Abstract
The actin cytoskeleton forms a membrane-associated network whose proper regulation is essential for numerous processes, including cell differentiation, proliferation, adhesion, chemotaxis, endocytosis, exocytosis, and multicellular development. In this report, we show that in Dictyostelium discoideum, paxillin (PaxB) and phospholipase D (PldB) colocalize and coimmunoprecipitate, suggesting that they interact physically. Additionally, the phenotypes observed during development, cell sorting, and several actin-required processes, including cyclic AMP (cAMP) chemotaxis, cell-substrate adhesion, actin polymerization, phagocytosis, and exocytosis, reveal a genetic interaction between paxB and pldB, suggesting a functional interaction between their gene products. Taken together, our data point to PldB being a required binding partner of PaxB during processes involving actin reorganization.
INTRODUCTION
Cytoskeletal networks determine cell shape, organelle distribution, and the cytoplasmic consistency of cells. Dynamic remodeling of the cytoskeleton is responsible for cell behavior and must be regulated precisely in space and time in response to both external and internal signals. One important component of the eukaryotic cytoskeleton is actin. Filamentous actin, in cooperation with accessory proteins, forms differently organized cytoskeletal networks that play a crucial role in cell adhesion, migration, cell shape changes, phagocytosis, and exocytosis (13, 23, 60). Structural and signaling proteins facilitate organization of actin filaments into appropriate networks in response to external signals. Mutations of these accessory proteins can cause defects in cellular processes that are based on actin cytoskeleton reorganization (32, 44, 50).
Two such proteins involved in actin-dependent processes are paxillin and phospholipase D (PLD). Paxillin acts as an adapter and anchor protein by recruiting diverse structural and signaling proteins into a complex to assist transmission of downstream signals (52). Paxillin provides binding sites at the plasma membrane for tyrosine kinases such as FAK and SRC and for cytoskeletal proteins such as vinculin and parvin (4, 38, 39, 53). Paxillin also serves as a docking protein to bring the enzymes PTP-PEST and CSK into close proximity to their targets (5, 21, 49). Paxillin is implicated in cell migration, but its precise role in this process has not been well established (1, 26, 59). In addition, it is a proposed player in phagocytosis and exocytosis (24, 40). PLD hydrolyzes phosphatidylcholine, resulting in the production of choline and the second messenger phosphatidic acid (PA). Like paxillin, it has been implicated in cytoskeletal reorganization, endocytosis, exocytosis, and cell migration (41). While paxillin and PLD both regulate the actin cytoskeleton, it has yet to be determined whether these proteins function separately or together to perform this task.
Like other eukaryotes, Dictyostelium discoideum depends upon proper actin regulation for survival. Dictyostelium discoideum is a soil amoeba that feeds on bacteria by phagocytosis and can grow axenically in nutrient broth by macropinocytosis, both of which are processes which depend upon actin (7, 25, 33). Upon nutrient depletion, solitary amoebae enter a multicellular developmental program. Starving cells start attracting each other by sensing and secreting cyclic AMP (cAMP). Highly polarized and elongated cells are connected head to tail, forming aggregation streams. The cells entering the aggregation center become amoeboid again and pile on top of each other to form a three-dimensional hemispherical structure called a mound (14). Cells differentiate into prestalk and prespore populations, after which cell sorting takes place. During cell sorting, prestalk cells form a tip on the top of the mound which acts as an organizer for culmination and fruiting body formation (46). Thus, normal development requires proper cell shape, cell-substrate adhesion, cell-cell adhesion, and locomotion, all of which depend upon proper actin regulation.
Like mammalian cells, Dictyostelium discoideum has paxillin and PLD. Dictyostelium discoideum paxillin, PaxB, is required for proper cell sorting and development past the mound stage (6) and is involved in regulating actin-required processes, including adhesion, endocytosis, and cell migration (15). One of three Dictyostelium discoideum PLD's, PldB, is implicated in multicellular development and cell migration (10, 37). Furthermore, PLD activity is essential for normal motility, F-actin distribution, endocytosis, and phagocytosis (61). Thus, like their mammalian counterparts, PaxB and PldB are implicated in regulating the actin cytoskeleton.
Dictyostelium discoideum is a well-established model for studying the mechanisms of actin cytoskeleton rearrangement. Various genetic approaches allow the identification and functional characterization of genes involved in actin-dependent processes (3, 32, 36, 44). Given the fact that homologous proteins exist, Dictyostelium discoideum serves as an excellent model system for studying the roles of paxillin and PLD and their potential interactions. Here we used genetic and biochemical approaches to elucidate a functional relationship between PaxB and PldB. We show that PaxB and PldB interact physically and functionally in a number of developmental and actin-based processes.
MATERIALS AND METHODS
Strains and culture conditions.
Dictyostelium discoideum cells of the wild-type strain AX2 were grown axenically at 22°C in HL5 nutrient medium (0.5% [wt/vol] yeast extract, 0.5% protease peptone, 0.5% Thiotone peptone, 1% dextrose, 4.7 mM Na2HPO4, 2.5 mM KH2PO4, pH 6.5) (48). HR30 cells (AX2 cells expressing β-galactosidase) (17) and pldB OE cells (AX2 cells overexpressing pldB) (10) were grown in HL5 supplemented with 20 μg/ml G418. paxB− cells were grown in HL5 supplemented with 10 μg/ml Blasticidin (6). paxB− pldB OE cells were created by transforming a plasmid containing the pldB gene under the control of the constitutively active actin 15 promoter (10) into paxB− cells (6) by electroporation as described previously (28, 29). Transformed cells were selected in the presence of 10 μg/ml Blasticidin and 20 μg/ml G418. Individual clones were isolated on GYP plates (0.2% peptone, 0.025% yeast extract, 2.2% agar, 0.1% dextrose, 19 mM Na2HPO4, 30 mM KH2PO4) containing 10 μg/ml Blasticidin and 20 μg/ml G418. To initiate development, cells at mid-log phase (2 × 106 to 5 × 106 cells/ml) were washed twice with PBM (20 mM KH2PO4, 10 μM CaCl2, 1 mM MgCl2, pH 6.1, with KOH) and then plated on nitrocellulose filter pads with a 2.4-cm diameter (Millipore, Billerica, MA) at 1 × 107 cells/pad at 22°C (2).
Fluorescence microscopy.
Cells were washed twice with PBM and then starved on an 8-well chambered slide for 6 h. Cells were fixed (3.7% formaldehyde solution in phosphate-buffered saline [PBS] for 5 min) and permeabilized (0.2% Triton X-100 solution in PBS for 5 min). PaxB and PldB were detected with peptide-purified anti-PaxB and anti-PldB antibodies (10, 15), respectively, and with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG. F-actin was labeled with 8 U/ml rhodamine-phalloidin (Invitrogen, Eugene, OR) for 1 h in the dark. Slow Fade Gold (Invitrogen, Eugene, OR) mounting solution was added prior to placing a coverslip onto the slide. Confocal immunofluorescence microscopy was performed with a Leica laser scanning microscope equipped with a ×100 (HCX Plan Apo; CS 1.40) objective and Leica confocal software.
Western blot analysis.
A total of 1 × 107 cells were collected and boiled for 3 min in SDS-PAGE sample buffer in a final volume of 500 μl. Proteins were separated by SDS-PAGE, transferred to Hybond-P membranes (Amersham Bioscience, Piscataway, NJ), immunoblotted with a peptide-purified anti-PaxB or anti-PldB antibody (10, 15), and visualized by use of an enhanced chemiluminescence substrate for horseradish peroxidase (HRP) detection (Pierce, Rockford, IL).
Immunoprecipitation.
A total of 1 × 107 cells were resuspended in 0.5 ml lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 2× Roche Complete protease inhibitor cocktail [minitablet] [Indianapolis, IN]), and the lysate was clarified by centrifugation at 1,200 × g for 5 min at 4°C. Cell lysates were incubated with 1 μg antibody overnight on a rotating shaker for 2 h at 4°C. Pure proteome protein A magnetic beads (Millipore, Temecula, CA) were added to the sample and incubated on a rotary shaker for 1 h at 25°C. Beads were collected with a magnetic rack (Millipore, Temecula, CA) and washed 3 times with PBS containing 0.1% Tween. Beads were resuspended in 50 μl PBS plus 0.1% Tween and 10 μl 6× protein sample buffer and then boiled for 10 min at 90°C. Beads were then removed from suspension with a magnetic rack. The samples were separated electrophoretically in SDS-10% polyacrylamide gels and immunoblotted with a peptide-purified anti-PaxB or anti-PldB antibody (10, 15).
Chimeras.
Chimeras were created and stained as previously described, with some adjustments (31). Chimeras consisting of 10% HR30 cells and 90% paxB−, pldB OE, or paxB− pldB OE cells were developed on white filter pads with a 0.8-mm pore size (Millipore, Billerica, MA). Chimeric structures were fixed with glutaraldehyde solution (0.1% glutaraldehyde, 0.1% Triton X-100 in Z buffer [60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4]), washed twice for 10 min in Z buffer, and then incubated in staining solution {5 mM K3[Fe(CN)6], 0.4 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and 0.5% Tween 20 in Z buffer} at 37°C overnight. Images were taken with a dissecting microscope equipped with a ×4.5 objective, utilizing the Spot Advanced program with a Spot Insight 3.2.0 color camera (Diagnostics Instruments).
Chemotaxis assay.
Under-agarose chemotaxis assays were performed as previously described, with some adjustments (58). For folate chemotaxis assay, vegetative cells were collected and resuspended to 1 × 106 cells/ml. One hundred microliters of cells was used in the assay. For cAMP chemotaxis assay, 1 × 107 cells were starved on filter pads for 6 h prior to the assay. Cells were collected and resuspended in 1 ml PBM. A 100-μl sample of cells was used in the assay. One hour after the cells were placed in the wells, images of the cells migrating under the agarose were taken every 30 s for 20 min on an inverted Nikon TE 200 Eclipse microscope using a Metafluor image system (Molecular Devices, Downingtown, PA) and a ×10 objective. The individual cells were tracked using Image J software. Directionality was defined as the absolute displacement divided by the total path. The chemotaxis index (CI) was defined as the cosine of the angle between the line up the gradient and the line drawn from the cell's start point to its endpoint.
Cell-substrate adhesion assay.
Cell-substrate adhesion assays were performed as described by Chen and Katz (10a), with slight modifications. A suspension of 1 × 106 cells in 1 ml of PBM was placed in a 25-ml glass culture flask and put on a gyratory shaker for 10 min at 120 rpm. The cells were allowed to adhere to the glass substrate for 2 h. The flasks were then gently agitated for 3 min at 60 rpm. The supernatant was collected, and the number of cells present was counted with a hemacytometer.
Actin polymerization assay.
cAMP-induced F-actin formation was measured as described previously, with some adjustments (27). A total of 1 × 107 cells were starved on filter pads for 6 h prior to the assay. Cells were collected and resuspended in 1 ml PBM. A 100-μl sample of cells was used in the assay. The cells were fixed (3.7% formaldehyde solution in PBS) before or after stimulation with cAMP. After staining with 8 U/ml rhodamine-phalloidin (Invitrogen, Eugene, OR) in the dark for 1 h, the fluorescence of individual cells was analyzed by flow cytometry. Cells were resuspended to 1 × 106 cells/ml in cold PBS containing 0.5% bovine serum albumin (BSA) and were passed through a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson, San Jose, CA). Cells were identified based on forward and side scatter, and their fluorescence was measured with an excitation wavelength of 488 nm and an emission wavelength of 572 nm. The mean fluorescence value was determined for at least 10,000 cells, after background autofluorescence of unstained cells was determined and subtracted.
Phagocytosis and exocytosis assays.
Phagocytosis was measured using a modification of a previously described assay (11). Cells grown to 2 × 106 cells/ml were harvested and resuspended in HL5. After 15 min of shaking, 1-μm latex beads conjugated with tetramethyl rhodamine isocyanate (TRITC; Sigma, St. Louis, MO) were added to a final concentration of 100 beads/cell. A total of 2 × 105 cells were collected at specific time points, and 2% formaldehyde was added to stop the uptake. The cells were then washed three times in PBS and lysed in 5 mM glycine-NaOH (pH 8.5) containing 100 mM sucrose and 0.2% Triton X-100. The fluorescence of the lysate was measured in a Typhoon 9410 imager (Amersham Bioscience, Piscataway, NJ), using excitation and emission wavelengths of 532 nm and 610 nm, respectively. Relative fluorescence against time was plotted, and the phagocytosis rate was determined. That of wild-type cells was set as 100%. The exocytosis assay was performed essentially as described previously (3). Cells grown to 2 × 106 cells/ml were harvested, resuspended in HL5 containing FITC-dextran (Sigma) at 2 mg/ml, and allowed to internalize the FITC-dextran for 3 h. The cells were then collected and resuspended in fresh HL5. At various time points, 1 ml of cells was collected and 2% formaldehyde was added to stop the uptake. The cells were then washed once in HL5 and twice in 5 mM glycine-NaOH (pH 8.5) containing 100 mM sucrose. The cells were then lysed in the same buffer containing 0.2% Triton X-100. The fluorescence of the lysate was measured in a Versa Fluor fluorometer (Bio-Rad, Hercules, CA), using excitation and emission wavelengths of 495 nm and 520 nm, respectively. Relative fluorescence against time was plotted, and the exocytosis rate was determined. That of wild-type cells was set as 100%.
Statistical analysis.
Statistical significance of differences was determined by one-way analysis of variance (ANOVA), followed by a t test for pairwise comparisons. P values of <0.05 were considered statistically significant. The absence of a star in figures means that there was no significant difference from the wild type.
RESULTS
PaxB and PldB colocalize with F-actin at the cell-substrate interface.
Paxillin and PLD colocalize in leukocytes (30). To determine whether their orthologs in Dictyostelium discoideum also colocalize, we labeled starved Dictyostelium cells with antibody to PaxB or PldB and with the F-actin stain rhodamine-phalloidin. We found overlapping localization of PaxB with F-actin-rich spots at the cell-substrate interface (Fig. 1 A). In addition, we found colocalization of PldB with F-actin-rich spots at the cell-substrate interface (Fig. 1B). Thus, both PaxB and PldB are found associated with F-actin at the cell-surface interface.
Fig. 1.
Colocalization of PaxB and PldB with F-actin by immunofluorescence analysis. Cells starved for 6 h were double labeled with anti-PaxB (A) or anti-PldB (B) antibody and rhodamine-phalloidin (A and B) to visualize F-actin. Bar, 10 μm.
PaxB and PldB are part of a protein complex.
The common localization of PaxB and PldB with F-actin is consistent with these proteins interacting physically. To investigate this possibility, coimmunoprecipitation assays were performed. Cells were starved for 14 h, and the lysates were immunoprecipitated with antibodies to PaxB and probed for the presence of PldB. As shown in Fig. 2, PldB was detected in wild-type AX2 cells immunoprecipitated with PaxB antibodies, suggesting that these proteins exist in a complex. PldB was not present in paxB− immunoprecipitated samples, demonstrating that coimmunoprecipitation of PldB is dependent upon PaxB. This suggests a physical interaction, either direct or indirect, between PaxB and PldB.
Fig. 2.
PaxB and PldB coimmunoprecipitate. Wild-type (WT) and paxB− cells were lysed, and the samples were immunoprecipitated with anti-PaxB antibodies. The immunoprecipitates were analyzed by Western blotting with anti-PldB and anti-PaxB antibodies.
Overexpression of pldB rescues the developmental block and cell sorting defect of paxB− cells.
Upon starvation, Dictyostelium discoideum cells enter a developmental phase that culminates in the formation of a fruiting body, consisting of dead stalk cells supporting live, viable spore cells. The process begins when starved cells use cAMP to aggregate, forming a mound which eventually undergoes morphogenesis to form the fruiting body. PaxB is required for this process, as paxB− cells are not able to progress fully through development and are arrested at the mound stage (Fig. 3 A) (6). As previously reported, overexpression of pldB prevents cells from aggregating (10). Interestingly, overexpression of pldB in paxB− cells resulted in multicellular development comparable to that seen in wild-type cells (Fig. 3A). Therefore, PaxB and PldB may act in a shared process during development, suggesting a functional interaction between PaxB and PldB.
Fig. 3.
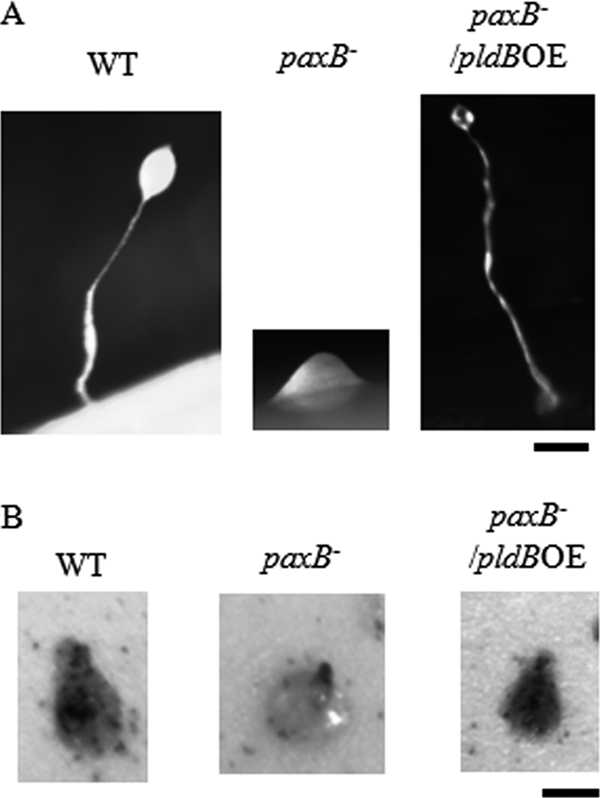
Development and cell sorting of paxB− pldB OE cells is normal. (A) Logarithmically growing wild-type, paxB−, and paxB− pldB OE cells were starved on filters. Pictures of structures were taken after 24 h. Bar, 0.2 mm. (B) Wild-type cells carrying an actin 15-lacZ reporter construct (HR30 cells) were mixed at a 10:90 ratio with wild-type, paxB−, and paxB− pldB OE cells and then starved on filters. Tipped mounds were fixed, stained, and photographed after 16 h. Bar, 0.2 mm.
In addition to their developmental arrest, paxB− cells are also defective in cell sorting, in that when mixed with wild-type cells, they are unable to sort to the tip of the mound (6). This sorting is essential for morphogenesis into the fruiting body. To determine whether the overexpression of pldB rescues this defect in paxB− cells, we examined the localization of HR30 cells (wild-type AX2 cells expressing β-galactosidase) in chimeras with wild-type, paxB−, pldB OE, and paxB− pldB OE cells. As expected, chimeras composed of 90% wild-type cells showed random distribution of the HR30 cells in the tipped mound, demonstrating that expression of β-galactosidase had no effect on cell sorting (Fig. 3B). In chimeras consisting of 90% paxB− cells, HR30 cells were found in the tip of the mound (Fig. 3B), consistent with the defect in cell sorting previously demonstrated for paxB− cells (6). Chimeras with 90% pldB OE cells failed to aggregate. Interestingly, in chimeras with 90% paxB− pldB OE cells, HR30 cells were randomly distributed in the tipped mound, suggesting that the cells carrying the double mutation were able to sort similarly to wild-type cells (Fig. 3B). Thus, much like the case for fruiting body formation, overexpression of pldB rescues the inability of paxB− cells to sort properly in the mound.
The production of PaxB or PldB is independent of that of the other.
The ability of pldB overexpression to rescue the developmental defect observed in paxB− cells could be due to paxB− cells not expressing pldB. To test this possibility, we examined production of PaxB and PldB in wild-type, paxB−, pldB OE, and paxB− pldB OE cells. Western blot analysis confirmed that the levels of PldB in paxB− cells and paxB− pldB OE cells were similar to those in wild-type and pldB OE cells, respectively (Fig. 4). In addition, the levels of PaxB were unchanged in pldB OE cells compared with wild-type cells (Fig. 4). This indicates that loss of paxB does not affect the production of PldB, nor does overexpression of pldB affect the production of PaxB. Thus, the rescue in development is not due to restoration of PldB production and is most likely indicative of a functional interaction between PaxB and PldB.
Fig. 4.
Production of PaxB or PldB is independent of that of the other. Total cell lysates from wild-type, paxB−, pldB OE, and paxB− pldB OE cells were analyzed by Western blotting for the presence of PldB and PaxB. Actin from the same lysates was probed to confirm equal loading amounts.
PaxB and PldB interact functionally during cAMP chemotaxis.
Since cAMP chemotaxis is required for aggregation and tip formation, the developmental block seen in pldB OE and paxB− cells could be due to inefficient chemotactic movement. To examine this possibility, we measured cAMP chemotaxis in wild-type, paxB−, pldB OE, and paxB− pldB OE cells, using an under-agarose assay. In this assay, cells move up a stable chemotactic gradient by crawling underneath a thin layer of agarose. Wild-type cells moved toward the cAMP source with an average speed of 7.9 ± 0.4 μm/min, which is consistent with previous reports (9, 47, 55). The speed of paxB− cells was increased 80% over that of wild-type cells. pldB OE cells had a speed similar to that of wild-type cells. Interestingly, paxB− pldB OE cells also had a speed similar to that of wild-type cells (Fig. 5 A), suggesting that overexpression of pldB rescues the defect in speed in paxB− cells.
Fig. 5.
PaxB and PldB interact functionally during cAMP chemotaxis. The speed (A), directionality (B), and chemotaxis index (C) were determined for cells starved for 6 h in an under-agarose assay. Data presented are means and standard errors of the means (SEM) for at least three independent experiments, with each experiment counting 15 cells. A star indicates a significant difference compared to wild-type cells (P < 0.05).
To analyze the efficiency of chemotaxis, we determined the directionality of cell movement as well as the chemotaxis index. Directionality is a measure of the deviation of cell movement from the straight path and is calculated as the ratio of the distance of a straight line from the cell's start point to its endpoint to the distance of the total path of the cell. Thus, cells that move in a straight line have the maximal directionality value of 1, while cells that make a random walk and end up back at their starting point have a directionality of 0. In accordance with previous work, wild-type cells moved with a directionality of 0.65 ± 0.05 (9, 55). For paxB− cells, the directionality was similar to that of wild-type cells. For pldB OE cells, it was drastically reduced, by 82%, compared to that of wild-type cells. Surprisingly, paxB− pldB OE cells moved with a directionality comparable to that of the wild-type cells (Fig. 5B).
The CI is a measure of how well the cell moves up the gradient and is defined as the cosine of the angle between the line up the gradient and the line drawn from the cell's start point to its endpoint. Thus, cells that move directly toward the cAMP source have a CI of 1. Cells that move perpendicular to the gradient have a CI of 0. Cells that move directly away from the cAMP source have a CI of −1. Wild-type and paxB− cells had similar CIs (0.80 ± 0.05 and 0.90 ± 0.01, respectively). For pldB OE cells, the CI was significantly decreased, to just 20% that of the wild-type cells. paxB− pldB OE cells had a CI comparable to that of wild-type cells (Fig. 5C). Thus, as measured by directionality and the chemotaxis index, overexpression of pldB leads to an impaired efficiency of chemotaxis to cAMP. This is rescued by removal of paxB. Taken together, these data suggest that overexpression of pldB rescues defects caused by loss of paxB and that the loss of paxB rescues defects caused by overexpression of pldB.
To determine whether the observed functional interaction between paxB and pldB is specific to cAMP chemotaxis or is a general, chemotaxis-related occurrence, we assayed folate chemotaxis. Both single mutants as well as the double mutant showed chemotaxis toward folate, with a speed, directionality, and chemotaxis index comparable to those of wild-type cells (data not shown). This indicated that PaxB and PldB do not play roles in chemotaxis toward folate and that the observed functional interaction between paxB and pldB is specific to developing cells responding to cAMP.
PaxB and PldB interact functionally during cell-substrate adhesion.
Since cells lacking paxB have adhesion defects (6), we decided to further explore the relationship between PaxB and PldB by examining the role of these proteins in cell-substrate adhesion. Cells were allowed to settle on a glass surface, which was then gently agitated to remove nonadhered cells. The percentage of cells attached to the surface was calculated as a measure of adhesion. As depicted in Fig. 6, approximately 80% of wild-type AX2 cells adhered to the surface in this assay. Consistent with a previous report (6), paxB− cells were significantly less adhesive than wild-type AX2 cells, with only 50% adhesion. pldB OE cells exhibited cell-substrate adhesion comparable to that of wild-type cells. The overexpression of pldB in cells lacking paxB returned adhesion to the wild-type level, suggesting that PldB overproduction compensates for the loss of PaxB in adhesion. The phenotypes observed in this assay suggest a functional interaction between PaxB and PldB in regulating adhesion.
Fig. 6.
PaxB and PldB interact functionally during cell-substrate adhesion. Wild-type, paxB−, pldB OE, and paxB− pldB OE cells were allowed to adhere to a glass surface for 2 h. After gentle agitation, the number of cells in the supernatant was counted and the percentage of adhered cells calculated. Data presented are means and SEM for at least three independent experiments. The star indicates a significant difference compared to wild-type cells (P < 0.05). paxB− pldB OE cells had slightly higher adhesion than wild-type cells. This difference was not significant (P > 0.05).
PaxB and PldB interact functionally to regulate actin.
Because proper organization of the actin cytoskeleton is necessary for chemotaxis and adhesion, one possible cause for the altered cAMP chemotaxis and adhesion of our mutant cells could be a defective actin cytoskeleton. To examine the distribution of F-actin in starved cells, wild-type and mutant cells were fixed and stained with rhodamine-phalloidin. In wild-type cells, F-actin was localized mainly to broad membrane protrusions and filopodia, as previously reported (Fig. 7). paxB− cells seemed to have impaired cortical staining and increased filopodia. In contrast, pldB OE cells had cortical staining but did not project filopodia at all. paxB− pldB OE cells were able to project filopodia and had normal cortical staining. Thus, while pldB OE cells exhibited an obvious defect in modulating the actin cytoskeleton, disruption of paxB in these cells restored a wild-type phenotype, indicating a functional interaction between PaxB and PldB in actin regulation.
Fig. 7.
Starved paxB− pldB OE cells have normal cell morphology and F-actin staining. Wild-type, paxB−, pldB OE, and paxB− pldB OE cells were starved in submerged cultures, fixed, and stained with rhodamine-phalloidin. Bar, 10 μm.
To further study F-actin regulation, we examined F-actin formation in response to cAMP stimulation. cAMP stimulation causes polymerization and reorganization of actin, which are crucial for the extension of new pseudopods during chemotaxis (27). To examine actin polymerization in response to cAMP, starved wild-type and mutant cells were fixed before or after cAMP stimulation. The amount of polymerized actin was determined by staining with rhodamine-phalloidin and measuring the fluorescence of individual cells by flow cytometry. The increased staining seen in wild-type cells upon cAMP stimulation was defined as 100%. Upon cAMP stimulation, paxB− cells exhibited slightly less stimulation than that seen in wild-type cells. This difference was not statistically significant (P > 0.05), but pldB OE cells showed drastically reduced stimulation compared to that seen in wild-type cells. The level of stimulation in the double mutant was similar to that seen in paxB− cells (P > 0.05) (Fig. 8). These data suggest that a lack of paxB does not seem to have a significant effect on actin polymerization in response to cAMP, while overexpression of pldB impairs the process. In addition, loss of paxB is able to rescue the defect in cAMP-induced F-actin formation seen in pldB OE cells, implying a functional interaction between PaxB and PldB in actin regulation.
Fig. 8.
PaxB and PldB interact functionally to regulate cAMP-induced actin polymerization. Wild-type, paxB−, pldB OE, and paxB− pldB OE cells starved for 6 h were fixed and stained with rhodamine-phalloidin before or after cAMP stimulation. Fluorescence was measured by flow cytometry. The data presented are means and SEM for at least three independent experiments, with each experiment counting 10,000 cells. The star indicates a significant difference compared to wild-type cells (P < 0.05).
PldB positively regulates PaxB function during phagocytosis and exocytosis.
Since our experiments suggested that PaxB and PldB interact functionally to regulate proper F-actin localization and actin polymerization, we wanted to determine whether this interaction also exists in two other actin-based processes, namely, phagocytosis and exocytosis. Phagocytosis was measured by monitoring the uptake of 1-μm fluorescent latex beads over 30 min. The rate of uptake was calculated, and that of wild-type cells was set at 100%. As shown in Fig. 9, the rate of phagocytosis for paxB− cells was 6 times higher than that for wild-type cells. pldB OE cells exhibited a phagocytosis rate similar to that of wild-type cells. The phagocytosis rate of paxB− pldB OE cells was similar to that seen for paxB− cells (6 times higher than that of wild-type cells). Thus, while PaxB negatively regulates phagocytosis, overexpression of pldB does not affect this process. In addition, the paxB− phenotype was able to override the pldB OE phenotype in the double mutant during phagocytosis.
Fig. 9.
paxB− pldB OE cells have a restored paxB− phenotype during phagocytosis and exocytosis. The rate of uptake of 1-μm fluorescent latex beads was determined for wild-type, paxB−, pldB OE, and paxB− pldB OE cells. That of wild-type cells was set at 100%. The rate of release of FITC-dextran was determined for wild-type, paxB−, pldB OE, and paxB− pldB OE cells. That of wild-type cells was set at 100%. The data presented are means and SEM for at least three independent experiments. A star indicates a significant difference compared to wild-type cells (P < 0.05).
Exocytosis was examined by loading cells with FITC-dextran and then observing its release over the course of 60 min. The rate of decreased fluorescence was calculated, and that of wild-type cells was set at 100%. paxB− cells displayed an impaired exocytosis rate compared to that of wild-type cells, whereas pldB OE cells had an increased exocytosis rate. The rate of exocytosis for paxB− pldB OE cells was similar to that for paxB− cells (P > 0.05) (Fig. 9). This suggests that PaxB and PldB are positive regulators of exocytosis and that these proteins interact functionally to regulate this process. Consistent with the phagocytosis results, the paxB− phenotype was able to override the pldB OE phenotype in the double mutant during exocytosis.
DISCUSSION
Previous studies with mammalian cell lines implied that paxillin and PLD associate functionally with the actin cytoskeleton (30, 54). Both paxillin and PLD are critical for efficient cellular migration, requiring the coordination of membrane trafficking and the reorganization of the actin cytoskeleton (20, 43), suggesting a possible interaction. While PLD has not been shown to bind paxillin, PLD activity has been associated with cell membrane fractions containing paxillin, and PLD1 partially colocalizes with paxillin at the cell-substrate interface in human macrophages, opening the possibility that these proteins do form a complex (30). Given the role of paxillin as an adapter molecule that provides a platform for an array of signaling and structural proteins (51), it is quite possible that PLD binds paxillin either directly or indirectly. However, the molecular mechanisms by which such paxillin-containing protein complexes function remain to be elucidated fully.
In this study, we provide evidence that much like the case in mammalian cells, paxillin and PLD regulate the actin cytoskeleton in Dictyostelium discoideum. We show here that loss of PaxB (paxillin) leads to defects in cAMP chemotaxis, adhesion, F-actin localization, phagocytosis, and exocytosis. We also demonstrate that overproduction of PldB (PLD) disrupts proper cAMP chemotaxis, F-actin formation and localization, and exocytosis. Even more interesting is the fact that the results from several experiments support the possibility that the two proteins function together to regulate the actin cytoskeleton. Previously reported protein expression profiles for paxB and pldB showed that the two proteins encoded by these genes are expressed during the same period of development and in the same cell type (6, 10, 40). In addition, we found that both proteins localize to F-actin-rich areas within the cell. More telling, however, is our discovery that PaxB and PldB can be coimmunoprecipitated. Thus, PaxB and PldB are present at the same time, in the same cells, and in the same locations and can form a complex, all of which are consistent with the two proteins working together.
Evidence that the two proteins function in the same pathways was found by examining the phenotypes of a paxB− pldB OE double mutant. Classical epistasis analysis can be used to determine whether two gene products function in the same pathway and, if so, their order of action. If the phenotypes associated with two mutations are different, then the phenotype of the double mutant dictates which gene product acts downstream of the other. For example, cells lacking paxB have decreased adhesion. Cells overexpressing pldB have normal adhesion. Double mutants which lack paxB and overexpress pldB have normal adhesion, just like pldB-overexpressing cells. Epistasis analysis indicates that since the pldB-overexpressing cell phenotype is dominant, PldB must act downstream of PaxB. According to this logic, PldB acts downstream of PaxB in regulating the speed of chemotaxis to cAMP and adhesion. In contrast, PldB acts upstream of PaxB in controlling directionality, the chemotactic index, cAMP-induced F-actin polymerization, phagocytosis, and exocytosis. Simple epistasis cannot explain the fact that the order of action of PaxB and PldB varies depending on the process measured. Thus, PaxB and PldB cannot be in a simple pathway where one protein activates the other.
To explain how PaxB and PldB work together to regulate cytoskeletal functions, both their physical and functional interactions must be taken into account. Our observations provide evidence in favor of the hypothesis that PaxB and PldB form a complex. PLD catalyzes the hydrolysis of phosphatidylcholine, producing PA, a second messenger (18). PldB uses PaxB as an adaptor to be recruited to specific subcellular locations, where production of PA can facilitate proper actin responses to environmental changes. This could trigger recruitment of other proteins, such as Phg2 and SadA, by PaxB at focal sites, which in turn controls adhesion, motility, and actin cytoskeleton organization (19, 22). In paxB− cells, PldB is unable to localize properly, leading to improper actin reorganization. In pldB OE cells, an inordinate amount of PldB is recruited to sites of action by PaxB, leading to hyperactive signaling and, therefore, to cytoskeletal defects. These phenotypes are ameliorated in the paxB− pldB OE double mutant because even though there is an abundance of PldB, there is no PaxB to efficiently localize it. A relatively small percentage of the overproduced PldB is able to localize to its sites of action, allowing it to perform its signaling function and to regulate the actin cytoskeleton.
From this point of view, the cAMP chemotaxis results can be explained easily. Efficient chemotaxis requires PaxB to recruit PldB and other proteins into a complex at sites of action at the plasma membrane and the cell-substrate interface. Without PaxB, these complexes cannot be formed. When pldB is overexpressed, recruitment of these protein complexes by PaxB is increased, to the point that they are mislocalized throughout the cell, preventing pseudopod extension and disrupting directed migration. In the absence of PaxB, PldB is not recruited efficiently, such that even when it is overexpressed, only a small portion is localized properly. This allows a rescued efficiency of cAMP chemotaxis and hence the full development of paxB− pldB OE cells. Interestingly, the role of PaxB and PldB in chemotaxis appears to be specific to cAMP, as all mutants were able to exhibit chemotaxis toward folate with an efficiency comparable to that of wild-type cells. Thus, the functional interaction between PaxB and PldB is specific to cAMP chemotaxis and does not occur in vegetative cells.
The rescued chemotaxis can also explain the developmental phenotypes exhibited by the mutants. Previously reported expression profiles for paxB and pldB showed that both protein products reached their peak production during mound and slug formation, suggesting their importance at these stages. In accordance with this interpretation, pldB OE cells cannot form mounds, and paxB− strains are not able to fully develop, arresting at the mound stage (Fig. 3A) (6). In response to cAMP, pldB OE cells have a low level of directionality and a low chemotactic index, explaining their inability to form mounds. paxB− cells have normal directionality and a normal chemotactic index, and thus they can form mounds. However, once in the mound, they cannot form tips (6). In fact, we found that in chimeric mounds with wild-type cells, the paxB− cells were excluded specifically from the tip. During Dictyostelium development, the tip forms by the sorting of prestalk cells toward the top of the mound (56, 57). The tip seems to direct all subsequent morphogenesis, exhibiting the properties of an organizer. Thus, the paxB− arrest at the mound stage is most likely due to an inability to form a tip. The details of the sorting mechanism are still unknown but may involve cell type-specific differences in cytoskeletal function (12, 42), adhesion (45), or chemotaxis to cAMP signals (16, 35). Interestingly, paxB− cells had increased chemotactic speed, which could explain why they were not able to form a tip. In contrast to the pldB OE and paxB− cells, the paxB− pldB OE double mutant cells had a normal speed, directionality, and chemotactic index. They were thus able to form mounds, to sort properly to form a tip, and eventually to form a fruiting body.
In addition to its role in chemotaxis, actin cytoskeleton remodeling provides the forces required for a variety of other cellular processes, such as adhesion, motility, phagocytosis, and endocytosis. These processes compete for coronin and other actin-binding proteins (34). Thus, the improved phagocytosis seen in the paxB− cells and paxB− pldB OE cells comes at the expense of exocytosis. On the other hand, the improved exocytosis exhibited by pldB OE cells prevents filopodium projection and proper responses to cAMP stimulation (seen as inefficient chemotaxis and actin polymerization).
Using Dictyostelium discoideum, we have described for the first time a functional and physical interaction between paxillin and PLD. We provide evidence that this interaction regulates multicellular development, cAMP chemotaxis, cell-substrate adhesion, actin distribution and polymerization, phagocytosis, and exocytosis. We hypothesize that PaxB controls PldB action by shuttling it to specific cellular locations. This is not to say that PaxB is the only protein regulating PldB. In mammals, PLD activity is regulated by protein kinases, Arf, and Rho family GTPases (18). The Dictyostelium genome encodes several of these proteins, some of which regulate the cytoskeleton (8). While their modes of action are still unknown, some of them may function through regulating PldB activity. Given that Dictyostelium discoideum has proven to be an excellent cellular model for studying the cytoskeleton and its regulation, it can be used to uncover other interactions involved in signaling to the cytoskeleton.
ACKNOWLEDGMENTS
This work was supported by NIGMS grant S06-GM606564 from the National Institutes of Health and by grant 0346975 from the National Science Foundation. The infrastructure and instrumentation of the Biological Sciences Department at Hunter College are supported by Research Centers in Minority Institutions award RR-03037 from the National Center for Research Resources of the National Institutes of Health.
Footnotes
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Amagasaki K., Kaneto H., Heldin C. H., Lennartsson J. 2006. c-Jun N-terminal kinase is necessary for platelet-derived growth factor-mediated chemotaxis in primary fibroblasts. J. Biol. Chem. 281:22173–22179 [DOI] [PubMed] [Google Scholar]
- 2. Bishop J. D., et al. 2002. A second UDP-glucose pyrophosphorylase is required for differentiation and development in Dictyostelium discoideum. J. Biol. Chem. 277:32430–32437 [DOI] [PubMed] [Google Scholar]
- 3. Brazill D. T., Meyer L. R., Hatton R. D., Brock D. A., Gomer R. H. 2001. ABC transporters required for endocytosis and endosomal pH regulation in Dictyostelium. J. Cell Sci. 114:3923–3932 [DOI] [PubMed] [Google Scholar]
- 4. Brown M. C., Turner C. E. 2004. Paxillin: adapting to change. Physiol. Rev. 84:1315–1339 [DOI] [PubMed] [Google Scholar]
- 5. Brown M. C., Turner C. E. 2002. Roles for the tubulin- and PTP-PEST-binding paxillin LIM domains in cell adhesion and motility. Int. J. Biochem. Cell Biol. 34:855–863 [DOI] [PubMed] [Google Scholar]
- 6. Bukharova T., et al. 2005. Paxillin is required for cell-substrate adhesion, cell sorting and slug migration during Dictyostelium development. J. Cell Sci. 118:4295–4310 [DOI] [PubMed] [Google Scholar]
- 7. Cardelli J. 2001. Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic 2:311–320 [DOI] [PubMed] [Google Scholar]
- 8. Chen P. W., Randazzo P. A., Parent C. A. 2010. ACAP-A/B are ArfGAP homologs in Dictyostelium involved in sporulation but not in chemotaxis. PLoS One 5:e8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen S., Segall J. E. 2006. EppA, a putative substrate of DdERK2, regulates cyclic AMP relay and chemotaxis in Dictyostelium discoideum. Eukaryot. Cell 5:1136–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y., Rodrick V., Yan Y., Brazill D. 2005. PldB, a putative phospholipase D homologue in Dictyostelium discoideum, mediates quorum sensing during development. Eukaryot. Cell 4:694–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a. Chen C. F., Katz E. R. 2000. Mediation of cell-substratum adhesion by RasG in Dictyostelium. J. Cell Biochem. 79:139–149 [PubMed] [Google Scholar]
- 11. Cornillon S., et al. 2002. Two members of the beige/CHS (BEACH) family are involved at different stages in the organization of the endocytic pathway in Dictyostelium. J. Cell Sci. 115:737–744 [DOI] [PubMed] [Google Scholar]
- 12. De Lozanne A., Spudich J. A. 1987. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236:1086–1091 [DOI] [PubMed] [Google Scholar]
- 13. Devreotes P. N., Zigmond S. H. 1988. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 4:649–686 [DOI] [PubMed] [Google Scholar]
- 14. Dormann D., Vasiev B., Weijer C. J. 2000. The control of chemotactic cell movement during Dictyostelium morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duran M. B., Rahman A., Colten M., Brazill D. 2009. Dictyostelium discoideum paxillin regulates actin-based processes. Protist 160:221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Early A., Abe T., Williams J. 1995. Evidence for positional differentiation of prestalk cells and for a morphogenetic gradient in Dictyostelium. Cell 83:91–99 [DOI] [PubMed] [Google Scholar]
- 17. Ennis H. L., Dao D. N., Wu M. Y., Kessin R. H. 2003. Mutation of the Dictyostelium fbxA gene affects cell-fate decisions and spatial patterning. Protist 154:419–429 [DOI] [PubMed] [Google Scholar]
- 18. Exton J. H. 2002. Regulation of phospholipase D. FEBS Lett. 531:58–61 [DOI] [PubMed] [Google Scholar]
- 19. Fey P., Stephens S., Titus M. A., Chisholm R. L. 2002. SadA, a novel adhesion receptor in Dictyostelium. J. Cell Biol. 159:1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foster D. A., Xu L. 2003. Phospholipase D in cell proliferation and cancer. Mol. Cancer Res. 1:789–800 [PubMed] [Google Scholar]
- 21. Garton A. J., Flint A. J., Tonks N. K. 1996. Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol. Cell. Biol. 16:6408–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gebbie L., et al. 2004. Phg2, a kinase involved in adhesion and focal site modeling in Dictyostelium. Mol. Biol. Cell 15:3915–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberg S. 1995. Signal transduction of phagocytosis. Trends Cell Biol. 5:93–99 [DOI] [PubMed] [Google Scholar]
- 24. Greenberg S., Chang P., Silverstein S. C. 1994. Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 269:3897–3902 [PubMed] [Google Scholar]
- 25. Hacker U., Albrecht R., Maniak M. 1997. Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 110:105–112 [DOI] [PubMed] [Google Scholar]
- 26. Hagel M., et al. 2002. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22:901–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hall A. L., Schlein A., Condeelis J. 1988. Relationship of pseudopod extension to chemotactic hormone-induced actin polymerization in amoeboid cells. J. Cell. Biochem. 37:285–299 [DOI] [PubMed] [Google Scholar]
- 28. Heikoop J. C., et al. 1998. Expression of a bioactive, single-chain choriogonadotropin in Dictyostelium discoideum. Eur. J. Biochem. 256:359–363 [DOI] [PubMed] [Google Scholar]
- 29. Howard P. K., Ahern K. G., Firtel R. A. 1988. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 16:2613–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iyer S. S., Kusner D. J. 1999. Association of phospholipase D activity with the detergent-insoluble cytoskeleton of U937 promonocytic leukocytes. J. Biol. Chem. 274:2350–2359 [DOI] [PubMed] [Google Scholar]
- 31. Jermyn K. A., Williams J. G. 1991. An analysis of culmination in Dictyostelium using prestalk and stalk-specific cell autonomous markers. Development 111:779–787 [DOI] [PubMed] [Google Scholar]
- 32. Konzok A., et al. 1999. DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis, and motility. J. Cell Biol. 146:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maniak M. 2001. Fluid-phase uptake and transit in axenic Dictyostelium cells. Biochim. Biophys. Acta 1525:197–204 [DOI] [PubMed] [Google Scholar]
- 34. Maniak M., Rauchenberger R., Albrecht R., Murphy J., Gerisch G. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein tag. Cell 83:915–924 [DOI] [PubMed] [Google Scholar]
- 35. Matsukuma S., Durston A. J. 1979. Chemotactic cell sorting in Dictyostelium discoideum. J. Embryol. Exp. Morphol. 50:243–251 [PubMed] [Google Scholar]
- 36. Mendoza M. C., Booth E. O., Shaulsky G., Firtel R. A. 2007. MEK1 and protein phosphatase 4 coordinate Dictyostelium development and chemotaxis. Mol. Cell. Biol. 27:3817–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagasaki A., Uyeda T. Q. 2008. Screening of genes involved in cell migration in Dictyostelium. Exp. Cell Res. 314:1136–1146 [DOI] [PubMed] [Google Scholar]
- 38. Nikolopoulos S. N., Turner C. E. 2001. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J. Biol. Chem. 276:23499–23505 [DOI] [PubMed] [Google Scholar]
- 39. Nikolopoulos S. N., Turner C. E. 2002. Molecular dissection of actopaxin-integrin-linked kinase-paxillin interactions and their role in subcellular localization. J. Biol. Chem. 277:1568–1575 [DOI] [PubMed] [Google Scholar]
- 40. Ohnishi H., et al. 2001. A src family tyrosine kinase inhibits neurotransmitter release from neuronal cells. Proc. Natl. Acad. Sci. U. S. A. 98:10930–10935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powner D. J., Pettitt T. R., Anderson R., Nash G. B., Wakelam M. J. 2007. Stable adhesion and migration of human neutrophils requires phospholipase D-mediated activation of the integrin CD11b/CD18. Mol. Immunol. 44:3211–3221 [DOI] [PubMed] [Google Scholar]
- 42. Rivero F., et al. 1996. The role of the cortical cytoskeleton: F-actin crosslinking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J. Cell Sci. 109:2679–2691 [DOI] [PubMed] [Google Scholar]
- 43. Schaller M. D. 2001. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459–6472 [DOI] [PubMed] [Google Scholar]
- 44. Seastone D. J., et al. 2001. The WASp-like protein scar regulates macropinocytosis, phagocytosis and endosomal membrane flow in Dictyostelium. J. Cell Sci. 114:2673–2683 [DOI] [PubMed] [Google Scholar]
- 45. Siu C. H., Kamboj R. K. 1990. Cell-cell adhesion and morphogenesis in Dictyostelium discoideum. Dev. Genet. 11:377–387 [DOI] [PubMed] [Google Scholar]
- 46. Strmecki L., Greene D. M., Pears C. J. 2005. Developmental decisions in Dictyostelium discoideum. Dev. Biol. 284:25–36 [DOI] [PubMed] [Google Scholar]
- 47. Sun B., Ma H., Firtel R. A. 2003. Dictyostelium stress-activated protein kinase alpha, a novel stress-activated mitogen-activated protein kinase kinase kinase-like kinase, is important for the proper regulation of the cytoskeleton. Mol. Biol. Cell 14:4526–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sussman M. 1987. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28:9–29 [DOI] [PubMed] [Google Scholar]
- 49. Thomas S. M., Hagel M., Turner C. E. 1999. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 112:181–190 [DOI] [PubMed] [Google Scholar]
- 50. Tsujioka M., et al. 2008. Overlapping functions of the two talin homologues in Dictyostelium. Eukaryot. Cell 7:906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turner C. E. 2000. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2:E231–E236 [DOI] [PubMed] [Google Scholar]
- 52. Turner C. E. 2000. Paxillin interactions. J. Cell Sci. 113:4139–4140 [DOI] [PubMed] [Google Scholar]
- 53. Turner C. E. 1994. Paxillin: a cytoskeletal target for tyrosine kinases. Bioessays 16:47–52 [DOI] [PubMed] [Google Scholar]
- 54. Turner C. E., West K. A., Brown M. C. 2001. Paxillin-ARF GAP signaling and the cytoskeleton. Curr. Opin. Cell Biol. 13:593–599 [DOI] [PubMed] [Google Scholar]
- 55. Veltman D. M., Keizer-Gunnik I., Van Haastert P. J. 2008. Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J. Cell Biol. 180:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams J. 1995. Morphogenesis in Dictyostelium: new twists to a not-so-old tale. Curr. Opin. Genet. Dev. 5:426–431 [DOI] [PubMed] [Google Scholar]
- 57. Williams J., Morrison A. 1994. Prestalk cell-differentiation and movement during the morphogenesis of Dictyostelium discoideum. Prog. Nucleic Acid Res. Mol. Biol. 47:1–27 [DOI] [PubMed] [Google Scholar]
- 58. Woznica D., Knecht D. A. 2006. Under-agarose chemotaxis of Dictyostelium discoideum. Methods Mol. Biol. 346:311–325 [DOI] [PubMed] [Google Scholar]
- 59. Yano H., et al. 2000. Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 97:9076–9081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zigmond S. H. 1996. Signal transduction and actin filament organization. Curr. Opin. Cell Biol. 8:66–73 [DOI] [PubMed] [Google Scholar]
- 61. Zouwail S., et al. 2005. Phospholipase D activity is essential for actin localization and actin-based motility in Dictyostelium. Biochem. J. 389:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]










