, and ergosterol biosynthesis in C. neoformans (3–5, 11). Among the seven sensor kinases, Tco1 and Tco2 are critical sensor kinases modulating a subset of Hog1-related phenotypes. Downstream of Tco1 and Tco2, Ssk1 is the major upstream regulator of the Hog1 MAPK module, whereas Skn7 exhibits a Hog1-independent phenotype (5). In the C. neoformans phosphorelay system, however, the role of the Ypd1 HPt protein has not yet been characterized, although a single Ypd1-like ortholog appears to exist, based on the Cryptococcus genome database.
In this study, we investigated the role of a Ypd1-like HPt protein in the phosphorelay system associated with the Hog1 MAPK pathway in C. neoformans. To characterize the correct exon-intron structure and protein coding sequence of YPD1, we performed rapid amplification of cDNA ends (RACE) and cDNA analysis by using a GeneRacer kit (Invitrogen) and Superscript II reverse transcriptase (Invitrogen), respectively, with total RNAs isolated from the C. neoformans H99 strain. Each RACE and cDNA product was cloned into the pCR2.1-TOPO vector and sequenced. C. neoformans YPD1 contains three exons that encode a protein of 209 amino acids (GenBank accession no. JF937063). Compared with HPt proteins in other fungi and plants, C. neoformans Ypd1 was shown to contain highly conserved His residues (H138) that are equivalent to the phospho-accepting His residue of S. cerevisiae Ypd1 (H64) and other HPt proteins (Fig. 1A). Furthermore, other evolutionarily conserved HPt residues that are critical for interaction with sensor kinases and response regulators, including M20, D21, F27, G68, and S69 in S. cerevisiae Ypd1 (13, 20, 21), are also conserved in C. neoformans Ypd1 (Fig. 1A). Therefore, it is highly likely that C. neoformans Ypd1 is a structural homolog of fungal HPt proteins.
A prior attempt to generate a ypd1Δ mutant in C. neoformans for functional analysis of Ypd1 was not successful, implying that Ypd1 may be essential for the viability of C. neoformans. To further verify this hypothesis, we constructed a YPD1 promoter replacement strain with the copper-regulated CTR4 promoter (PCTR4::YPD1), as illustrated in Fig. 1B. To replace the native YPD1 promoter with the CTR4 promoter, the YPD1 promoter replacement cassette was generated as follows. The left flanking region (YPD1 promoter region spanning from −824 to −118 relative to the ATG start codon at ∼+1 to +3) and the right flanking region (YPD1 gene, +1 to +957 region) were PCR amplified (ExTaq; Takara Co.) with primer pairs J13976 (5′-CGAAAGAGCCTCCATAAAG-3′)/B366 (5′-CACTCGAATCCTGCATGCCAACCAACCACCGACTATTAC-3′) and B367 (5′-CGACAACGACTTCACCAATCATGCCAGACCAGGCCAGATC-3′)/J13978 (5′-TGACCATCCAGTTCTTAGCC-3′), respectively. The underlined sequences indicate the reverse-complementary sequences of B354 (5′-GCATGCAGGATTCGAGTG-3′) and B355 (5′-GATTGGTGAAGTCGTTGTCG-3′), respectively, that were used for PCR amplification of the NAT-CTR4 promoter fragment in the plasmid pNAT-CTR4-2 (provided by John Perfect at Duke University). The YPD1 promoter replacement cassette was produced by overlap PCR with the two primers J13976 and J13978 and introduced into the serotype A C. neoformans strain H99 via biolistic transformation as previously described (8, 10). The targeted promoter replacement was confirmed by diagnostic PCR with primer pair B365 (5′-CAGAAAGCGGACAAAGTAAC-3′)/B79 (5′-TGTGGATGCTGGCGGAGGATA-3′) and Southern hybridization (data not shown).
First, we examined the growth defect of the PCTR4::YPD1 strain. When grown in YNB medium containing BCS (bathocuproine disulfonate, a copper chelator), which induces the CTR4 promoter, the PCTR4::YPD1 strain showed no growth defects compared to the growth of the wild-type (WT) strain. In contrast, the PCTR4::YPD1 strain clearly exhibited growth defects in YNB medium containing CuSO4, which represses the CTR4 promoter, compared to the growth of the WT strain (Fig. 1C). These data suggest that Ypd1 is essential for the viability of C. neoformans.
Previously, we had shown that hyperactivation of Hog1 by fludioxonil caused lethality in C. neoformans due to the overaccumulation of intracellular glycerol (12). Therefore, we examined whether Ypd1 also influences the viability of C. neoformans via the Hog1 MAPK pathway. To prove the hypothesis, we disrupted the HOG1 gene in the PCTR4::YPD1 strain. The hog1Δ mutant allele using the NEOR marker (hog1::NEO) was generated by overlap PCR as previously described (4) and introduced into the PCTR4::YPD1 strain by biolistic transformation. The positive PCTR4::YPD1 hog1Δ strain was confirmed by both diagnostic PCR and Southern blot analysis (data not shown). Deletion of HOG1 restored the normal growth of the PCTR4::YPD1 strain in CuSO4-containing medium (Fig. 1C), strongly suggesting that Ypd1 governs the viability of C. neoformans via the Hog1 MAPK. This finding indicated that the deletion of YPD1 could be possible in the hog1Δ mutant background, unlike in the WT background. To prove this possibility, the YPD1 gene disruption cassette was generated by using overlap PCR with the primer pairs B1295 (5′-CGAAAGAGCCTCCATAAAG-3′)/B1703 (5′-CTGGCCGTCGTTTTACTGTCATCTTTAGCGGGTTG-3′) and J13974 (5′-GTCATAGCTGTTTCCTGACAAGGCGGCTAAGAACTGG-3′)/J13975 (5′-CGAGAGGAAAGTTCTACCCC-3′) for the 5′ and 3′ regions, respectively, and the M13Re (5′-CAGGAAACAGCTATGACCATG-3′) and M13Fe (5′-GTAAAACGACGGCCAGTGAGC-3′) primers for Natr dominant selectable markers of plasmid pNATSTM#242, and the cassette was introduced into the hog1Δ mutant (MATa YSB81) (4) by biolistic transformation. We successfully constructed three independent ypd1Δ hog1Δ mutants (YSB779, YSB780, and YSB781), which were confirmed by both diagnostic PCR and Southern blot analysis (data not shown), further demonstrating that Ypd1 controls the viability of C. neoformans via the Hog1 MAPK pathway.
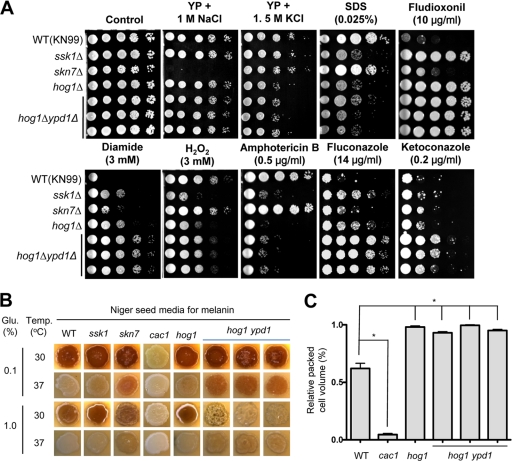
To address whether Ypd1 plays additional roles independent of the HOG pathway, we performed phenotypic analysis of ypd1Δ hog1Δ mutants in comparison to the WT and the hog1Δ, ssk1Δ, and skn7Δ mutants. The ypd1Δ hog1Δ mutant exhibited hyperosmosensitivity similar to that of the hog1Δ mutant and did not show extreme sensitivity to high sodium salt, unlike the skn7Δ mutant (Fig. 2 A). Furthermore, the ypd1Δ hog1Δ mutant was as sensitive to the cell membrane destabilizer sodium dodecyl sulfate (SDS) and as resistant to fludioxonil as the hog1Δ mutant (Fig. 2A). These data suggest that Ypd1 mainly controls the Hog1-dependent pathway for maintaining osmotic balance and cell membrane integrity. However, Ypd1 appears to have Hog1-independent roles in C. neoformans as well. The ypd1Δ hog1Δ mutant exhibited even higher resistance to diamide than the hog1Δ, ssk1Δ, and skn7Δ mutants (Fig. 2A). Furthermore, increased H2O2 sensitivity of the ssk1Δ and hog1Δ mutants was slightly suppressed by the YPD1 mutation (Fig. 2B), indicating that Ypd1 could be involved in the oxidative stress response in both a Hog1-dependent and -independent manner. Recently, we have shown that the HOG pathway negatively regulates ergosterol biosynthesis and, as a result, affects susceptibility to polyene and azole drugs. In the hog1Δ mutant (Fig. 2A), the deletion of YPD1 further increased azole resistance but not amphotericin B sensitivity. Previously, we have shown that inhibition of Skn7 increases resistance to azole drugs in an Erg11-independent manner, whereas inhibition of Ssk1 increases ERG11 expression and ergosterol synthesis and thereby promotes resistance to azole drugs in C. neoformans (11). Therefore, the further-enhanced azole resistance observed in the hog1Δ ypd1Δ double mutant may result from inhibition of both Skn7 and Ssk1. Although its reason is not clear at this point, it is possible that the activity or expression of azole drug efflux pumps could be affected by the inhibition of Ypd1 and Skn7.
Fig. 2.
Comparative phenotypic analysis of the ypd1Δ hog1Δ mutant. (A) The role of Ypd1 in stress and antifungal drug responses. Each C. neoformans strain (MATa WT [KN99a] and ssk1Δ [YSB429], skn7Δ [YSB433], hog1Δ [YSB81], and ypd1Δ hog1Δ [YSB779, YSB780, and YSB781] mutant strains) was 10-fold serially diluted (1 to 104 dilutions) and spotted (4 μl of each dilution) on YP or YPD agar containing the indicated concentration of NaCl, KCl, SDS, diamide, hydrogen peroxide (H2O2), fludioxonil, amphotericin B, fluconazole, or ketoconazole. Cells were incubated at 30°C for 2 to 3 days and then photographed. (B) The role of Ypd1 in melanin biosynthesis. For melanin production, each C. neoformans strain (MATa WT [KN99a] and ssk1Δ [YSB429], skn7Δ [YSB433], cac1Δ [YSB79], hog1Δ [YSB81], and ypd1Δ hog1Δ [YSB779, YSB780, and YSB781] mutant strains) was grown for 16 h in YPD medium, spotted on solid Niger seed medium containing either 0.1% or 1% glucose (Glu.), incubated at either 30°C or 37°C for 2 days, and then photographed. (C) The role of Ypd1 in capsule biosynthesis. Capsule synthesis levels for each C. neoformans strain were quantitatively measured by using hematocrit as described before (10). Error bars show standard deviations. *, P < 0.001.
Finally, we have examined the role of Ypd1 in the regulation of two major virulence factors, capsule and melanin, in C. neoformans. At a low glucose concentration (0.1%), the deletion of YPD1 further increased melanin production in the hog1Δ mutant, to levels equivalent to those of the skn7Δ mutant (Fig. 2B). This finding was more evident when melanin production was induced at a high temperature (37°C) (Fig. 2B). At a high glucose concentration (1%), however, the deletion of YPD1 repressed melanin production in the hog1Δ mutant, which was particularly evident when cells were placed at 30°C (Fig. 2B). These data indicate that Ypd1 plays differential roles in controlling melanin synthesis in both a Hog1-dependent and -independent manner. In contrast to melanin biosynthesis, the ypd1Δ hog1Δ mutant exhibited an increased capsule volume, similar to that of the hog1Δ mutant (Fig. 2C).
In conclusion, Ypd1 is required for the viability of C. neoformans via the Hog1-dependent signaling pathway. However, Ypd1 also plays Hog1-independent roles in controlling the oxidative stress response, azole drug resistance, and melanin biosynthesis.
Acknowledgments
This work was supported by a Korea Research Foundation grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund, KRF-2008-331-C00245) and a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST, no. 2008-0061963).
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Aoyama K., Mitsubayashi Y., Aiba H., Mizuno T. 2000. Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator. J. Bacteriol. 182:4868–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahn Y. S. 2008. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot. Cell 7:2017–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahn Y. S., Geunes-Boyer S., Heitman J. 2007. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot. Cell 6:2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahn Y. S., Kojima K., Cox G. M., Heitman J. 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 16:2285–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahn Y. S., Kojima K., Cox G. M., Heitman J. 2006. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol. Biol. Cell 17:3122–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bahn Y. S., et al. 2007. Sensing the environment: lessons from fungi. Nat. Rev. Microbiol. 5:57–69 [DOI] [PubMed] [Google Scholar]
- 7. Burbulys D., Trach K. A., Hoch J. A. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552 [DOI] [PubMed] [Google Scholar]
- 8. Davidson R. C., et al. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607–2615 [DOI] [PubMed] [Google Scholar]
- 9. Hwang I., Chen H. C., Sheen J. 2002. Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 129:500–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim M. S., et al. 2010. Comparative transcriptome analysis of the CO2 sensing pathway via differential expression of carbonic anhydrase in Cryptococcus neoformans. Genetics 185:1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko Y. J., et al. 2009. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot. Cell 8:1197–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kojima K., Bahn Y. S., Heitman J. 2006. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152:591–604 [DOI] [PubMed] [Google Scholar]
- 13. Krantz M., Becit E., Hohmann S. 2006. Comparative analysis of HOG pathway proteins to generate hypotheses for functional analysis. Curr. Genet. 49:152–165 [DOI] [PubMed] [Google Scholar]
- 14. Kruppa M., Calderone R. 2006. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 6:149–159 [DOI] [PubMed] [Google Scholar]
- 15. Laub M. T., Goulian M. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121–145 [DOI] [PubMed] [Google Scholar]
- 16. Lu J. M., Deschenes R. J., Fassler J. S. 2003. Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot. Cell 2:1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizuno T. 2005. Two-component phosphorelay signal transduction systems in plants: from hormone responses to circadian rhythms. Biosci. Biotechnol. Biochem. 69:2263–2276 [DOI] [PubMed] [Google Scholar]
- 18. Nguyen A. N., Lee A., Place W., Shiozaki K. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11:1169–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ory J. J., Griffith C. L., Doering T. L. 2004. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21:919–926 [DOI] [PubMed] [Google Scholar]
- 20. Porter S. W., West A. H. 2005. A common docking site for response regulators on the yeast phosphorelay protein YPD1. Biochim. Biophys. Acta 1748:138–145 [DOI] [PubMed] [Google Scholar]
- 21. Porter S. W., Xu Q., West A. H. 2003. Ssk1p response regulator binding surface on histidine-containing phosphotransfer protein Ypd1p. Eukaryot. Cell 2:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Posas F., et al. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875 [DOI] [PubMed] [Google Scholar]
- 23. Song H. K., et al. 1999. Insights into eukaryotic multistep phosphorelay signal transduction revealed by the crystal structure of Ypd1p from Saccharomyces cerevisiae. J. Mol. Biol. 293:753–761 [DOI] [PubMed] [Google Scholar]
- 24. Xu Q., West A. H. 1999. Conservation of structure and function among histidine-containing phosphotransfer (HPt) domains as revealed by the crystal structure of YPD1. J. Mol. Biol. 292:1039–1050 [DOI] [PubMed] [Google Scholar]