Abstract
Release of dipicolinic acid (DPA) and its fluorescence with terbium (Tb3+) allow rapid measurement of the germination and viability of spores of Bacillus and Clostridium species. However, germination of coat-deficient Bacillus spores was strongly inhibited by Tb3+ and some other multivalent cations. Tb3+ also inhibited germination of coat-deficient Clostridium perfringens spores.
TEXT
Many Bacillus and Clostridium species can form dormant, resistant spores under conditions unfavorable for growth (24, 25). These spores can resist extreme conditions and survive for years in the absence of nutrients. However, when nutrients return, these spores can germinate and become growing cells (16, 23, 25). Some spore-forming bacteria can also cause food spoilage, food poisoning, and disease, and spores of Bacillus anthracis are a potential bioterrorism agent (9, 13, 25). Therefore, rapid, sensitive assays for bacterial spores have applied importance.
An assay has been developed to detect low spore concentrations, based on spores' high concentration (∼10% [dry weight]) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) (9). DPA forms a strong 1:1 highly fluorescent complex with terbium (Tb3+) ions, and spore quantification by Tb3+-DPA fluorescence takes only a few minutes (4, 12). DPA is also released in the first minutes of spore germination (23), and since germination is essential for spore viability, Tb3+-DPA fluorescence has been used to detect spore germination, and there is a good correlation between DPA release and spore viability (4, 26, 28, 29, 31, 32). However, we report a flaw in the latter assay of spore viability, as Tb3+ and some other multivalent metal cations strongly inhibit germination of coat-deficient spores. The coats comprise the outermost layer of spores of many species, including Bacillus subtilis, and protect spores against many agents, including enzymes and reactive chemicals (8, 10, 11, 24).
The B. subtilis strains used are isogenic derivatives of a laboratory 168 strain and are (i) PS533 (wild type), carrying plasmid pUB110, encoding resistance to kanamycin (22); (ii) PS3328 (ΔcotE mutant), in which much of the cotE coding sequence was replaced with a tetracycline resistance (Tcr) cassette (15); and (iii) PS3738 (ΔsafA mutant), which carries a Tcr cassette replacing much of the safA coding sequence (10). These strains were sporulated at 37°C on 2× Schaeffer's-glucose (SG) agar plates without antibiotics, and spores were harvested and cleaned as described previously (14). The Bacillus cereus strain used was strain T obtained from H. O. Halvorson, and its spores were prepared at 30°C in a defined liquid medium and were purified as described previously (3). The Bacillus megaterium strain used was strain ATCC 12782, and its spores were prepared at 30°C in liquid supplemented nutrient broth and were harvested and cleaned as described previously (5). The Clostridium perfringens strain used was MRS101 (Δcpe::catP mutant) in which the enterotoxin gene (cpe) in the C. perfringens food poisoning isolate SM101 has been inactivated by insertion of a chloramphenicol resistance gene (18). The C. perfringens spores were prepared and purified as described previously (17, 18). All spores used in this work were free (>98%) of growing or sporulating cells, germinated spores, and cell debris, as determined by phase-contrast microscopy. For chemical decoating, spores at an optical density at 600 nm (OD600) of ∼25 were incubated at 70°C for 2 h in 1% SDS-0.1 M NaOH-0.1 M NaCl-0.1 M dithiothreitol, and decoated spores were washed and stored as described previously (1).
Prior to germination, spores at an OD600 of ∼10 were heat activated at 75°C for 30 min (B. subtilis), 70°C for 20 min (B. cereus), or 60°C for 15 min (B. megaterium); activated spores were cooled on ice before germination. Spores were routinely germinated at an OD600 of 0.5 at 37°C in a 96-well plate in 200 μl of 25 mM K-HEPES buffer (pH 7.4) (B. subtilis and C. perfringens), 25 mM Tris-HCl buffer (pH 8.8) (B. cereus), or 25 mM Tris-HCl buffer (pH 7.4) (B. megaterium), with or without 50 μM TbCl3 (30). Spore germination was initiated by the addition of germinants to final concentrations of 2 mM l-valine (B. subtilis) (valine was used instead of l-alanine to preclude germination inhibition by d-alanine generated by spore-associated alanine racemase [23, 25]), 40 mM NH4Cl plus 10 mM l-alanine (B. cereus), or 10 mM glucose (B. megaterium). Dodecylamine germination did not require heat activation and was carried out at 45°C in 40 mM dodecylamine with 25 mM K-HEPES buffer, pH 7.4 (19). For wild-type spores, germination was measured in the presence of 50 μM TbCl3 by quantifying DPA release in a fluorescence plate reader by measuring the formation of Tb3+-DPA (30). To measure germination of coat-deficient spores, 50 μM TbCl3 was added to germination incubations at various times and the fluorescence intensity was recorded. However, to measure whether Tb3+ inhibited DPA release from coat-deficient spores, 10 μM TbCl3 was added to germination incubations unless otherwise specified. The percentages of germinated spores at the end of incubations were checked by examination of ≥100 spores by phase-contrast microscopy. All germination experiments were repeated at least twice with similar results (less than or equal to ±10% of all values reported).
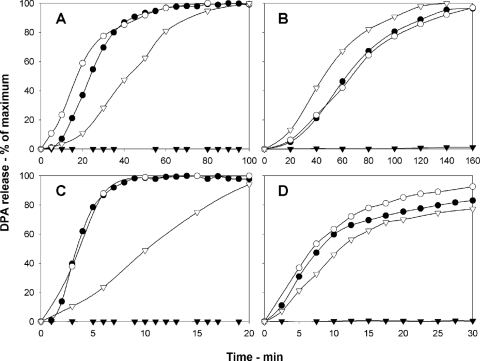
To evaluate the effects of Tb3+ on germination of coat-deficient spores, the valine germination of intact and chemically decoated wild-type B. subtilis spores was measured with or without Tb3+. To measure germination plus Tb3+, TbCl3 was added at the beginning of germination, and DPA release was determined by continuously monitoring Tb3+-DPA fluorescence. To measure spore germination without Tb3+, TbCl3 was added at various times during germination, and the fluorescence immediately after TbCl3 addition was considered the DPA released by that time point. Intact wild-type B. subtilis spores released DPA similarly with or without 50 μM Tb3+ (Fig. 1A), and ≥95% of these spores germinated after 2.5 h, as determined by phase-contrast microscopy. Without Tb3+, decoated B. subtilis spores released DPA slower with l-valine than did intact spores (Fig. 1A), but 85% of the decoated spores germinated in 2.5 h, as determined by phase-contrast microscopy. However, with 10 μM Tb3+ present continuously, no DPA release was detected from decoated spores incubated with l-valine (Fig. 1A), and ≥99% of these spores remained phase bright after 2.5 h (data not shown).
Fig. 1.
Effect of Tb3+ on DPA release during spore germination. DPA release during germination of intact (○, •) or chemically decoated (▿, ▾) spores in the presence (•, ▾) or absence (○, ▿) of TbCl3 was measured as described in the text. In panels A, B, and C, the TbCl3 concentration was either 50 μM (•) or 10 μM (▾), and in panel D TbCl3 was at 50 μM (•, ▾). (A) B. subtilis PS533 (wild-type) spores germinating in 2 mM l-valine; (B) B. subtilis PS533 (wild-type) spores germinating in 40 mM dodecylamine; (C) B. cereus spores germinating in 40 mM NH4Cl plus 10 mM l-alanine; and (D) B. megaterium spores germinating in 10 mM d-glucose. With intact spores germinating with nutrient germinants, the amounts of fluorescence in the TbCl3 assay were similar with 10 μM and 50 μM TbCl3 present throughout the assay (data not shown).
We also investigated if Tb3+ interfered with germination triggered by the cationic surfactant dodecylamine that triggers germination independent of spores' nutrient germinant receptors (GRs), in contrast to germination by l-valine that is GR dependent (19, 23). Intact wild-type B. subtilis spores germinated similarly with dodecylamine and with or without Tb3+ and released most of their DPA within 3 h (Fig. 1B). Without Tb3+ present continuously, dodecylamine germination of decoated wild-type B. subtilis spores was like that of intact spores (Fig. 1B), consistent with previous work (19). However, 10 μM Tb3+ present continuously inhibited the dodecylamine germination of decoated wild-type B. subtilis spores.
The effects of Tb3+ on the germination of spores of other species were also examined. Intact B. cereus and B. megaterium spores incubated with NH4Cl plus l-alanine and glucose, respectively, released almost all DPA within 10 to 30 min with or without Tb3+ (Fig. 1C and D). Without Tb3+, decoated B. cereus and B. megaterium spores germinated normally (B. megaterium) or germinated somewhat slower (B. cereus) than intact spores. However, 10 and 50 μM Tb3+ completely inhibited DPA release from decoated B. cereus and B. megaterium spores, respectively (Fig. 1C and D); this inhibition of germination was confirmed by phase-contrast microscopy (data not shown). We also tested effects of Tb3+ on the dodecylamine germination of intact and decoated C. perfringens spores (Table 1). The effects of Tb3+ on germination by KCl, a particularly effective germinant for C. perfringens MRS101 spores (17), were not tested, because KCl germination of these decoated spores was extremely slow (data not shown); however, dodecylamine germination of these decoated spores was relatively normal. Tb3+ gave only minimal inhibition of the dodecylamine germination of intact C. perfringens spores but inhibited dodecylamine germination of the decoated spores, albeit not as strongly as decoated B. subtilis spores (Fig. 1B; Table 1).
Table 1.
Effect of various Tb3+ concentrations on the dodecylamine germination of intact and decoated C. perfringens sporesa
| Tb3+ concn (μM) | % spore germination in 2.5 h |
|
|---|---|---|
| Intact spores | Decoated spores | |
| 0 | 100 | 100 |
| 50 | 100 | 56 |
| 500 | 92 | 32 |
| 2,500 | 83 | 21 |
Intact and chemically decoated spores of C. perfringens were germinated with dodecylamine plus various TbCl3 concentrations, and the extent of germination was assessed by monitoring DPA release fluorometrically as described in the text. Use of 2.5 mM TbCl3 in the fluorometric DPA assay did not alter the fluorescence compared to that with the standard 50 μM TbCl3 normally present.
Since Tb3+ strongly inhibited the germination of at least Bacillus spores that had been chemically decoated, we then asked if Tb3+ inhibited the germination of spores that were coat deficient due to specific mutations. Spores of B. subtilis were used that lacked either CotE or SafA, morphogenic proteins essential for the proper spore coat assembly and for coats' protection against enzymes and reactive chemicals (8, 10). As expected, there was no significant effect on valine germination of intact wild-type B. subtilis spores with ≤200 μM Tb3+ (Table 2). However, valine germination of chemically decoated wild-type spores and of intact ΔcotE and ΔsafA spores was completely inhibited by 10 μM Tb3+ (Table 2).
Table 2.
Effect of various Tb3+ concentrations on the valine germination of intact and coat-deficient B. subtilis sporesa
| Spore type used | % spore germination in 2.5 h at a Tb3+ concentration of: |
|||||
|---|---|---|---|---|---|---|
| 0 | 500 nM | 2 μM | 10 μM | 50 μM | 200 μM | |
| Intact wild type | 96 | 92 | 96 | 94 | 96 | 95 |
| Decoated wild type | 80 | 80 | 84 | 0 | 0 | 0 |
| ΔcotE mutant | 76 | 68 | 78 | 0 | 0 | 0 |
| ΔsafA mutant | 78 | 86 | 78 | 0 | 0 | 0 |
Spores of various B. subtilis strains, including intact and chemically decoated wild-type (PS533) spores, were germinated for 2.5 h at 37°C with 2 mM l-valine plus various TbCl3 concentrations, and the degree of spore germination was determined by phase-contrast microscopy as described in the text.
Other multivalent metal cations were also examined for their ability to inhibit germination of coat-deficient spores (Table 3). With intact wild-type B. subtilis spores, all cations tested had no significant effect on germination except for 5 mM Zn2+ (Table 3). However, with decoated spores, two lanthanide cations completely inhibited germination at all concentrations tested, and Zn2+ had a similar inhibitory effect, although Mg2+ and Ca2+ were relatively ineffective.
Table 3.
Effects of various multivalent cations on the valine germination of intact and decoated wild-type B. subtilis sporesa
| Cation | % spore germination in: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intact spores at cation concn of: |
Decoated spores at cation concn of: |
|||||||||
| 0 | 50 μM | 500 μM | 2.5 mM | 5 mM | 0 | 50 μM | 500 μM | 2.5 mM | 5 mM | |
| Gd3+ | 96 | 92 | 94 | 84 | 85 | 0 | 0 | 0 | ||
| Dy3+ | 96 | 92 | 94 | 96 | 84 | 0 | 0 | 0 | ||
| Ca2+ | 94 | 88 | 92 | 88 | 82 | 72 | 64 | 60 | ||
| Mg2+ | 94 | 88 | 90 | 82 | 82 | 76 | 64 | 48 | ||
| Zn2+ | 94 | 90 | 88 | 20 | 82 | 0 | 0 | 0 | ||
Intact and chemically decoated wild-type B. subtilis spores (PS533) were germinated for 2.5 h at 37°C with 2 mM l-valine plus various concentrations of divalent and trivalent cation chlorides, and the percentages of germinated spores were determined by phase-contrast microscopy as described in the text.
This communication shows that low concentrations of Tb3+ and some other multivalent metal ions strongly inhibit the germination of coat-deficient spores. This finding is notable, as these same low Tb3+ concentrations are used to assay viable spores by measuring DPA release in spore germination (26, 28, 29, 31, 32). Since coat-deficient spores have viability comparable to that of intact spores (8, 15), Tb3+-based assays of spore viability could yield false-negative results with coat-deficient spores. Consequently, the Tb3+ inhibition of spore germination may be significant when Tb3+-based assays are used to quantitate viable spores in either old environmental samples or by the food industry.
While the Tb3+ inhibition of germination of coat-deficient spores is of applied importance, the mechanism of this inhibition is not clear. One possibility is that spore coat defects facilitate access of cations such as Tb3+ to inner spore layers not accessible in an intact spore, and cation binding to some now accessible target inhibits germination. One such target could be the GRs in spores' inner membranes, and GR inhibition could abolish nutrient germination (16, 23). However, while Tb3+ and similar cations might inhibit GR function, this cannot explain these cations' effects on spore germination, since Tb3+ also inhibits dodecylamine germination, and dodecylamine germination is GR independent (19). Other possible targets of Tb3+ are the cortex-lytic enzymes (CLEs) that hydrolyze spores' peptidoglycan cortexes, a process essential to complete spore germination. However, while Tb3+ and similar multivalent cations may inhibit CLEs, such inhibition again cannot explain such cations' inhibition of DPA release during germination, because spores of Bacillus species lacking CLEs release DPA completely during germination, albeit slower than wild-type spores, and dodecylamine germination is completely CLE independent (7, 19, 20, 21). Since effects of Tb3+ and similar cations on spores' GRs or CLEs cannot explain these cations' inhibition of DPA release during spore germination, the only other likely target is the channel through which DPA is released during germination. This DPA channel has not been definitively identified but seems likely to contain proteins encoded by the spoVA operon, and if such channels were blocked, this would block not only DPA release during germination but also almost certainly subsequent germination events (16, 23, 27). How Tb3+ and similar cations might inhibit such DPA channels is not clear, but it is interesting that the efficacy of the various cations' inhibition of the germination of coat-deficient spores of Bacillus species parallels the values of the logarithms of the stability constants for M2/3+-DPA complexes (where M is any metal), and the stability constants for the formation of multi-M2/3+ ion-DPA complexes also have this same relative order (2, 6) (data not shown). These observations suggest that DPA channels may be blocked by formation of M2/3+ complexes that act like a cork to plug the DPA channel. While this mechanism is certainly speculative, and Tb3+ was not as effective in blocking C. perfringens spore germination, this suggestion seems worth further study.
Acknowledgments
This work was supported by a Multi-University Research Initiative award from the U.S. Department of Defense (PS/MRS).
Footnotes
Published ahead of print on 17 June 2011.
REFERENCES
- 1. Bagyan I., Noback M., Bron S., Paidhungat M., Setlow P. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212:179–188 [DOI] [PubMed] [Google Scholar]
- 2. Chung L., Rajan K. S., Merdinger E., Grecz N. 1971. Coordinative binding of divalent cations with ligands related to bacterial spores—equilibrium studies. Biophys. J. 11:469–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clements M. O., Moir A. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fichtel J., Köster J., Rullkötter J., Sass H. 2007. Spore dipicolinic acid contents used for estimating the number of endospores in sediments. FEMS Microbiol. Ecol. 61:522–532 [DOI] [PubMed] [Google Scholar]
- 5. Goldrick S., Setlow P. 1983. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J. Bacteriol. 155:1459–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grenthe I. 1961. Stability relationships among the rare earth dipicolinates. J. Amer. Chem. Soc. 83:360–364 [Google Scholar]
- 7. Heffron J. D., Lambert E. A., Sherry N., Popham D. L. 2010. Contributions of four cortex lytic enzymes to germination of Bacillus anthracis spores. J. Bacteriol. 192:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henriques A. O., Moran C. P., Jr 2007. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 61:555–588 [DOI] [PubMed] [Google Scholar]
- 9. Hindle A. A., Hall E. A. 1999. Dipicolinic acid (DPA) assay revisited and appraised for spore detection. Analyst 124:1599–1604 [DOI] [PubMed] [Google Scholar]
- 10. Klobutcher L. A., Ragkousi K., Setlow P. 2006. The Bacillus subtilis spore coat provides “eat resistance” during phagosomal predation by the protozoan Tetrahymena thermophila. Proc. Natl. Acad. Sci. U. S. A. 103:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laaberki M. H., Dworkin J. 2008. Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J. Bacteriol. 190:6197–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Q., Dasgupta P. K., Temkins H. K. 2008. Airborne bacterial spore counts by terbium-enhanced luminescence detection: pitfalls and real values. Environ. Sci. Technol. 42:2799–2804 [DOI] [PubMed] [Google Scholar]
- 13. Mock M., Fouet A. 2001. Anthrax. Annu. Rev. Microbiol. 55:647–671 [DOI] [PubMed] [Google Scholar]
- 14. Nicholson W. L., Setlow P. 1990. Sporulation, germination and outgrowth, p. 391–450 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 15. Paidhungat M., Ragkousi K., Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paredes-Sabja D., Setlow P., Sarker M. R. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94 [DOI] [PubMed] [Google Scholar]
- 17. Paredes-Sabja D., Torres J. A., Setlow P., Sarker M. R. 2008. Clostridium perfringens spore germination: characterization of germinants and their receptors. J. Bacteriol. 190:1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarker M. R., Carman R. J., McClane B. A. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens endotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946–958 [DOI] [PubMed] [Google Scholar]
- 19. Setlow B., Cowan A. E., Setlow P. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637–648 [DOI] [PubMed] [Google Scholar]
- 20. Setlow B., Melly E., Setlow P. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 183:4894–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Setlow B., et al. 2009. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J. Appl. Microbiol. 107:318–328 [DOI] [PubMed] [Google Scholar]
- 22. Setlow B., Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 24. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 25. Setlow P., Johnson E. A. 2007. Spores and their significance, p. 35–67 In Doyle M. P., Beuchat L. R. (ed.), Food microbiology: fundamentals and frontiers, 3rd edition. ASM Press, Washington, DC [Google Scholar]
- 26. Shafaat H. S., Ponce A. 2006. Applications of a rapid endospore viability assay for monitoring UV inactivation and characterizing arctic ice cores. Appl. Environ. Microbiol. 72:6808–6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vepachedu V. R., Setlow P. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71–77 [DOI] [PubMed] [Google Scholar]
- 28. Yang W. W., Ponce A. 2009. Rapid endospore viability assay of Clostridium sporogenes spores. Int. J. Food Microbiol. 133:213–216 [DOI] [PubMed] [Google Scholar]
- 29. Yang W. W., Ponce A. 2011. Validation of a Clostridium endospore viability assay and analysis of Greenland ices and Atacama Desert soils. Appl. Environ. Microbiol. 77:2352–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yi X., Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yung P. T., Ponce A. 2008. Fast sterility assessment by germinable-endospore biodosimetry. Appl. Environ. Microbiol. 74:7669–7674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yung P. T., Shafaat H. S., Cannon S. A., Ponce A. 2007. Quantification of viable endospores from a Greenland ice core. FEMS Microbiol. Ecol. 59:300–306 [DOI] [PubMed] [Google Scholar]