Abstract
The scarcity of fresh water in the Mediterranean region necessitates the search for halotolerant agents of biological control of plant diseases that can be applied in arid-zone agriculture irrigated with saline water. Among 29 Trichoderma strains previously isolated from Mediterranean Psammocinia sp. sponges, the greatest number of isolates belong to the Trichoderma longibrachiatum-Hypocrea orientalis species pair (9), H. atroviridis/T. atroviride (9), and T. harzianum species complex (7), all of which are known for high mycoparasitic potential. In addition, one isolate of T. asperelloides and two putative new species, Trichoderma sp. O.Y. 14707 and O.Y. 2407, from Longibrachiatum and Strictipilosa clades, respectively, have been identified. In vitro salinity assays showed that the ability to tolerate increasing osmotic pressure (halotolerance) is a strain- or clade-specific property rather than a feature of a species. Only a few isolates were found to be sensitive to increased salinity, while others either were halotolerant or even demonstrated improved growth in increasingly saline conditions. In vitro antibiosis assays revealed strong antagonistic activity toward phytopathogens due to the production of both soluble and volatile metabolites. Two marine-derived Trichoderma isolates, identified as T. atroviride and T. asperelloides, respectively, effectively reduced Rhizoctonia solani damping-off disease on beans and also induced defense responses in cucumber seedlings against Pseudomonas syringae pv. lachrimans. This is the first inclusive evaluation of marine fungi as potential biocontrol agents.
INTRODUCTION
Plant diseases play a significant role in the destruction of agricultural resources. In particular, soilborne pathogens cause important losses, with fungi being among the most aggressive. Biological control, the use of specific organisms that interfere with plant pathogens and pests, is a nature-friendly ecological approach to overcome problems caused by standard chemical methods of plant protection. Trichoderma spp. are opportunistic fungi found in a large variety of ecosystems. Some strains have the ability to reduce the severity of plant diseases by inhibiting plant pathogens in the soil or on plant roots through their high antagonistic and mycoparasitic potential (47). Trichoderma spp. produce numerous bioactive secondary metabolites which are species and strain dependent, including volatile and nonvolatile antifungal substances (38, 46). Some Trichoderma rhizosphere-competent strains that can colonize root surfaces have been shown to have direct effects on plants, increasing their growth potential and nutrient uptake, fertilizer efficiency utilization, percentage and rate of seed germination, and induced systemic resistance (ISR) to diseases (37).
Environmental factors associated with climate change, especially temperature and soil salinity, may influence plant pathogens, biocontrol agents, and mechanisms of their interactions. Due to the scarcity of fresh water (usually with an electric conductivity [EC] of 1 dS m−1 or less), especially in arid and semiarid areas, some agricultural crops are irrigated with saline water (EC up to 5 dS m−1). Crops tolerant to salt, like cotton and barley, can withstand EC levels above 7 dS m−1; most soils have an EC ranging from 2 to 4 dS m−1, and seawater has an EC of 54 dS m−1.
High salinity may increase the severity of diseases caused by a variety of plant pathogens (15, 43), and the search for new Trichoderma strains capable of overcoming extreme environmental conditions is timely.
Although Trichoderma is widely used for biological control in many climatic zones (49), its ability to tolerate salinity has not been extensively investigated. Different degrees of salinity were found to have a different impact on the efficacy of the diverse antagonistic modes of action of T. harzianum in controlling Verticillium wilt in tomato (32).
Significant progress has been made on the observation and identification of marine-derived fungi (18). Many fungi isolated from various marine habitats have been found to be taxonomically closely related to species that are well known from terrestrial environments (16, 29, 31, 48, 50). Paz et al. (29) isolated 85 fungal taxa from the Mediterranean Sea sponge Psammocinia sp., mainly by using a sample-compressing method in combination with fungicide-amended media. Abundant terrestrial taxa, such as Acremonium and Penicillium, were found along with potentially undescribed Phoma and Trichoderma species. The role (if any) of the fungi found in the Psammocinia species has yet to be elucidated. Since sponges are filter feeders, it is conceivable that fungal propagules have been filtered from the water (and some of these may originate from land, via dust and runoff) and are lodged in the sponge tissues, thus having no active role in the biology of the sponge (as has been suggested in the case of the coral pathogen Aspergillus sydowii, which is found in Spongia obscura [11]). The aim of this study was to characterize the marine Trichoderma isolates to assess the potential of marine-derived isolates as halotolerant biocontrol agents.
MATERIALS AND METHODS
Microbial strains and plants.
Twenty-eight Hypocrea/Trichoderma strains isolated from the Mediterranean sponge Psammocinia species (29) and the established biocontrol strain T203 (T. asperelloides [35]) were routinely grown on potato dextrose agar (PDA; Difco). The list of marine-derived Hypocrea/Trichoderma strains is given in Table 1 . The plant pathogens Botrytis cinerea (BO5.10), Rhizoctonia solani (TP6), and Alternaria alternata (tobacco pathovar from I. Chet, Israel), all from the Department of Plant Pathology and Microbiology, The Hebrew University of Jerusalem, Israel, were routinely grown on PDA and used as test pathogens in antagonistic in vitro and in vivo assays.
Table 1.
Strains of Trichoderma species associated with the Mediterranean sponge Psammocinia species used in this study
| Section and species | Designation |
Origina | NCBI GenBank accession no. for: |
||||
|---|---|---|---|---|---|---|---|
| O.Y. | C.P.K. | tef1 | cal1 | chit18-5 | rbp2 | ||
| Longibrachiatum | |||||||
| Trichoderma longibrachiatum | 6607 | 3003 | 10 | JF619248 | |||
| 7407 | 3005 | 2 | JF421259 | ||||
| 7507 | 3008 | 3 | JF421262 | ||||
| 14207 | 3012 | 2 | JF421264 | ||||
| 2607 | 3025 | 3 | FJ619254 | ||||
| 5007 | 3028 | 7 | JF421273 | ||||
| 7807 | 3016 | 3 | JF421267 | ||||
| 4607 | 3006 | 3 | JF421260 | ||||
| 10107 | 2999 | 7 | JF421254 | ||||
| Trichoderma sp. O.Y. 14707 | 14707 | 3030 | 1 | FJ619256 | JF421249 | JF421251 | JF421253 |
| sensu Paz et al. 2010 | 7107 | 3021 | 1 | FJ619252 | JF421248 | JF421250 | JF421252 |
| Trichoderma | |||||||
| T. asperelloides | 1607 | 3013 | 5 | ||||
| H. atroviridis/T. atroviride | 4407 | 3001 | 1 | JF421256 | |||
| 8607 | 3004 | 7 | JF421258 | ||||
| 12907 | 3027 | 3 | JF421272 | ||||
| 4007 | 3018 | 5 | JF421268 | ||||
| 4807 | 3007 | 5 | JF421261 | ||||
| 5107 | 3024 | 1 | JF421271 | ||||
| 3807 | 3020 | 1 | JF421274 | ||||
| 10307 | 3029 | 3 | JF421257 | ||||
| Pachybasium | |||||||
| T. harzianum sensu latob | 1107 | 3023 | 2 | FJ619253 | |||
| 7307 | 3000 | 2 | JF421255 | ||||
| 3207 | 3009 | 7 | FJ619249 | ||||
| 3907 | 3015 | 7 | JF421266 | ||||
| 5507 | 3014 | 3 | JF421265 | ||||
| 5707 | 3010 | 7 | JF421263 | ||||
| 12407 | 3022 | 5 | JF421270 | ||||
| Trichoderma sp. O.Y. 2407 sensu Paz et al. 2010 | 2407 | 3017 | 3 | FJ619250 | JF461453 | JF461454 | JF461455 |
Numbers indicate sponge numbers from specimens collected as described by Paz et al. (29).
T. harzianum sensu Chaverri et al. 2003=H. pseudoharzianum nom. prov. sensu Druzhinina et al. 2010.
Cucumis sativus L. cv. Kfir (Gedera Seeds, Israel) seedlings were used for induced resistance assays to the bacterial leaf pathogen Pseudomonas syringae pv. lachrimans (51), and Phaseolus vulgaris cv. Gloria (Ben-Shahar Seeds, Israel) seedlings were used in greenhouse biocontrol assays.
Halotolerance assays.
Halotolerance was determined by cultivating fungi at a range of different NaCl concentrations (0 to 3%) in agar-free SNA medium (poor-nutrition agar [27]) and its derivate enriched by carbon sources (1% glucose and 1% sucrose). Spore suspensions were adjusted to a final concentration of 105 spores per ml using a turbidimeter set at 590 nm and with sterile distilled water supplemented with traces of Tween 80 (0.01%). Ninety-six-well microtiter plates were prefilled with 2-fold-concentrated nutritional medium (50 μl per well), with columns corresponding to manipulated conditions (nutrients versus NaCl concentration) and rows to identical replicates. In total, 50 μl of a 2-fold-concentrated spore suspension was added to each well using an electronic multichannel pipette. Microplates were incubated at 25°C in the dark. Spore germination and mycelial growth were monitored spectrophotometrically by measuring the optical density at 750 nm every 24 h for 4 days (9).
DNA extraction, PCR amplification, and phylogenetic analysis.
Mycelia were harvested after 2 to 4 days of growth on 3% malt extract agar (MEA; Merck, Germany) at 25°C, and genomic DNA was extracted using a Qiagen DNeasy plant minikit (Hilden, Germany) by following the manufacturer's protocol. The amplification of fragments of the large fourth intron of tef1 (translation elongation factor 1-α) and cal1 (calmodulin) genes, using primer pairs EF1728F (4) and TEF1LLErev (5′-AAC TTG CAG GCA ATG TGG-3′) as well as CAL-228F (5′-GAGTTCAAGGAGGCCTTCTCCC-3′) and CAL-737R (5′-CATCTTTCTGGCCATCATGG-3′) (cal1) (4), was performed as described previously (8). The amplification of chi18-5 (GH18 chitinase CHI18-5; previously ech42) and rpb2 (encoding RNA polymerase subunit II ß) was performed using the primer pair ech42-1a and ech42-2a as described by Lieckfeldt et al. (21) and fRPB2-5f and fRPB2-7cr (22), respectively. PCR products were purified with the QIAquick PCR purification kit (Qiagen, Germany) and were subjected to automated sequencing at MWG (Ebersberg, Germany).
For phylogenetic analysis, DNA sequences were aligned with the CLUSTAL X program (version 1.81) (42) and visually verified with GeneDoc software (version 2.6) (http://www.psc.edu/biomed/genedoc). The interleaved NEXUS file was formatted using PAUP*4.0b10 (40). The phylogenetic position of the putative new species was inferred using the Bayesian phylogenetic method implemented in MrBayes v. 3 (34) with 1,000,000 mcmc generation and a general time-reversible model for nucleotide substitutions (GTR) (41) as described previously (2, 10). Posterior probability above 0.94 was considered significant (20). Phylogenetic analysis for the identification of strains, but not inferring their evolutionary history, was performed using the maximum-parsimony method (observed p-distance, no evolutionary modeling required) implemented in PAUP*4.0b10 applying a heuristic search (n=1,000) with the random addition of sequences and the TBR tree-swapping algorithm. The reliability of the obtained clades was tested by 500 bootstrap replications. Bootstrap values of >75% were considered significant.
Chitinase activity assays.
Trichoderma isolates were grown on SM medium (28) with 0.2% (wt/vol) colloidal chitin as the sole carbon source to induce chitinolytic activity in the presence of 2% (wt/vol) NaCl. A fluorimetric chitinase assay kit (Sigma, St. Louis, MO) was used to determine chitinase activity according to the manufacturer's instructions. Total protein (0.5 μg) from mycelial extracts or concentrated extracellular medium was diluted in 0.05 M K-acetate buffer (pH 4.5) containing substrate (4-methylumbelliferyl N,N′-diacetyl-β-d-chitobioside or 4-methylumbelliferyl N-acetyl-β-d-glucosaminide) to a total final volume of 100 μl. Assays were carried out in 96-well assay plates. After incubation at 37°C for 15 min, the reaction was stopped by adding sodium carbonate solution (0.3 M), and fluorescence was measured by a 96-well microtiter plate reader (Synergy 2; BioTek) using excitation and emission filters of 360 and 450 nm, respectively. Chitinase activity was expressed in nanomoles of methylumbelliferone (4-MU) released from substrate per hour per microgram of soluble protein.
In vitro antagonistic assays.
The antagonistic ability of the isolates was tested in dual plate confrontation assays against the plant pathogens A. alternata, R. solani, and B. cinerea on solidified SM medium (28). To measure the ability to produce water-soluble inhibitors, petri dishes with PDA were covered with cellophane disks (Bio-Rad) and inoculated in the center with a 5-mm-diameter mycelial disk of Trichoderma isolates (T203, O.Y. 1607, O.Y. 14707, O.Y. 7107, and O.Y. 3807). After 2 days of incubation at 28°C, the cellophane was removed and the plates were inoculated with a 5-mm-diameter mycelial disk of the different plant pathogenic test fungi. The pathogen colony radius was measured periodically. For the antagonistic evaluation of emitted volatiles, a PDA petri dish with a 5-mm-diameter mycelial disk of a phytopathogen culture was fastened to another dish (using parafilm) on which a 2-day-old Trichoderma strain was growing. The pathogen colony radius was measured periodically.
HS-SPME-GC-MS analysis.
An Agilent 7890A gas chromatograph (GC) equipped with a CombiPAL autosampler (CTC Analytics) and coupled to an Agilent 5975C VL MSD mass spectrometer (MS) (Agilent Technologies) was used for headspace solid-phase microextraction-GC-MS (HS-SPME-GC-MS) analysis. Trichoderma isolates were grown on PDA at 28°C for 7 days. After harvesting, 3,500 spores in 50 μl water were placed in the center of slants consisting of 5 ml of PDA in 20-ml-HS vials. The vials were sealed and incubated at 28°C for 3 days. PDA-filled and empty HS vials were used to record background (blank) chromatograms to evaluate substances originating from the culture medium, bleeding of the GC stationary phase, or the SPME fiber coating. The analytical procedure and conditions used were the following: samples were placed in 20-ml-headspace vials and equilibrated in the incubator at 50°C for 10 min; to sample the volatile organic compounds (VOCs), an SPME fiber (65 μm polydimethylsiloxane-divinylbenzene [PDMS-DVB]; Supelco) was exposed in the incubated vial at 50°C for 20 min, and the analytes then were desorbed from the fiber at 240°C in a split/splitless injector equipped with a 0.75-mm-inner-diameter liner in split (1:5) mode. The separation of VOCs was carried out on a BPX-Volatiles (60 m by 0.25 mm; 1.4 μm; SGE) capillary column using helium as the carrier gas (1 ml min−1) and the following temperature gradient: initially 50°C for 1 min, then ramped at 10°C min−1 to 240°C and held for 10 min. The mass spectrometer was operated in electron ionization mode with automatically tuned parameters; the acquired mass range was 15 to 350 atomic mass units (amu). The ChemStation (Agilent Technologies) software package was used for instrument control and data analysis. VOCs were tentatively identified (>95% match) based on the National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health (NIST/EPA/NIH) Mass Spectral Library (data version NIST 05, software version 2.0d) using the XCALIBUR v1.3 program (ThermoFinnigan) library.
Assessment of in vivo antagonistic activity and induction of plant systemic resistance.
The antagonistic activity of the selected strains was evaluated against the pathogen R. solani in greenhouse experiments with bean seedlings as described in Carsolio et al. (3). The induction of systemic resistance was evaluated in cucumber seedling leaves infected with the foliar pathogen Pseudomonas syringae pv. lachrimansas as described by Yedidia et al. (51). Briefly, cucumber seeds were surface sterilized in 2% (vol/vol) NaOCl for 2 min and thoroughly washed with sterile distilled water. Growth containers with plant growth medium (PGM) were aerated through 0.45-μm-pore-size filters. Plants were grown in a controlled environment: 26°C, 80% relative humidity, light at 300 μE/m2/s, and a circadian cycle of 14 h of light and 10 h of darkness. Trichoderma isolates T203, O.Y. 3807, and O.Y. 1607 were added to the root compartment 72 h prior to pathogen challenge. After 3 days, leaves were collected for bacteria proliferation evaluation (51).
Root colonization assays.
Roots were detached at the end (after 14 days) of the greenhouse experiments described above and extensively washed in water. After sterilization in 1% NaOCl for 2 min, the roots were washed with sterile distilled water, weighed, and homogenized using an ULTRA-TURRAX apparatus (Janke and Kunkel) in 20 ml of water for 1 min. Serial dilutions were plated for CFU counts on PDA supplemented with rose bengal (20 mg liter−1), streptomycin (100 mg liter−1), and chloramphenicol (300 mg liter−1) (44) at 28°C.
Statistical analysis.
JMP8 software (SAS Institute, Inc., Cary, NC) and Statistica 6.0 (StatSoft, Inc., Tulsa, OK) were used for statistical analyses.
Nucleotide sequence accession numbers.
Newly determined nucleotide sequence accession numbers, all beginning with the designation JF, are shown in Table 1.
RESULTS
Diversity of Hypocrea/Trichoderma spp. of marine origin is represented by highly opportunistic cosmopolitan species but also contains at least two new taxa.
Paz et al. (29) used sequences of the internal transcribed spacers 1 and 2 (ITS1 and ITS2) of the rRNA gene cluster and the fourth intron of the translation elongation factor 1-alpha-encoding gene (tef1) to attribute three of their strains to the two putatively new phylogenetic species Trichoderma sp. O.Y. 14707 (2) and Trichoderma sp. O.Y. 2407 (1), respectively. To verify this conclusion, we analyzed sequences of the partial RNA polymerase subunit B II-encoding gene (rpb2), a partial sequence of the endochitinase-encoding gene chi18-5, and the sequence of the calmodulin gene (cal1) (Table 1). A sequence similarity search confirmed that Trichoderma sp. O.Y. 2407 is a new phylogenetic species which is most closely related to Hypocrea strictipilosa (with 95 and 92% similarity for rpb2 and cal1, respectively; data not shown). The concordant phylogeny of rpb2, chi18-5, and cal1 loci for the two strains assigned the name Trichoderma sp. O.Y. 14707 (Fig. 1 and Table 1) fulfill the genealogical concordance criteria of Dettman et al. (6) to be recognized as a new phylogenetic species. Both new species will be formally taxonomically described elsewhere.
Fig. 1.
Bayesian circular phylograms inferred from the data set of partial exons of chi18-5, rpb2, and cal1 phylogenetic markers analyzed separately. Symbols at the nodes correspond to posterior probabilities of >0.94. New phylogenetic species are underlined, belonging strains are marked in boldface. Sequences for Trichoderma species section Longibrachiatum used in this analysis are retrieved from GenBank, and the accession numbers are listed on the phylograms.
Twenty-six other isolates were safely identified by the analysis of tef1 as members of already-established common species (Fig. 2): H. atroviridis/T. atroviride (nine isolates), T. harzianum sensu lato species complex (seven), the T. longibrachiatum-H. orientalis species pair (four and five, respectively), and T. asperelloides (one).
Fig. 2.
Molecular identification phylograms for Trichoderma strains of marine origin belonging to H. atroviridis/T. atroviride (A), T. longibrachiatum and H. orientalis (B), and Harzianum complex (C) based on the maximum-parsimony analysis of partial sequences of the tef1 gene. Nodes supported by bootstrap values of >75% are indicated by black dots. The color code indicates the geographic region from which the isolates were obtained, as explained in the right top inset. Isolates with dark blue color indicate organisms of marine origins and are underlined. Reference sequences for Trichoderma species used for this analysis were retrieved from GenBank and are given by the assigned strain numbers. Red arrows indicate established taxonomic species; asterisks mark halotolerant strains as revealed in this study.
Biogeographical analysis reveals that marine Hypocrea/Trichoderma strains represent common African and European populations.
As the majority of strains isolated from the marine sponge belong to the cosmopolitan and widely distributed species of Hypocrea/Trichoderma, we wanted to test whether these strains represent new (i.e., putatively specialized to the marine environment) populations or correspond to known terrestrial clades. For this purpose, we compared the sequences of the most polymorphic phylogenetic marker, tef1, to previously published records (Fig. 2).
The dominant holomorphic (reproducing sexually and asexually) species, H. atroviridis/T. atroviride, has a complex infraspecific structure defined in both the original description of the taxon (7) (Fig. 2A) and in later studies (26). Four marine isolates showing two distinct alleles have been attributed to the clade A, while clades C and D were represented by one and two strains, respectively. Interestingly, two marine strains, O.Y. 4407 and O.Y. 8607, isolated from different sponge samples, represent a new subclade of this species (Fig. 2A).
T. longibrachiatum recently has been shown to be a clonal species which therefore has only minor infraspecific polymorphism of any character, including tef1 introns (8). Four of the marine strains showed two tef1 alleles (one unique and one represented by three isolates). Both of these alleles have been detected previously in various African and European ecosystems (Fig. 2B). The sister species, sexually recombining and therefore more polymorphic H. orientalis, also was represented by two distinct alleles. One allele (four isolates) is known from Africa, Europe, and South America, while the remaining strain is most similar to the other South American variant of tef1 (Fig. 2B).
Three distinct alleles of tef1 sequences were detected for strains attributed to T. harzianum sensu lato. Three different alleles (seven isolates) correspond to the three subclades of “H. pseudoharzianum” nom. prov. (three, one, and three strains, respectively), all proposed by Druzhinina et al. (10). For all of these subclades, marine Trichoderma isolates exhibit tef1 identity to African (Ethiopia, Egypt, and South Africa) populations (Fig. 2C).
T. asperelloides is a putatively clonal species and expresses almost no infraspecific polymorphism of any phylogenetic marker (35), therefore no phylograms where constructed for isolate O.Y. 1607.
The majority of marine Hypocrea/Trichoderma isolates are either halotolerant or halostimulated, but these traits are not species specific.
Seventeen strains representing major species and infraspecific clades were assessed for their ability to tolerate salinity. No resolution was obtained on agar-free starvation medium (liquid SNA), while SNA supplemented with carbon sources resulted in diversification. Although the majority of strains were able to tolerate 1% NaCl (EC of 15 dS m−1), seven strains demonstrated statistically confirmed improved mycelial growth on 3% NaCl-amended medium compared to the growth of the control (P > 0.05 by analysis of variance [ANOVA]) (Fig. 3; also see Fig. S1 in the supplemental material). Among these strains, two belong to H. pseudoharzianum nom. prov. (Fig. 2C) and two are from T. atroviride clade D (Fig. 2B). Others represent single strains of T. atroviride clade A, T. longibrachiatum, H. orientalis, and T. asperelloides. Three isolates of T. atroviride, one H. pseudoharzianum nom. prov., and one putative new species showed haloinhibited growth (P > 0.05 by ANOVA); other strains in the experiment expressed a versatile (statistically unconfirmed) or neutral response.
Fig. 3.
Results of the canonical variance analysis applied to the mycelial growth measured as the optical density at 750 nm after 96 h of cultivation in darkness at 25°C on optimized synthetic nutrition medium supplemented with 0, 1, and 3% NaCl. Experiments were performed in eight replicates. Filled squares, diamonds, and stars indicate H. atroviridis/T. atroviride, H. pseudoharzianum, and T. longibrachiatum, respectively. Open stars and squares correspond to H. orientalis and T. asperelloides, respectively. Circled and noncircled asterisks show Trichoderma sp. O.Y. 2407 and Trichoderma sp. O.Y. 14707, respectively.
Chitinolytic activity of halotolerant Trichoderma isolates.
To provide an initial assessment of the potential halotolerant mycoparasitic activity of strains O.Y. 1607 and O.Y. 3807, endochitinolytic and exochitinolytic activities were measured. This process was performed in mycelial extracts obtained from cultures grown in chitinase-inducing medium containing colloidal chitin in control and saline (2% [wt/vol] NaCl) conditions. The terrestrial strain (T203) was used as a control. In control media without the addition of salt, chitinolytic activities in strain O.Y. 3807 (identified as T. atroviride) were lower than those measured in strains O.Y. 1607 and T203 (T. asperelloides), in which similar levels of activities were measured (Table 2). The addition of 2% NaCl revealed a strong inhibitory effect (70 to 90%) on the chitinolytic activity of all strains, with the exception of the intracellular exochitinolytic activity of strains O.Y. 1607 and T203, which were affected to a lower extent (40%) (Table 2).
Table 2.
Effect of 2% NaCl on the chitinolytic activity of marine-derived strains O.Y. 3807 and O.Y. 1607 and the terrestrial strain T203a
| Isolate and activity type | 4-MU-(GlcNAc) |
4-MU-(GlcNAc)2 |
||||
|---|---|---|---|---|---|---|
| Chitinolytic act (nmol MU/min/μg proteins) |
Inhibition (%) | Chitinolytic act (nmol MU/min/μg proteins) with |
Inhibition (%) | |||
| Control | NaCl (2%) | Control | NaCl (2%) | |||
| Intracellular | ||||||
| T203 | 1.52 ± 0.01 | 0.86 ± 0.05 | 43 | 0.13 ± 0.04 | 0.03 ± 0.008 | 79 |
| O.Y. 3807 | 1.06 ± 0.14 | 0.22 ± 0.05 | 79 | 0.03 ± 0.01 | 0.003 ± 0.05 | 89 |
| O.Y. 1607 | 1.36 ± 0.18 | 0.85 ± 0.17 | 38 | 0.13 ± 0.004 | 0.03 ± 0.01 | 77 |
| Extracellular | ||||||
| T203 | 0.1 ± 0.007 | 0.01 ± 0.004 | 84 | 0.013 ± 0.004 | 0.003 ± 0.001 | 77 |
| O.Y. 3807 | 0.04 ± 0.002 | 0.004 ± 0.00 | 91 | 0.008 ± 0.001 | 0.002 ± 0.007 | 70 |
| O.Y. 1607 | 0.13 ± 0.02 | 0.027 ± 0.004 | 80 | 0.011 ± 0.007 | 0.002 ± 0.007 | 77 |
Results (± standard deviations) are averages from three independent experiments with three replicates for each isolate and treatment.
Marine-derived isolates are antagonistic to phytopathogens.
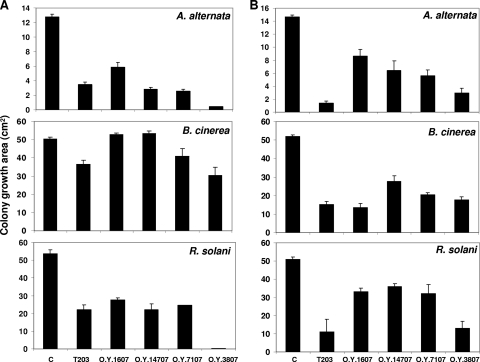
All of the Trichoderma sponge-derived isolates tested showed antagonistic activity toward the three tested phytopathogens in dual confrontation plate assays. Some strains (e.g., O.Y. 3807 and O.Y. 1607) overgrew the tested pathogen, while others (like O.Y. 7407 and O.Y. 7507) first produced a clear growth inhibition zone, indicating the secretion of antimicrobial metabolites followed by mycelial overgrowth (data not shown). The microscopic evaluation of the contact zone in most cases revealed the mycelial attachment. No direct physical interaction could be observed between isolate O.Y. 3807 and the pathogens R. solani and A. alternata. Interestingly, this strain is one of the few isolates in which direct interactions were observed in dual confrontation assays on marine agar against marine-derived pathogens (data not shown). All of the tested strains secrete soluble and volatile compounds with strong antagonistic activities, although different strains showed different inhibition rates against the tested pathogens (Fig. 4 A). Isolate O.Y. 3807 showed the strongest inhibiting activity of soluble metabolites against all three pathogens, while its volatiles showed less inhibition toward B. cinerea than toward the other two pathogens (Fig. 4B). Secreted soluble metabolites from O.Y. 14707 and O.Y. 1607 were not effective against B. cinerea (Fig. 4A). The volatile profile of the most active strains, O.Y. 1607 and O.Y. 3807, was analyzed by GC-MS and compared to the profile of T203, which, according to its molecular identification, belongs to the same species as O.Y. 1607. The compounds identified in the different isolates and their relative abundances are listed in Table 3. β-Phelandrene and 6-pentyl-2H-pyran-2-one are unique for O.Y. 3807 (T. atroviride), and since the latter compound was found to be 85% of all emitted volatiles, it is reasonable to assume that this is the main active antimicrobial compound produced by this isolate. Interestingly, the two T. asperelloides isolates present higher relative amounts of sesquiterpene-like compounds and alcohols.
Fig. 4.
Growth inhibition of the phytopathogens A. alternata, B. cinerea, and R. solani by soluble metabolites secreted in the agar plate (A) or volatiles compounds (B) emitted by isolates T203, O.Y. 3807, O.Y. 1607, O.Y. 7107, and O.Y. 14707. Results show colony area inhibition after 120 h and are an average from six replicates for each strain. Bars indicate standard errors.
Table 3.
Microbial VOCs of T203, O.Y. 1607, and O.Y. 3807 isolates detected by HS-SPME-GC-MS in culture grown for 3 days on PDAa
| Volatile metabolite | RI | Peak area (%) of HSb |
|||
|---|---|---|---|---|---|
| T203 | O.Y. 1607 | O.Y. 3807 | |||
| Ethanol | 522 | 29 | 37 | 4 | |
| Isobutanol | 575 | 1.2 | 2.3 | ND | |
| Ethylacetate | 675 | 0.8 | 1.3 | ND | |
| Isopentanol | 699 | 2.6 | 2.9 | 0.7 | |
| 2-Methyl-1-butanol | 787 | 2.34 | 4.4 | 0.54 | |
| β-Phellandrene | 1,081 | ND | ND | 0.6 | |
| Phenylethyl alchol | 1,228 | 5.9 | 11.6 | 1 | |
| p-Methoxystyrene | 1,236 | 2.4 | 3.5 | 0.7 | |
| Phenylacetic acid ethyl ester | 1,325 | 0.35 | 0.5 | ND | |
| 2-Phenylethylacetate | 1,342 | 0.76 | 1.12 | ND | |
| 2-Undecanone | 1,351 | 0.35 | 0.5 | 0.2 | |
| 2-Acetoxyalkane | 1,455 | 1.43 | 1.54 | ND | |
| β-Farnesene | 1,482 | ND | ND | 0.26 | |
| α-Bergamotene | 1,496 | 2.43 | 6.0 | 3.7 | |
| α-Langipinene | 1,534 | 2.1 | 2.45 | ND | |
| Curcumene | 1,543 | ND | 2.15 | ND | |
| Zingiberene | 1,548 | 18.3 | 6.8 | 0.56 | |
| β-Himachalene | 1,555 | 2.50 | 3.3 | 0.35 | |
| β-Bisabolene | 1,562 | 1.95 | 2.16 | 0.37 | |
| β-Sesquiphellandrene | 1,590 | 25.4 | 10.3 | 1.8 | |
| 6-Pentyl-pyran-2-one | 1,623 | ND | ND | 85.2 | |
The retention index (RI) was calculated according to the retention index guide (http://massfinder.com/wiki/Retention_index_guide) using an alkaline standard solution (C8-C20) as the reference.
Results are listed as peak area (%) of the headspace. Peak areas of individual compounds were calculated as percentage of the total area of the compounds appearing on the chromatogram and identified using a reference (NIST/EPA/NIH) mass spectral library. ND, not detected.
Marine-derived Trichoderma isolates exhibit in vivo biocontrol activity against Rhizoctonia solani and the ability to induce systemic defense responses in plants.
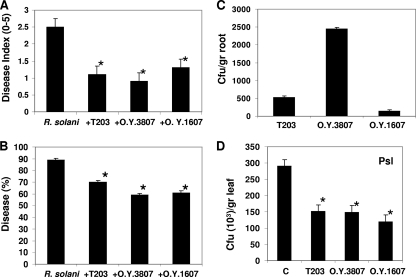
The isolates O.Y. 3807 and O.Y. 1607, exhibiting the most pronounced antagonistic activity in vitro, were chosen for further characterization of their potential biocontrol ability in soil. As determined in greenhouse experiments, both strains reduced the disease index of R. solani root rot on bean seedlings at levels comparable to those achieved by the terrestrial control strain T203 (Fig. 5 A). Specifically, disease incidence was reduced to 50% by the marine-derived isolates, which is an even higher rate than that observed with the terrestrial strain T203 (where 70% of the plants displayed disease symptoms) (Fig. 5B).
Fig. 5.
Biocontrol activity against Rhizoctonia solani under greenhouse conditions and ability to induce defense responses of isolates T203, O.Y. 3807, and O.Y. 1607. (A) Disease index. (B and C) Disease percentage (B) and Trichoderma root colonization ability (C) were measured 2 weeks after planting. Disease indexes, ranging from 0 (no symptoms) to 5 (severe lesions), were assessed according to the disease symptoms of 90 seedlings in three independent experiments. (D) P. syringae pv. lachrimans (Psl) proliferation inhibition in leaves of Trichoderma-treated and untreated (control) plants. Bars indicate standard errors. An asterisk indicates significantly different results (P < 0.05) by Tukey-Kramer honestly significant difference multiple-range tests.
To assess whether the success in reducing disease incidence involves features of plant-biocontrol agent interactions, we first determined the extent of bean seedling root colonization by the Trichoderma strains. When evaluated 2 weeks after application, both strains were found to be efficient root system colonizers (Fig. 5C). Strain O.Y. 3807 showed an exceptionally high ability to colonize the root system, significantly higher than that of the terrestrial T203 strain (Fig. 5C). However, when the kinetics of the colonization was examined, isolate O.Y. 3807 was recovered from the root tissues first after 48 h, while strains O.Y. 1607 and T203 were found to be able to penetrate the epidermis layers (examined microscopically) 12 h after the inoculation (data not shown).
On the basis of these results, we examined the ability of these strains to induce defense responses to the foliar bacterial pathogen P. syringae pv. lachrimans in hydroponic cultures of cucumber seedlings. Strains O.Y. 1607 and O.Y. 3807 both significantly reduced bacterial proliferation in leaves (to 43 and 50%, respectively) in a manner similar to that of T203 (Fig. 5D). Based on these results and the fact that these Trichoderma isolates do not colonize upper plant tissue (data not shown), we concluded that these strains activate an induced systemic resistance response in plants (as has been demonstrated previously with other isolates [37]).
DISCUSSION
Reports on the presence of fungi in marine environments are rapidly accumulating (with more than 500 species described already [18]). Whether or not marine fungi (as an accepted ecological group) have evolved in the sea or have secondarily adapted to life in the marine environment is still an open question (discussed in reference 18). The question is further complicated when distinctions between so-called true marine fungal species (obligates of the marine environment) and those not restricted to marine habitats are made. Based on the diversity of fungal species present in marine environments (ranging from Chytrids to Dikarya), it is likely that the answer to this question is not conclusive.
The presence of Hypocrea/Trichoderma species in marine niches has been observed (29, 30, 48). In some cases, unique traits have been identified for these species from marine environments (12, 33). The studies of Hypocrea/Trichoderma from marine habitats often reveal high applied value of the isolates or their compounds to, e.g., catalyze the hydrolysis of benzyl glycidyl ether (24), and they reveal unique aminolipopeptides active against dormant mycobacteria (30) as well as several other novel compounds (39, 52). In most studies of Hypocrea/Trichoderma from marine environments, isolates have been identified only to the genus level, so further applied values and characteristics of the species remain unknown.
In this study, we report on the diversity of the Trichoderma species isolated from a marine sponge. We suggest that their special characteristics, which differentiate them from their terrestrial counterparts, are advantageous in the biological control of plant pathogens in arid agricultural zones where saline water irrigation is becoming more common. The isolates were identified as members of four established common species, most of which exhibit high opportunistic activity. Strains of H. atroviridis/T. atroviride and T. harzianum sensu lato species complex (H. pseudoharzianum nom. prov.) (10) are well known to be applied as biocontrol agents due to their high antagonistic potential. Moreover, the T. longibrachiatum-H. orientalis species pair has been frequently identified as the causal agent of invasive Trichoderma mycoses (8, 19). Considering the high numbers of marine sponge T. longibrachiatum-H. orientalis isolates, we can hypothesize that the associations between this species pair and animals are not uncommon, but are until now, except with humans, not well studied.
All of the isolated species generally are found in environmental samples worldwide (Fig. 2). Regarding the number of identified species, the diversity of Trichoderma species in the Psammocinia sp. sponge samples is relatively low. However, considering the extreme environmental conditions, e.g., osmotic pressure and the physical effects of the water filtration through the sponge's oscula, the sponge may well be defined as an extreme environment for fungal inhabitation. The strains isolated from a large number of Psammocinia sponges indicate that the biodiversity in marine sponges is not random and could be determined by the low species variability, so only the aggressive opportunistic species survive in these extreme conditions. However, it is interesting that, despite this low species richness, three of the isolates represent two putatively new phylogenetic species, Trichoderma sp. O.Y. 14707 and Trichoderma sp. O.Y. 2407. The latter species is most closely related to H. strictipilosa, while Trichoderma sp. O.Y. 14707 is recognized as a new phylogenetic species. In both cases, none of the strains belonging to these two species revealed special traits that would lead us to speculate that they are limited to this environment. Furthermore, given the fact that some Trichoderma strains have been reported to be potential agents of human mycosis in immunocompromised individuals (8, 19), proper risk assessment analyses is warranted prior to biocontrol use in agriculture.
We have shown that isolates O.Y. 3807 and O.Y. 1607, identified as T. atroviride and T. asperelloides, respectively, can inhibit three major plant pathogens in vitro and are effective in reducing R. solani damping off in beans in vivo. These isolates also exhibit a very good growth adaptation to saline environments in vitro and therefore are promising candidates for the development of biocontrol formulations based on Trichoderma for the amelioration of plant growth in saline soils. Fungi have been shown to develop extrusion systems to keep levels of intracellular sodium below concentrations toxic to the cell (13). Transcriptional data indicate important differences in the cellular processes of osmoadaptation between halotolerant and salt-sensitive fungi (45).
Furthermore, even slight alterations in conserved modules involved in salt stress tolerance, as has been found in halotolerant fungi (e.g., in the high-osmolarity glycerol [HOG] signaling pathway), can contribute to adaptation to saline environments (17).
Changes in the membrane properties also have important functions in the adaptation to different stress situations, and different sterol-to-phospholipid ratios and fatty acid compositions in different fungi can have important effects on the properties of membranes. In Saccharomyces cerevisiae, the main pathway involved in sensing changes in Na+ concentrations and in responding to them is known as the HOG signaling pathway, which controls the expression of others osmoresponsive genes. The hog1 gene homologue was isolated and characterized from T. harzianum (5) and was shown to be important for the hyperosmotic stress response but also for fungus-fungus interactions.
The involvement of chitinases in mycoparasitism (including in Trichoderma) is still an open question (36, 47). Based on our results, the chitinolytic activities of the tested isolates did not show any adaptation to high salt, as they all were strongly inhibited by the addition of 2% NaCl (Table 2), a concentration which otherwise stimulates the growth of these fungi (Fig. 3; also see Fig. S1 in the supplemental material). These data may indicate that the chitinolytic activities are not of primary importance for the antagonistic potential of these strains in a marine environment but still are sufficient to allow fungal development, as it is known that some chitinases are required for hyphal growth (1).
As the biocontrol activity of the tested strains against terrestrial phytopathogens is not based primarily on their mycoparasitism, as has been shown by our in vitro assays, the significance of a lack in chitinase adaptation to salt may be small. Furthermore, the salt concentrations used in our in vitro assays are extremely high (15 dS m−1) compared to the salinity levels that can be reached in soils and still allow plant growth (5 dS m−1 or less). Hence, there also may be a difference in the contribution of chitinase activities in fungus-fungus interactions in marine and terrestrial environments.
Both of these strains secrete antimicrobial water-soluble and volatile metabolites, and since no direct interaction could be observed between O.Y. 3807 and R. solani or A. alternata, antibiosis and ISR are the most likely prevailing mechanisms of the antagonism of this strain to these diseases. As shown in the volatile GC-MS analysis, T. atroviride O.Y. 3807 is a very strong producer of 6-pentyl-2H-pyran-2-one, a compound well known for its antifungal and plant growth-promoting activities (46).
Trichoderma spp. able to interact with plant roots now are regarded as endophytic plant symbionts and general anti-stress factors. This close interaction with the roots induce increased resistance of the plant to various biotic stresses through induced or acquired systemic resistance and also to abiotic stresses, such as water deficit/excess, high salinity, and extreme temperature (23, 25).
We have shown that strains O.Y. 3807 and O.Y. 1607 are good root colonizers (Fig. 5C). Hence, they can be used as potential inducers of more general defense responses in plants, as exemplified in this study by inducing resistance against the bacterial foliar pathogen P. syringae pv. lachrimans (Fig. 5D).
Saline-tolerant Trichoderma strains may be implemented in plant disease management in extreme conditions together with resistant plants created by traditional breeding or genetic manipulation efforts. The extent of physiological modifications accompanying the adaptation of these fungi to the marine environment has yet to be fully elucidated. The implications of the changes may extend beyond halotolerance and may include additional characteristics advantageous to biocontrol in changing environments. These may be differentially expressed on the basis of the host-pathogen system and the surrounding conditions. Such advantages may prove beneficial in light of the complexity of the alterations accompanying the use of saline water (including variations among sources of saline water) for irrigation, ranging from effects on soil properties to rhizosphere communities to the physiological state of the plant as well as pathogens. Regardless, adaptation to higher salt concentrations can be used to reduce the probability of fungal/bacterial contamination in Trichoderma stocks intended for liquid formulations in the on-site cultivation of strains (14). Thus, the maintenance and/or cultivation of such strains in salt-amended medium also may be an avenue for exploiting the characteristics of marine-derived Trichoderma spp.
Our results support evidence that, in addition to traditional potential biotechnological applications (31), the marine ecosystem is a valuable source for potential antagonists of plant pathogens. The marine fungal community is comprised of obligate and facultative fungal species, and some of the latter are found in terrestrial ecosystems as well. Fungi have evolved biologically and biochemically in a diverse manner that has allowed them to utilize various substrates, but key questions that remain unanswered are whether or not fungi are living in sponges, whether the sponges only harbor spores, and whether fungal hyphae remain dormant until proper nutrient conditions are encountered for growth.
Supplementary Material
ACKNOWLEDGMENTS
We thank Udi Landau for technical assistance and statistical analysis. We also express special thanks to Christian P. Kubicek for the discussion of intermediate results and valuable comments on the manuscript.
This research was supported by the Israel Science Foundation (ISF), the Austrian Agency for international cooperation in education and research (OeAD-GmbH), and the Academic Cooperation and Mobility unit (ACM).
Footnotes
Present address: Austrian Institute of Industrial Biotechnology (ACIB) GmBH, c/o Institute of Chemical Engineering, University of Technology of Vienna, Gumpendorferstraße 1a, A-1060 Vienna, Austria.
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Adams D. J. 2004. Fungal cell wall chitinases and glucanases. Microbiology 150:2029–2035 [DOI] [PubMed] [Google Scholar]
- 2. Atanasova L., Jaklitsch W. M., Komoń-Zelazowska M., Kubicek C. P., Druzhinina I. S. 2010. Clonal species Trichoderma parareesei sp. nov. likely resembles the ancestor of the cellulase producer Hypocrea jecorina/T. reesei. Appl. Environ. Microbiol. 76:7259–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carsolio C., et al. 1999. Role of the Trichoderma harzianum endochitinase ech42, in mycoparasitism. Appl. Environ. Microbiol. 65:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaverri P., Castlebury L. A., Samuels G. J., Geiser D. 2003. Multilocus phylogenetic structure within the Trichoderma/Hypocrea lixii complex. Mol. Phylogenet. Evol. 27:302–313 [DOI] [PubMed] [Google Scholar]
- 5. Delgado-Jarana J., Sousa S., González F., Rey M., Llobell A. 2006. ThHog1 controls the hyperosmotic stress response in Trichoderma harzianum. Microbiology 152:1687–1700 [DOI] [PubMed] [Google Scholar]
- 6. Dettman J. R., Jacobson D. J., Taylor J. W. 2003. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57:2703–2720 [DOI] [PubMed] [Google Scholar]
- 7. Dodd S. L., Lieckfeldt E., Samuels G. J. 2003. Hypocrea atroviridis sp. nov., the teleomorph of Trichoderma atroviride. Mycologia 95:27–40 [DOI] [PubMed] [Google Scholar]
- 8. Druzhinina I. S., et al. 2008. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 154:3447–3459 [DOI] [PubMed] [Google Scholar]
- 9. Druzhinina I. S., Schmoll M., Seiboth B., Kubicek C. P. 2006. Global carbon utilization profiles of wild-type, mutant, and transformant strains of Hypocrea jecorina. Appl. Environ. Microbiol. 72:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Druzhinina I. S., Kubicek C. P., Komoń-Zelazowska M., Mulaw T. B., Bissett J. 2010. The Trichoderma harzianum demon: complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evol. Biol. 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ein-Gil N., et al. 2009. Presence of Aspergillus sydowii, a pathogen of gorgonian sea-fans in the marine sponge Spongia obscura. ISME J. 3:752–755 [DOI] [PubMed] [Google Scholar]
- 12. El-Kassas H. Y., Khairy H. M. 2009. A trial for biological control of a pathogenic fungus (Fusarium solani) by some marine microorganisms. Am.-Euras. J. Agric. Environ. Sci. 5:434–440 [Google Scholar]
- 13. Gunde-Cimerman N., Ramos J., Plemenitaš A. 2009. Halotolerant and halophilic fungi. Mycol. Res. 113:1231–1241 [DOI] [PubMed] [Google Scholar]
- 14. Harman G. E., Obregón M. A., Samuels G. J., Lorito M. 2010. Changing models for commercialization and implementation of biocontrol in the developing and the developed world. Plant Dis. 94:928–939 [DOI] [PubMed] [Google Scholar]
- 15. Hasegawa P. M., Bressan R. A., Zhu J. K., Bohnert H. J. 2000. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51:463–499 [DOI] [PubMed] [Google Scholar]
- 16. Höller H. W., et al. 2000. Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol. Res. 104:1354–1365 [Google Scholar]
- 17. Jin Y., Weining S., Nevo E. 2005. MAPK gene from Dead Sea fungus confers stress tolerance to lithium salt and freezing-thawing: prospects for saline agriculture. Proc. Natl. Acad. Sci. U. S. A. 102:18992–18997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones E. B. G., Sakayaroj J., Suetrong S., Somrithipol S., Pang K. L. 2009. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 35:1–187 [Google Scholar]
- 19. Kredics L., et al. 2003. Clinical importance of the genus Trichoderma. A review. Acta Microbiol. Immunol. Hung. 50:105–117 [DOI] [PubMed] [Google Scholar]
- 20. Leaché A. D., Reeder T. W. 2002. Molecular systematics of the Eastern Fence lizard (Sceloporus undulatus): a comparison of parsimony, likelihood and Bayesian approaches. Syst. Biol. 51:44–68 [DOI] [PubMed] [Google Scholar]
- 21. Lieckfeldt E., Cavignac Y., Fekete C., Börner T. 2000. Endochitinase gene-based phylogenetic analysis of Trichoderma. Microbiol. Res. 155:7–15 [DOI] [PubMed] [Google Scholar]
- 22. Liu Y. L., Whelen S., Hall B. D. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 16:1799–1808 [DOI] [PubMed] [Google Scholar]
- 23. Lorito M., Woo S. L., Harman G. E., Monte E. 2010. Translational research on Trichoderma: from 'omics to the field. Annu. Rev. Phytopathol. 48:395–417 [DOI] [PubMed] [Google Scholar]
- 24. Martins M. P., et al. 2011. Marine fungi Aspergillus sydowii and Trichoderma sp. catalyze the hydrolysis of benzyl glycidyl ether. Mar. Biotechnol. doi:10.1007/s10126-010-9302-2 [DOI] [PubMed] [Google Scholar]
- 25. Mastouri F., Björkman T., Harman G. E. 2010. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 100:1213–1221 [DOI] [PubMed] [Google Scholar]
- 26. Mulaw T.B., Kubicek C. P., Druzhinina I. S. 2010. The rhizosphere of Coffea Arabica in its native highland forests of Ethiopia provides a niche for a distinguished diversity of Trichoderma. Diversity 2:527–549 [Google Scholar]
- 27. Nirenberg H. I. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt. Biol. Bundesanstalt Land Forstwirtschaft Berlin-Dahlem 169:1–117 [Google Scholar]
- 28. Okon Y., Chet I., Henis Y. 1973. Effects of lactose, ethanol and cyclohexamide on the translation pattern of radioactive compounds and on sclerotium formation of Sclerotium rolfsii. J. Gen. Microbiol. 74:251–258 [Google Scholar]
- 29. Paz Z., et al. 2010. Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Divers. 42:17–26 [Google Scholar]
- 30. Pruksakorn P., et al. 2010. Trichoderins, novel aminolipopeptides from a marine sponge-derived Trichoderma sp. , are active against dormant mycobacteria. Bioorg. Med. Chem. Lett. 20:3658–3663 [DOI] [PubMed] [Google Scholar]
- 31. Raghukumar C. 2008. Marine fungal biotechnology: an ecological perspective. Fungal Divers. 31:19–35 [Google Scholar]
- 32. Regragui A., Lahlou H. 2005. Effect of salinity on in vitro Trichoderma harzianum antagonism against Verticillium dahliae. Pak. J. Biol. Sci. 8:872–876 [Google Scholar]
- 33. Ren J. W., et al. 2009. Asperelines A-F, peptaibols from the marine-derived fungus Trichoderma asperellum. J. Nat. Prod. 72:1036–1044 [DOI] [PubMed] [Google Scholar]
- 34. Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 35. Samuels G. J., Ismaiel A., Bon M. C., De Respinis S., Petrini O. 2010. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102:944–966 [DOI] [PubMed] [Google Scholar]
- 36. Seidl V., Huemer B., Seiboth B., Kubicek C. P. 2005. A complete survey of Trichoderma chitinases reveals three distinct subgroups of family 18 chitinases. FEBS J. 272:5923–5939 [DOI] [PubMed] [Google Scholar]
- 37. Shoresh M., Harman G. E., Mastouri F. 2010. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 48:21–43 [DOI] [PubMed] [Google Scholar]
- 38. Sivasithamparam K., Ghisalberti E. L. 1998. Secondary metabolism in Trichoderma and Gliocladium, p. 139–191 In Harman G. E., Kubicek C. P. (ed.), Trichoderma and Gliocladium, vol. 1. Taylor and Francis Ltd., London, United Kingdom [Google Scholar]
- 39. Sun S., et al. 2009. Two new compounds from fermentation liquid of the marine fungus Trichoderma atroviride G20-12. J. Asian Nat. Prod. Res. 11:898–903 [DOI] [PubMed] [Google Scholar]
- 40. Swofford D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, MA [Google Scholar]
- 41. Tavaré S. 1986. Some probabilistic and statistical problems in the analysis of DNA sequences. Some mathematical questions in biology-DNA sequence analysis. Am. Math. Soc. Miura RM 17:57–86 [Google Scholar]
- 42. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Triky-Dotan S., Yermiyahu U., Katan J., Gamliel A. 2005. Development of crown and root diseases of tomato under irrigation with saline water. Phytopathology 95:1438–1444 [DOI] [PubMed] [Google Scholar]
- 44. Vargas Gil S., Pastor S., March G. J. 2009. Quantitative isolation of biocontrol agents Trichoderma spp., Gliocladium spp. and Actinomycetes from soil with culture media. Microbiol. Res. 164:196–205 [DOI] [PubMed] [Google Scholar]
- 45. Vaupotič T., Plemenitaš A. 2007. Differential gene expression and Hog1 interaction with osmoresponsive genes in the extremely halotolerant black yeast Hortaea werneckii. BMC Genomics 8:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vinale F., et al. 2008. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 72:80–86 [Google Scholar]
- 47. Viterbo A., Horwitz B. 2010. Mycoparasitism, p. 676–693 In Borkovich K., Ebbole D. (ed.), Cellular and molecular biology of filamentous fungi. ASM Press, Washington, DC [Google Scholar]
- 48. Wang G., Li Q., Zhu P. 2008. Phylogenetic diversity of culturable fungi associated with the Hawaiian sponges Suberites zeteki and Gelliodes fibrosa. Antonie Van Leeuwenhoek 93:163–174 [DOI] [PubMed] [Google Scholar]
- 49. Woo S. L., Scala F., Ruocco M., Lorito M. 2006. The molecular biology of the interactions between Trichoderma spp. , phytopathogenic fungi, and plants. Phytopathology 96:181–185 [DOI] [PubMed] [Google Scholar]
- 50. Yarden O., et al. 2007. Increased prevalence of ubiquitous ascomycetes in an acropoid coral (Acropora formosa) exhibiting symptoms of brown band syndrome and skeletal eroding band. Appl. Environ. Microbiol. 73:2755–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yedidia I., et al. 2003. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 69:7343–7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. You J., et al. 2010. Trichoderone, a novel cytotoxic cyclopentenone and cholesta-7, 22-diene-3 beta, 5 alpha, 6 beta-triol, with new activities from the marine-derived fungus Trichoderma sp. J. Ind. Microbiol. Biotechnol. 37:245–252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.