Abstract
Very little is known about rhizobia that form nodules on Thermopsis spp. We report the isolation of a Mesorhizobium huakuii strain with a unique nodA gene that form nodules on Thermopsis lupinoides in Kamtchatka, Russia. The isolate did not form nodules on Thermopsis chinensis or Thermopsis caroliniana, which suggests it may be host specific.
TEXT
Biological nitrogen fixation through the symbiotic interaction between leguminous plants and soil bacteria collectively called rhizobia is very important for sustainable plant production in natural ecosystems and in agriculture. Consequently, there has been an increase in research toward rhizobia nodulating legumes worldwide in order to improve upon the benefits associated with this interaction. Despite these efforts, relatively little is known about rhizobial populations associated with some native legumes in northern temperate regions. The legume genus Thermopsis which is found exclusively in the Northern hemisphere comprises about 23 species, eight of which are reported to form nodules with α-rhizobia (14). The root nodule bacteria of Thermopsis appear to have received relatively little research attention. Available information suggests that species within this genus may form nodules with rhizobia ascribed to the genera Rhizobium and/or Agrobacterium (8, 16). In Kamtchatka, Russia, Thermopsis is represented by Thermopsis lupinoides (L.) Link. This species has an Asiatic Pacific distribution and is considered to occur on seashores, but in Kamtchatka, it also occurs inland (9). We had the unique opportunity to study rhizobia nodulating T. lupinoides growing naturally in this region. Nodules from four well-spaced and randomly selected T. lupinoides plants in full flower (Fig. 1, left panel) were sampled on July 2005 on a gravel roadside near Utka, southwestern Kamtchatka (53°14.9′N, 156°50.6′E) in Russia. The plants, which were collected within a stretch of about 50 m, were sampled by digging clumps of soil down to 10 to 15 cm. Roots were carefully released from the soil, and randomly selected nodules were detached and preserved by drying over silica gels until root nodule bacteria were isolated (13). A total of 20 isolates (TM1 to TM20), each from an individual nodule, which were representative of the sampled plants were obtained using the procedure described by Ampomah and Huss-Danell (2). All the isolates formed colonies within 3 or 4 days after streaking on yeast extract-mannitol agar medium kept in a 28°C growth chamber. Pure cultures of isolates were stored at −80°C.
Fig. 1.
Thermopsis lupinoides at collection site (left; photo by Kerstin Huss-Danell), roots with nodules formed with TM1 in the greenhouse (top right) and the interior of the nodules (bottom right panel) (both panels on right courtesy of Kjell Olofsson).
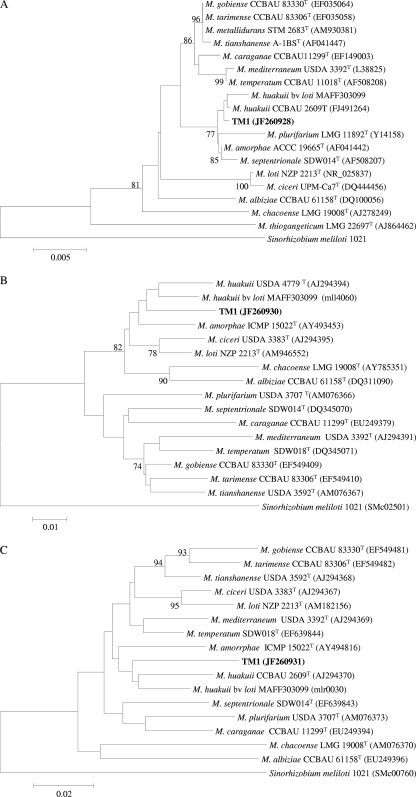
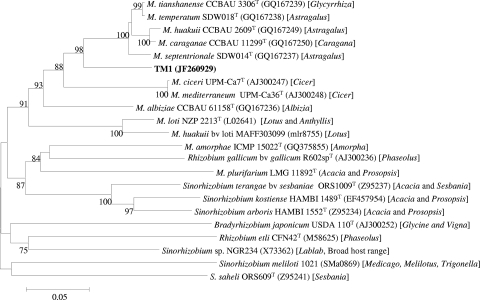
We studied the genetic diversity among the 20 isolates at the chromosomal and nodulation gene level by amplifying and comparing sequence information from the housekeeping genes atpD and recA and the nodA gene, respectively. DNA for PCR was obtained by using the method described by Rodriguez-Echeverria and associates (12). Partial atpD and recA sequences were amplified with the primer pairs atpD255F/atpD782R (at the end of the primer designations, F stands for forward and R stands for reverse) (17) and recA6F/recA504R (5), respectively, with PCR cycling conditions similar for amplifying the intergenic spacer region of the rRNA of rhizobia (11). A partial nodA gene was also amplified with the primer pair nodA-1 and nodA-2 with procedures described by Haukka and associates (7). Aliquots (10 μl) of amplified products examined by electrophoresis in 1% agarose gel stained with SYBR safe (Invitrogen) showed that the products obtained were of the expected size. The remnants of the PCR products were purified using the NucleoSpin extract II column (Machery-Nagel, Duren, Germany) according to the manufacturer's recommendations and sequenced at Macrogen Inc., Seoul, South Korea. Nucleotide alignments of the sequenced atpD, recA, and nodA genes for all the isolates that were constructed with the ClustalW 1.83 program imported into Bioedit 4.8.4 (6) revealed that the isolates were identical at all the studied loci. Enterobacterial repetitive intergenic consensus sequences (ERIC) PCR (4) run for all the isolates showed similar profiles for the isolates (data not shown), which further confirmed that indeed one strain was present in all 20 nodules studied. The lack of diversity in occupants of our sampled nodules obtained from different plants well separated from each other was quite a surprise, because it is very common to find some diversity among nodule bacteria within a site. Some of the possible reasons for our results could be that the strain obtained in our work may be the most dominant strain in that site or may have spread along the roadside in connection with maintenance of the road as the plants grew along the road or this strain may be very competitive for nodulation. BLASTN searches (1) indicated that atpD, recA, and nodA sequences of the Thermopsis lupinoides bacteria matched reference strains in the genus Mesorhizobium. However, at this time, the phylogeny of bacteria, including rhizobia, to a large extent is influenced by the 16S rRNA sequence information (10). Because our isolates were practically one strain, we arbitrarily selected an isolate (TM1) and obtained a nearly full-length 16S rRNA sequence (18). Phylogenetic analysis of type strains of Mesorhizobium species indicated that TM1 grouped with Mesorhizobium huakuii (Fig. 2). Further inclusion of some previously described M. huakuii strains (3) with TM1 in a 16S rRNA and recA tree confirmed the affiliation of TM1 to M. huakuii (information available from authors upon request). To our knowledge, this is the first report of an M. huakuii strain nodulating Thermopsis lupinoides, as M. huakuii is commonly associated with Acacia and Astragalus (14). Even though the nodA tree showed that TM1 possessed nodA, which was typical of the genus Mesorhizobium, it branched well from the other type strains, which also suggested that it was a unique type (Fig. 3).
Fig. 2.
Neighbor-joining phylogenetic tree showing the relationship between TM1 and the different Mesorhizobium type strains based on the 1,309-nucleotide sequence of the 16S rRNA (A), 399-nucleotide sequence of the atpD gene (B), and 401-nucleotide sequence of the recA gene (C). The tree was constructed using MEGA4 software (15). The numbers at branch points are the significant bootstrap values (expressed as a percentage based on 1,000 replicates; only values that are >70% are shown). The horizontal branch lines are proportional and indicate p-distances. The scale bar in each panel represents the number of nucleotide substitutions per 100 nucleotides. bv, biovar.
Fig. 3.
Neighbor-joining phylogenetic tree based on nodA sequences (539 nucleotides [nt]), showing the relationship between TM1, the different Mesorhizobium type strains, and some other type strains of rhizobia. The legume genus (or genera) in brackets is the common host(s) associated with that rhizobial type strain. The tree was constructed using MEGA4 software (15). The numbers at branch points are the significant bootstrap values (expressed as a percentage based on 1,000 replicates; only values that are >70% are shown). The horizontal branch lines are proportional and indicate p-distances. The scale bar represents the number of nucleotide substitutions per 100 nucleotides.
We examined whether nodulation of TM1 was specific to Thermopsis lupinoides or extends to other hosts. Therefore, besides Thermopsis lupinoides (seeds from Kamtchatka, Russia), we also included Thermopsis chinensis S. Moore and Thermopsis caroliniana M. A. Curtis (seeds from Chiltern Seeds, United Kingdom), originating in China and North America, respectively, which are known to form nodules, as well as Lotus corniculatus L. (seeds from Sweden), in a nodulation test performed on seedlings raised from surface sterile seeds established in sterile growth pouches (Mega International, St. Louis Park, MN) with N-free solution in a greenhouse. We included the model L. corniculatus symbiont M. huakuii MAFF303099 in our tests, because in addition to its closer relation to TM1, it also served as a positive control for nodulation of L. corniculatus. TM1 formed pink nodules only on T. lupinoides (Fig. 1, right panel), not on the other tested hosts, an indication that it has a narrow host range. The inability of TM1 to form nodules on the two other tested Thermopsis species suggests that its nodA gene may be unique and strengthens the inferences from the nodA phylogenetic tree. M. huakuii MAFF303099 formed nodules only on L. corniculatus.
In conclusion, we have demonstrated that T. lupinoides forms nodules only with a M. huakuii strain with a unique nodA gene which may be host specific.
Nucleotide sequence accession numbers.
The nucleotide sequences of TM1 have been deposited in the GenBank database under the following accession numbers: JF260928 for the 16S rRNA fragment, JF260929 for the nodA gene, JF260930 for the atpD gene, and JF260931 for the recA gene.
Acknowledgments
This work was supported financially by The Swedish Research Council FORMAS (to K.H.-D.). The Swedish Polar Research Secretariat supported the field work by K.H.-D. in Kamtchatka, Russia, during Beringia 2005, a part of the research program SWEDARCTIC.
We thank Kjell Olofsson for the nodule photos in Fig. 1.
Footnotes
Published ahead of print on 7 June 2011.
REFERENCES
- 1. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. 1990. Basic local alignment research tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Ampomah O. Y., Huss-Danell K. 2011. Genetic diversity of root nodule bacteria nodulating Lotus corniculatus and Anthyllis vulneraria in Sweden. Syst. Appl. Microbiol. 34:267–275 [DOI] [PubMed] [Google Scholar]
- 3. Chen W. F., et al. 2008. Different Mesorhizobium species associated with Caragana carry similar symbiotic genes and have common host ranges. FEMS Microbiol. Lett. 283:203–209 [DOI] [PubMed] [Google Scholar]
- 4. de Bruijn F. J. 1992. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergenic consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl. Environ. Microbiol. 58:2180–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaunt M. W., Turner S. L., Rigottier-Gois L., Lloyd-Macgilp S. A., Young J. P. W. 2001. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Evol. Microbiol. 51:2037–2048 [DOI] [PubMed] [Google Scholar]
- 6. Hall T. A. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 7. Haukka K., Lindström K., Young J. P. W. 1998. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl. Environ. Microbiol. 64:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hou B. C., et al. 2009. Rhizobial resources associated with epidemic legumes in Tibet. Microb. Ecol. 57:69–81 [DOI] [PubMed] [Google Scholar]
- 9. Hultén E. 1929. Flora of Kamtchatka and the adjacent islands III. Dicotyledoneae: Droseraceae - Cornaceae. Kungl. Sven. Vetenskapsakademiens Handlingar 8:93–95 [Google Scholar]
- 10. Kuykendall L. D. 2005. Family I. Rhizobiaceae, p. 324–361 In Brenner D. J., Krieg N. R., Staley J. T., Garrity G. M. (ed.), Bergey's manual of systematic bacteriology. Springer, New York, NY [Google Scholar]
- 11. Laguerre G., et al. 1996. Typing of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: application to Rhizobium leguminosarum and its different biovars. Appl. Environ. Microbiol. 62:2029–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez-Echeverria S., Perez-Fernandez M. A., Vlaar S., Finan T. 2003. Analysis of the legume-rhizobia symbiosis in shrubs from central western Spain. J. Appl. Microbiol. 95:1367–1374 [DOI] [PubMed] [Google Scholar]
- 13. Somasegaran P., Hoben H. J. 1994. Handbook for Rhizobia. Methods in legume-Rhizobium technology. Springer-Verlag, New York, NY [Google Scholar]
- 14. Sprent J. I. 2009. Legume nodulation: a global perspective. Wiley-Blackwell, Oxford, United Kingdom [Google Scholar]
- 15. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 16. Thórsson ÆT, Sverrisson H., Anamthawat-Jónsson K. 2000. Genotyping Icelandic isolates of rhizobia based on rDNA-RFLP. Icel. Agric. Sci. 13:17–25 [Google Scholar]
- 17. Vinuesa P., Silva C., Werner D., Martinez-Romero E. 2005. Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol. Phylogenet. Evol. 34:29–54 [DOI] [PubMed] [Google Scholar]
- 18. Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]