Abstract
Bacillus cereus can use swarming to move over and colonize solid surfaces in different environments. This kind of motility is a collective behavior accompanied by the production of long and hyperflagellate swarm cells. In this study, the genome-wide transcriptional response of B. cereus ATCC 14579 during swarming was analyzed. Swarming was shown to trigger the differential expression (>2-fold change) of 118 genes. Downregulated genes included those required for basic cellular metabolism. In accordance with the hyperflagellate phenotype of the swarm cell, genes encoding flagellin were overexpressed. Some genes associated with K+ transport, phBC6A51 phage genes, and the binding component of the enterotoxin hemolysin BL (HBL) were also induced. Quantitative reverse transcription-PCR (qRT-PCR) experiments indicated an almost 2-fold upregulation of the entire hbl operon during swarming. Finally, BC1435 and BC1436, orthologs of liaI-liaH that are known to be involved in the resistance of Bacillus subtilis to daptomycin, were upregulated under swarming conditions. Accordingly, phenotypic assays showed reduced susceptibility of swarming B. cereus cells to daptomycin, and Pspac-induced hyper-expression of these genes in liquid medium highlighted the role of BC1435 and BC1436 in the response of B. cereus to daptomycin.
INTRODUCTION
The quiet soil dweller Bacillus cereus is a well known human pathogen causing two distinct types of gastrointestinal diseases, the emetic and diarrheal syndromes, and it is increasingly recognized to be involved in a variety of local and systemic infections (10, 14, 17, 32, 49). The pathogenicity that B. cereus exerts is primarily due to the ability of vegetative cells to produce an array of virulence factors. Emesis is triggered by a single, heat-stable peptide (cereulide), which is preformed in contaminated food (14). One or more enterotoxins, such as hemolysin BL (HBL), nonhemolytic enterotoxin (Nhe), and cytotoxin K (CytK), are responsible for the diarrheal symptoms (49). The tripartite toxin HBL (with components B, L1, and L2) is also one of the major B. cereus virulence determinants in nongastrointestinal infections. In fact, this toxin possesses hemolytic, cytotoxic, dermonecrotic, and vascular permeability activity, as demonstrated by in vitro and in vivo assays (5, 6). HBL genes are part of a virulence regulon that is controlled by the pleiotropic regulator PlcR, whose expression is autoinduced and starts shortly before the onset of the stationary phase of growth (20, 35).
The ubiquitous nature of B. cereus, combined with the ability to form highly adhesive endospores, explains why this organism is frequently isolated from soil, vegetation, and food, as well as food processing equipment (12). The presence of B. cereus in the food industry is of great relevance, since this organism has been implicated in food spoilage, thus leading to decreased food quality and safety, which affects public health as well as the economy. Furthermore, B. cereus has been shown to be able to form multicellular communities, such as biofilms and swarming colonies (40, 47), which facilitate microbial persistence in the environment and surface colonization. Swarming is a specialized form of surface translocation enabling flagellated bacteria to coordinately move across moist surfaces (25). As described for other species, swarming by B. cereus is accompanied by a dramatic change in cell morphology leading planktonic cells to give rise to long and hyperflagellate swarm cells (47, 48). These cells move as multicellular rafts until they stop and revert to the vegetative phenotype. In Proteus mirabilis, swarming migration characteristically occurs in cyclic rounds, alternating with phases of active cell division, and results in the appearance of characteristic terraced colonies (23, 45). Morphogenesis of swarming colonies varies substantially among swarming-proficient microorganisms depending on the bacterial species and the growth conditions under which swarming occurs. Most often, swarming colonies are characterized by a unique migration front of swarm cells localized at the colony border (53).
In some bacterial species, swarming motility leads to increased expression/secretion of virulence determinants (2, 16, 19, 41, 47), as well as decreased susceptibility to various antibiotics and other antimicrobial agents (30, 34, 41). Some evidence suggests that production of swarm cells can also be considered an additional virulence attribute for B. cereus strains. In fact, swarming by B. cereus is accompanied by a significant increase in the secretion of the HBL enterotoxin (19) and contributes to the severity of experimental endophthalmitis in rabbits, allowing faster bacterial migration from the vitreous to the anterior segment of the eye (11).
Considering that swarming is a complex process contributing to the virulence and persistence of B. cereus in the host/environment, this study was designed to shed light on the physiological and molecular responses of B. cereus cells grown under swarming conditions. To this end, a genome-wide transcriptional approach was used, and phenotypic analyses have been performed in order to support the transcriptome changes observed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The nonemetic type strain ATCC 14579 of Bacillus cereus was used in this study. It was propagated in TrB broth (10 g/liter tryptone, 5 g/liter NaCl), TrB solidified with 0.7% (swarm plates) or 2% (nonswarm plates) (wt/vol) Difco granulated agar (Becton Dickinson, Franklin Lakes, NJ), Mueller Hinton broth (MH) (Oxoid, Hampshire, United Kingdom), or MH solidified with 1% agar.
The Escherichia coli strain TOP10 (Invitrogen, Carlsbad, CA) was used for general cloning strategies, while strain SCS110 (Stratagene, La Jolla, CA) was used as an intermediary host in B. cereus transformation experiments. E. coli strains were grown at 37°C in Luria-Bertani broth (LB) (Fluka, Buchs, Switzerland) or LB solidified with 1% agar. Staphylococcus aureus ATCC 6538 was used as the standard in daptomycin susceptibility assays. The following antibiotics were used for selection when necessary: ampicillin at 80 μg/ml or kanamycin at 30 μg/ml.
Construction of strain MP24.
The genomic region of B. cereus ATCC 14579 containing the genes BC1435 and BC1436 was amplified using the primers LiaIHup1 and LiaIHdw1 (see Table S1 in the supplemental material). The 1,191-bp PCR product was purified, digested with HindIII and SphI restriction enzymes, and ligated into the HindIII and SphI sites of the expression vector pDG148 (50). The recombinant plasmid (pDGLiaIH), carrying both genes under the transcriptional control of the inducible spac promoter (Pspac), was verified by sequencing and used to transform B. cereus ATCC 14579. After selection for kanamycin resistance, the recombinant strain MP24 was isolated. Pspac was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to the bacterial cultures at a final concentration of 4 mM.
Swarming and nonswarming cultures.
For each experiment, swarm and nonswarm plates were prepared fresh daily and allowed to sit at room temperature overnight before use. Plates were centrally inoculated with ∼105 cells of B. cereus ATCC 14579 from a freshly grown overnight colony and incubated at 37°C in a humidified chamber. The presence and percentage of long and hyperflagellate swarm cells inside a colony were evaluated as previously described (46). For the analysis of B. cereus growth rate on swarm or nonswarm plates, multiple cultures were initiated; at each time interval after the inoculum (0, 2, 4, 6, 8, and 10 h), colony diameters were measured and cells were harvested from duplicate plates with 1 ml of cold sterile water. Samples were gently vortexed to obtain monodispersed cell suspensions that were serially diluted before being plated for viable cell counts.
RNA isolation and cDNA production.
Total RNA was purified from B. cereus ATCC 14579 propagated for 8 h on swarm or nonswarm plates. Cells were harvested from plates by washing the agar surface with cold diethylpyrocarbonate-treated water. Cell suspensions were added to an equal volume of ice-cold methanol and incubated at room temperature for 5 min. Bacteria were collected by centrifugation at 4,000 × g at 4°C for 5 min, and the cell pellets (∼109 bacterial cells) were then rapidly frozen on dry ice and stored at −80°C until use. RNA extraction (performed with the RNeasy Midi kit; Qiagen, Hilden, Germany), RNA quantification, and cDNA synthesis and labeling were done as described previously (18, 20).
Microarray design, sample processing, and data analysis.
Microarray slides were prepared as previously described (20). The chip is a combined Bacillus anthracis/B. cereus microarray containing 7,787 features from B. anthracis strains Ames and A2012 and B. cereus strain ATCC 14579 (33). cDNA samples from swarming populations of B. cereus ATCC 14579 were hybridized against cDNA of B. cereus ATCC 14579 nonswarm cells. The microarray slides were prehybridized, hybridized with labeled cDNA, and washed before scanning (20). Data collection and analysis were performed as previously described (20). The raw data were filtered and weighted by quality (8), and the four technical replicates on each slide were averaged to increase data robustness. P values were computed using a false-discovery rate of 0.05. The analysis is based on four slides, all biological replicates.
qRT-PCR.
Prior to reverse transcription (RT), total RNA was subjected to PCR using primers specific to the rpoA gene (see Table S1 in the supplemental material) to exclude the possibility of genomic DNA contamination. RNA (40 ng) was converted into cDNA using the QuantiTec reverse transcription kit (Qiagen) as recommended by the manufacturer. Quantitative real-time RT-PCRs (qRT-PCRs) were performed using the QuantiFast SYBR green PCR kit (Qiagen) according to the manufacturer's protocol and were run in a LightCycler instrument (Roche, Basel, Switzerland). Primers were designed to give products of between 130 and 180 bp (see Table S1 in the supplemental material). The rpoA gene was used as housekeeping control; melting curve analysis was performed to confirm the specificities of amplification reactions. qRT-PCR for each gene was done in duplicate, and for each reaction, the calculated threshold cycle (CT) was normalized to the CT of rpoA amplified from the corresponding sample. The entire experiment was repeated two times on RNA samples extracted from independent cultures grown on swarm and nonswarm plates. Changes in mRNA levels were calculated using the 2−ΔΔCT method (36).
Daptomycin susceptibility assays.
The susceptibility of B. cereus to daptomycin was evaluated by agar diffusion assays on swarm and nonswarm plates and by the broth microdilution technique in MH (39). To ensure daptomycin activity, media were supplemented with 50 mg/liter Ca2+ (22). All assays were performed in duplicate and repeated four times.
Precise MICs for daptomycin in solid media were determined using ε-test strips (bioMérieux, Marcy l'Etoile, France). Swarm plates were inoculated with ∼105 cells from an overnight culture at four opposite spots, and the ε-test strip was placed in the middle of the plate between the four inocula. Nonswarm plates were inoculated with bacterial suspensions at an optical density at 625 nm (OD625) of 0.09 ± 0.01 in physiological salt solution by the use of a sterile swab in order to yield a semiconfluent growth zone (3). Plates were dried briefly at room temperature before the ε-test strip was placed in the middle. Bacteria were allowed to grow for 18 h at 37°C before MIC values were recorded. The MICs of daptomycin against S. aureus were obtained on swarm and nonswarm plates by seeding bacteria as described above for B. cereus on the nonswarm plates.
To perform liquid growth inhibition experiments, a stock solution containing 50 mg/ml (wt/vol) daptomycin was obtained by suspending the commercially available powder Cubicin 350 mg (Novartis International, Basel, Switzerland) in 7 ml sterile water according to the manufacturer's instructions. Serial dilutions of the antibiotic stock solution were prepared in MH using 96-well microtiter plates. When necessary, 4 mM IPTG was added to the medium. Bacterial cells grown on MH plates at 37°C for 18 h were suspended in MH (turbidity of a 0.5 McFarland standard) and diluted 1:20 in fresh MH before being added to each well (20 μl/well in a final volume of 200 μl). After 18 h of incubation at 37°C, bacterial susceptibility to daptomycin was expressed as the mode of the lowest antibiotic concentration that led to complete growth inhibition (MIC).
Statistical analyses.
Statistical analyses were performed with GraphPad InStat software unless otherwise specified.
Microarray data accession number.
The raw data files are available in the ArrayExpress database under accession number E-TABM-914.
RESULTS AND DISCUSSION
Comparison of B. cereus growth under swarming and nonswarming conditions.
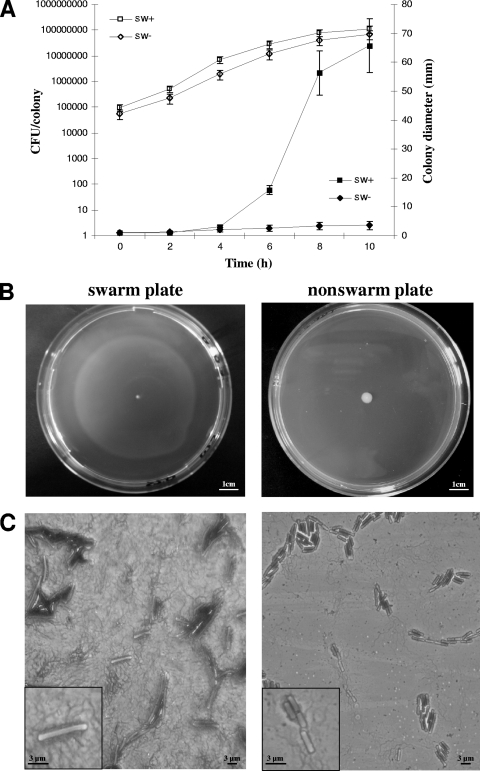
B. cereus is able to swarm under a wide range of growth conditions, and swarming colonies are most often characterized by a migration front of swarm cells and a unique consolidation phase at the colony center (48). For B. cereus ATCC 14579, active swarming migration has been previously demonstrated on TrB solidified with 0.7% agar (19). However, no data are available on the growth rate of a swarming population compared to a nonswarming community. Hence, for characterization of the global transcriptional patterns of such populations, we first determined the growth dynamics of B. cereus ATCC 14579 under swarming and nonswarming conditions. Cells were inoculated on TrB solidified with 0.7% agar for swarming and under the same growth conditions except for a harder surface (2% agar) as a nonswarming control. Changes in colony diameter and number of CFU were monitored over time. A slight and similar increase in the size of growing colonies was observed within the first 4 h postinoculation on both swarm and nonswarm plates. Thereafter, colonies were significantly wider on swarm plates than on the nonswarm ones (P < 0.0001 at 6 h) (Fig. 1A and B). Despite the differences in size observed, swarming and nonswarming communities always contained comparable numbers of bacteria (Fig. 1A), indicating no difference in the growth kinetics of B. cereus ATCC 14579.
Fig. 1.
Growth of B. cereus ATCC 14579 on swarm and nonswarm plates. (A) Growth of swarmer (sw+) and nonswarmer (sw−) populations was monitored over time by measuring colony diameters (▪, ⧫) and CFU numbers (□, ⋄). (B) Swarming (left) and nonswarming (right) colonies produced at 8 h after the inoculum. (C) Examples of swarm (left) and vegetative (right) cells collected from the swarming halo and the nonswarming colony, respectively. Samples were treated with flagellar staining and photographs taken by phase-contrast microscopy (magnification, ×1,000). Insets show higher-magnification views of swarm and nonswarm cells.
Analysis of the cell length and level of flagellation of bacteria picked up from the growing colonies revealed long and hyperflagellate cells only on TrB containing 0.7% agar at 6 to 10 h after the inoculation (Fig. 1C). Such conditions allowed B. cereus ATCC 14579 to generate swarming communities characterized by high percentages of swarm cells. These cells were isolated from both the colony margin (68 to 75%) and throughout the migration halo (35 to 48%). Since it is not possible to physically separate swarm and vegetative cells from a B. cereus swarming community, we decided to extract total RNA for transcriptome analysis from entire swarming colonies grown on 0.7% agar for 8 h. Total RNA from nonswarming communities was extracted from bacteria grown on 2% agar for 8 h.
Transcriptional analysis of B. cereus during swarming.
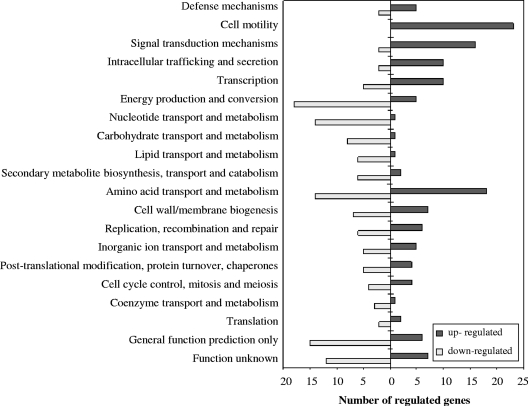
To explore gene regulation events associated with swarming motility in B. cereus, whole-genome transcriptomes of swarming and nonswarming populations were compared directly. Microarray data revealed that 290 genes (4% of all B. cereus ATCC 14579 genes), including 61 hypothetical or conserved hypothetical genes with no currently defined function, had a significantly diverse level of transcription (P < 0.05) under the two conditions (see Table S2 in the supplemental material). Among these genes, 145 were downregulated and 145 were upregulated. Figure 2 shows the differentially regulated genes divided into classes based on the Clusters of Orthologous Groups of proteins (COG) classification (51). At total of 118 genes out of these 290 were at least 2-fold differentially expressed in the swarming community compared to the nonswarming population (see Table S2 in the supplemental material).
Fig. 2.
Number of differentially expressed genes (P < 0.05) during swarming motility reported on the basis of COG classification. Some genes belong to more than one category. Genes that have not been assigned to a COG category (listed in Table S2 in the supplemental material) are not included in the figure.
Flagellation.
Swarm cells of swarming-proficient bacteria, including B. cereus, display a hyperflagellate phenotype (13, 24, 25, 29, 42, 48). In B. cereus ATCC 14579, many of the motility-associated genes are located in a 45-kb region ranging from motA (BC1625) to flgG (BC1671), which encode a flagellar motor protein and the flagellar basal-body rod protein, respectively. In the present study, the expression of almost all motility genes was slightly increased during swarming (see Table S2 in the supplemental material). Nevertheless, transcription of three loci (BC1657, BC1658, and BC1659) inside this region was about 4-fold higher during active swarming (Table 1). These open reading frames (ORFs) are located on the opposite strand compared to neighboring genes and encode different flagellin proteins (21). In most bacteria, synthesis of flagella is a highly ordered process which arises, at least in part, from the hierarchical expression of flagellar genes, with the flagellin gene being the last flagellar gene expressed (1). For B. cereus, the temporal expression and regulatory network of the flagellar genes have not yet been described. The higher expression of the gene cluster encoding flagellins than of other motility genes suggests that flagellin transcription is, at least in part, independently regulated under swarming conditions in B. cereus ATCC 14579.
Table 1.
Selected B. cereus genes that are differently expressed under swarming and nonswarming conditions
| Classification | Locus tag | Gene name | Putative function | Fold change (swarming/nonswarming) |
|---|---|---|---|---|
| Flagellation | BC1657 | Flagellin | +3.73 | |
| BC1658 | Flagellin | +3.25 | ||
| BC1659 | Flagellin | +4.00 | ||
| Metabolism | BC2778 | acoB | Thiamine PPi-dependent acetoin dehydrogenase E1 beta subunit | −2.29 |
| BC2779 | acoA | Thiamine PPi-dependent acetoin dehydrogenase E1 alpha subunit | −2.29a | |
| BC4870 | ldh2 | l-Lactate dehydrogenase | −3.03 | |
| BC4996 | ldh3 | l-Lactate dehydrogenase | −3.25a | |
| BC3541 | Flavodoxin | −2.29 | ||
| BC4792 | cydA2 | Cytochrome d ubiquinol oxidase subunit I | −4.29 | |
| BC0406 | arcA | Arginine deiminase | −4.00 | |
| BC0407 | arcB | Ornithine carbamoyltransferase | −10.56 | |
| BC0408 | arcD | Arginine/ornithine antiporter | −6.96 | |
| BC0409 | arcC | Carbamate kinase | −3.48 | |
| BC0410 | Transcriptional regulator, Crp/Fnr family | −4.00 | ||
| BC0491 | pfl | Formate acetyltransferase | −4.92 | |
| BC0492 | pflA | Pyruvate formate-lyase-activating enzyme | −6.50 | |
| BC2220 | adhA | Alcohol dehydrogenase | −3.48 | |
| BC0668 | (R,R)-Butanediol dehydrogenase | −2.64 | ||
| BC2134 | Uroporphyrin-III C-methyltransferase | −3.48 | ||
| BC2128 | narK | Nitrite extrusion protein | −3.03 | |
| Virulence | BC3526 | Collagen adhesion protein | −3.73 | |
| BC3698 | Endopeptidase, family M23/M37 | −2.64 | ||
| BC3101 | hblB | Hemolysin BL binding component precursor | +2.00 | |
| Other | BC0753 | kdpA | Potassium-transporting ATPase A subunit | +3.48 |
| BC0754 | kdpB | Potassium-transporting ATPase B subunit | +3.73 | |
| BC0755 | kdpC | Potassium-transporting ATPase C subunit | +3.73 | |
| BC0756 | kdpD | Sensor histidine kinase KdpD | +2.83 | |
| BC1435 | Conserved hypothetical protein | +6.50 | ||
| BC1436 | Phage shock protein A | +6.06 |
P value of between 0.05 and 0.1.
Metabolism.
Genome- and proteome-scale studies performed on Gram-negative bacteria have previously attempted to elucidate the metabolic state of a swarming population (26, 31, 41, 42, 55). Metabolic circuits specific to swarm cells have not yet been reported. This could be due to the intrinsic diversities in the bacterial species analyzed and to the different growth conditions adopted, which can deeply alter expression of metabolic genes. Our microarray data show that the expression of several genes for basic cellular metabolism of B. cereus ATCC 14579 is influenced but not dramatically altered during active swarming migration (see Table S2 in the supplemental material). A number of genes for energy production and conversion (COG category C), including several genes involved in both fermentation and oxidative respiration, were shown to be downregulated (Table 1; see Table S2 in the supplemental material). In particular, the arginine deiminase operon arcABDC (BC0406 to BC0409) and its regulator (BC0410) and genes involved in various forms of fermentation (BC0491, BC0492, and BC2220), oxidative phosphorylation (BC4792), and anaerobic respiration (BC2128 and BC2134), were downregulated in the swarming compared to the nonswarming population (Table 1; see Table S2 in the supplemental material).
Potassium uptake.
Potassium ions play an essential role in bacterial physiology, as demonstrated by their involvement in osmoregulation, maintenance of cellular pH, signaling, and stabilizing the structures and functions of numerous enzymes (15). During swarming by B. cereus ATCC 14579, transcripts for proteins putatively involved in K+ uptake, such as kdpA, kdpB, and kdpC, were found to be almost 4-fold upregulated (Table 1). As described for other organisms (52, 54), these genes appear to constitute an operon regulated by KdpD, a membrane-associated sensor kinase. Furthermore, the level of expression of kdpD in B. cereus ATCC 14579 swarm cells was upregulated accordingly (Table 1). It has recently been shown that a temporary decrease in intracellular potassium levels is a signal that triggers a multicellular behavior, biofilm formation, in B. subtilis (37). The finding that B. cereus hyperexpresses a K+ uptake system under swarming conditions suggests that modulation of intracellular potassium levels can also have a role in the development of swarming communities. Further studies will aim to elucidate the role of K+ in swarming motility by B. cereus.
Virulence.
B. cereus is well known for its ability to produce many toxins and degradative enzymes potentially acting against mammalian cells, such as phospholipases, hemolysins, enterotoxins, and proteases (44). Under the swarming conditions tested, a reduction in the expression of BC3526 and BC3698, which encode a collagen adhesion protein and an endopeptidase, respectively, was registered. The expression of the gene encoding the binding component precursor (BC3101) of the tripartite toxin HBL, which is the first gene of the hbl operon, was increased 2-fold (Table 1). Only a slight upregulation of the other hbl genes (BC3102 to BC3104) was observed in swarming B. cereus (see Table S2 in the supplemental material). In a liquid environment, the expression of the hbl operon as well as many virulence-associated genes is under the control of the pleiotropic transcriptional regulator PlcR (20). During swarming by B. cereus, a 1.5-fold reduction of plcR expression was found, and expression of the majority of PlcR-regulated virulence genes was slightly reduced accordingly (see Table S2 in the supplemental material).
To verify that the trends we observed with microarray analysis were reproducible by a complementary approach, quantitative real-time RT-PCRs were conducted on RNA samples obtained from B. cereus swarming and nonswarming populations, using primers for plcR and each of the hbl operon genes (see Table S1 in the supplemental material). qRT-PCR results for the five genes were consistent with the microarray data, and good correlation between the two assays was obtained (see Fig. S1 in the supplemental material). In fact, under swarming conditions, the relative expression of plcR was found to be −1.89 ± 0.33, and upregulation of the hbl operon was confirmed (hblB precursor, +2.30 ± 0.28; hblB, +2.03 ± 0.17; hblL1, +1.95 ± 0.24; hblL2, +2.06 ± 0.24). These findings suggest that regulatory factors in addition to PlcR act in modulating the expression of hbl genes during swarming. HBL secretion was previously shown to be significantly increased in the swarmer cells of swarming-proficient B. cereus strains (19). The results obtained in this study suggest that the higher level of HBL exported by B. cereus during swarming is due to increased expression of hbl genes.
Resistance to daptomycin.
Analysis of the microarray data demonstrated that two genes, BC1435 and BC1436, were markedly upregulated during active swarming (Table 1). BC1435 and BC1436 have not been previously characterized in B. cereus. BLAST searches in data banks revealed that these two loci are highly conserved in all completely sequenced members of the B. cereus group and that proteins encoded by BC1435 and BC1436 show sequence similarity to B. subtilis LiaI and LiaH, respectively (34% identity and 50% positivity for BC1435; 32% identity and 53% positivity for BC1436). In B. subtilis, liaI and liaH constitute an operon whose expression is completely dependent on the LiaRS two-component system (TCS), which can be activated by the presence of antibiotics acting on the cell wall (28, 38, 43). It has been recently suggested that the lia operon protects against daptomycin, a compound belonging to the new class of cyclic lipopeptide antibiotics used against multidrug-resistant Gram-positive pathogens (22). On the basis of these observations, we questioned whether swarm cell differentiation in B. cereus was coupled with increased resistance to daptomycin. In this regard, we compared the susceptibilities of vegetative and swarm cells using ε-test strips containing daptomycin in an agar diffusion assay on swarm and nonswarm plates. Under these growth conditions, a 4-fold higher MIC of daptomycin was recorded for swarming B. cereus than for its nonswarming counterpart (see Fig. S2 in the supplemental material). To exclude the possibility that different agar concentrations in our assay could affect antibiotic diffusion kinetics, the assay was repeated using the nonswarming strain S. aureus ATCC 6538. The same MIC value (0.064 μg/ml) was obtained for this organism on swarm and nonswarm plates, thus indicating that the results obtained for B. cereus were not due to an effect of the agar concentration on the function of the ε-test strip.
Resistance to daptomycin of swarming B. cereus could be due to the higher levels of expression of BC1435 and BC1436 as well as to the high cell densities reached within the swarming colony, as reported for other swarming species (9). Therefore, we performed growth inhibition experiments in liquid medium, which is a culture condition not allowing swarming. In this assay, strains ATCC 14579 and MP24 of B. cereus and S. aureus ATCC 6538 as a control were used. Strain MP24 is an ATCC 14579 derivative carrying both BC1435 and BC1436 under the transcriptional control of the inducible Pspac promoter, which is hyperstimulated by the addition of IPTG to the culture. Our results showed an MIC of 3.125 μg/ml for B. cereus ATCC 14579 and MP24 without IPTG induction. Overexpression of BC1435 and BC1436 in strain MP24 led to increased daptomycin resistance (MIC, 6.25 μg/ml). The same MIC value (0.195 μg/ml) was registered for S. aureus ATCC 6538 with or without IPTG, thus indicating that IPTG addition has no effect on the assay.
Together, these data indicate that BC1435 and BC1436 are related to the B. cereus response to daptomycin and that the increased resistance of swarm cells to this antibiotic is due to the upregulation of these genes. Further studies will aim to elucidate the roles of these two genes in B. cereus resistance to daptomycin and development of the swarming community.
Phage.
Many sequenced bacterial genomes contain prophage elements that may be associated with important functions, such as pathogenesis, serotype conversion, and phage immunity (4, 7). The chromosome of B. cereus ATCC 14579 harbors several probacteriophages, mostly uncharacterized in the B. cereus group (27). The phBC6A51 prophage spans a region of 61,395 bp that contains 75 ORFs (BC1847 to BC1921), many of which are described as encoding hypothetical proteins (58). Most of the genes in this prophage region are significantly upregulated in B. cereus ATCC 14579 under swarming conditions (see Table S2 in the supplemental material). It is of some interest to note that other reports showed an association between bacterial multicellularity and induction of phage-associated genes (56, 57). It could be speculated, therefore, that activation of phage genes could provide some evolutionary advantage to bacteria living in multicellular communities.
Concluding remarks.
In this study, the transcriptional profile of B. cereus swarm cells has been studied. Our data indicate that swarming B. cereus ATCC 14579 alters the expression of genes involved in several cellular functions. Swarm cells tune but do not dramatically modify the expression of genes involved in basic cellular metabolism. Although swarming motility has been recognized as a B. cereus pathogenicity determinant in vivo (11), we found that swarming B. cereus slightly reduces the expression of genes for toxins and degradative enzymes. However, in agreement with the high level of secretion of the tripartite enterotoxin HBL by swarm cells (19), the hbl operon is somewhat induced during swarming. Phenotypic assays suggest a link between hyperexpression of genes with sequence similarity to B. subtilis liaI and liaH and the response of B. cereus to daptomycin. Microarray data also reveal altered expression of genes associated with K+ transport, prophage genes, and conserved hypothetical genes with no currently defined function. An important challenge for the future will be to determine how these genes are involved in B. cereus swarming migration.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kim Susanna, Department of Molecular Cell Biology, University of Groningen, for her contribution to the initial microarray work.
This study was supported by research grant 2005058814 from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca and by funding from the Functional Genomics (CAMST, FUGE) initiative, Norwegian Research Council.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Aldridge P., Hughes K. T. 2002. Regulation of flagella assembly. Curr. Opin. Microbiol. 5:160–165 [DOI] [PubMed] [Google Scholar]
- 2. Allison C., Lai H. C., Hughes C. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583–1591 [DOI] [PubMed] [Google Scholar]
- 3. Andrews J. M. 2001. BSAC standardized disc susceptibility testing method. J. Antimicrob. Chemother. 48: 43–57 (Erratum 49:1049, 2002.) [DOI] [PubMed] [Google Scholar]
- 4. Banks D. J., Beres S. B., Musser J. M. 2002. The fundamental contribution of phages to GAS evolution, genome diversification and strain emergence. Trends Microbiol. 10:515–521 [DOI] [PubMed] [Google Scholar]
- 5. Beecher D. J., Schoeni J. L., Wong A. C. L. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beecher D. J., Wong A. C. L. 1994. Improved purification and characterization of hemolysin BL: a hemolytic, dermonecrotic vascular permeability factor from Bacillus cereus. Infect. Immun. 62:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyd E. F., Brüssow H. 2002. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 10:521–529 [DOI] [PubMed] [Google Scholar]
- 8. Bruland T., et al. 2007. Optimization of cDNA microarrays procedures using criteria that do not rely on external standards. BMC Genomics 8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butler M. T., Wang Q., Harshey R. M. 2010. Cell density and mobility protect swarming bacteria against antibiotics. Proc. Natl. Acad. Sci. U. S. A. 107:3776–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Callegan M. C., et al. 2007. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog. Retin. Eye Res. 26:189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Callegan M. C., Novosad B. D., Ramirez R., Ghelardi E., Senesi S. 2006. Role of swarming migration in the pathogenesis of Bacillus endophthalmitis. Invest. Ophthalmol. Vis. Sci. 47:4461–4467 [DOI] [PubMed] [Google Scholar]
- 12. Carlin F., et al. 2000. Research on factors allowing a risk assessment of spore-forming pathogenic bacteria in cooked chilled foods containing vegetables: a FAIR collaborative project. Int. J. Food Microbiol. 60:117–135 [DOI] [PubMed] [Google Scholar]
- 13. Eberl L., Molin S., Givskov M. 1999. Surface motility of Serratia liquefaciens MG1. J. Bacteriol. 181:1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehling-Schulz M., Fricker M., Scherer S. 2004. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol. Nutr. Food Res. 48:479–487 [DOI] [PubMed] [Google Scholar]
- 15. Epstein W. 2003. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75:293–320 [DOI] [PubMed] [Google Scholar]
- 16. Fraser G. M., Hughes C. 1999. Swarming motility. Curr. Opin. Microbiol. 2:630–635 [DOI] [PubMed] [Google Scholar]
- 17. Gaur A. H., et al. 2001. Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin. Infect. Dis. 32:1456–1462 [DOI] [PubMed] [Google Scholar]
- 18. Ghelardi E., et al. 2002. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated protein in Bacillus thuringiensis. J. Bacteriol. 184:6424–6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghelardi E., et al. 2007. Swarming behavior and hemolysin BL secretion in Bacillus cereus. Appl. Environ. Microbiol. 73:4089–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gohar M., et al. 2008. The PlcR virulence regulon of Bacillus cereus. PLoS One 3:e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gohar M., et al. 2002. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2:784–791 [DOI] [PubMed] [Google Scholar]
- 22. Hachmann A. B., Angert E. R., Helmann J. D. 2009. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 53:1598–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harshey R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249–273 [DOI] [PubMed] [Google Scholar]
- 24. Harshey R. M., Matsuyama T. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. U. S. A. 91:8631–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henrichsen J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoue T., et al. 2007. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J. Bacteriol. 189:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ivanova N., et al. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87–91 [DOI] [PubMed] [Google Scholar]
- 28. Jordan S., Junker A., Helmann J. D., Mascher T. 2006. Regulation of LiaRS-dependent gene expression in Bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system. J. Bacteriol. 188:5153–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kearns D. B., Losick R. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581–590 [DOI] [PubMed] [Google Scholar]
- 30. Kim W., Surette M. G. 2003. Swarming populations of Salmonella represent a unique physiological state coupled to multiple mechanisms of antibiotic resistance. Biol. Proced. Online 5:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim W., Surette M. G. 2004. Metabolic differentiation in actively swarming Salmonella. Mol. Microbiol. 54:702–714 [DOI] [PubMed] [Google Scholar]
- 32. Kotiranta A., Lounatmaa K., Haapasalo M. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189–198 [DOI] [PubMed] [Google Scholar]
- 33. Kristoffersen S. M., et al. 2007. Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereus ATCC 14579. J. Bacteriol. 189: 5302–5313 (Erratum 189:6741.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lai S., Tremblay J., Déziel E. 2009. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ. Microbiol. 11:126–136 [DOI] [PubMed] [Google Scholar]
- 35. Lereclus D., Agaisse H., Gominet M., Salamitou S., Sanchis V. 1996. Identification of a Bacillus thuringiensis gene that positively regulates transcription of the phosphatidylinositol-specific phospholipase C gene at the onset of the stationary phase. J. Bacteriol. 178:2749–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 37. López D., Fischbach M. A., Chu F., Losick R., Kolter R. 2009. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 106:280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mascher T., Zimmer S. L., Smith T. A., Helmann J. D. 2004. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis. Antimicrob. Agents Chemother. 48:2888–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., vol. 20, no. 2. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 40. Oosthuizen M. C., et al. 2002. Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Appl. Environ. Microbiol. 68:2770–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Overhage J., Bains M., Brazas M. D., Hancock R. E. 2008. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 190:2671–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearson M. M., Rasko D. A., Smith S. N., Mobley H. L. 2010. Transcriptome of swarming Proteus mirabilis. Infect. Immun. 78:2834–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pietiäinen M., et al. 2005. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151:1577–1592 [DOI] [PubMed] [Google Scholar]
- 44. Rasko D. A., Altherr M. R., Han C. S., Ravel J. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29:303–329 [DOI] [PubMed] [Google Scholar]
- 45. Rather P. N. 2005. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 7:1065–1073 [DOI] [PubMed] [Google Scholar]
- 46. Salvetti S., Celandroni F., Ceragioli M., Senesi S., Ghelardi E. 2009. Identification of non flagellar genes involved in swarm-cell differentiation using a Bacillus thuringiensis mini-Tn10 mutant library. Microbiology 155:912–921 [DOI] [PubMed] [Google Scholar]
- 47. Senesi S., et al. 2002. Swarming motility in Bacillus cereus and characterization of a fliY mutant impaired in swarm cell differentiation. Microbiology 148:1785–1794 [DOI] [PubMed] [Google Scholar]
- 48. Senesi S., Salvetti S., Celandroni F., Ghelardi E. 2010. Features of Bacillus cereus swarm cells. Res. Microbiol. 161:743–749 [DOI] [PubMed] [Google Scholar]
- 49. Stenfors Arnesen L. P., Fagerlund A., Granum P. E. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 50. Stragier P., Bonamy C., Karmazyn-Campelli C. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697–704 [DOI] [PubMed] [Google Scholar]
- 51. Tatusov R. L., Koonin E. V., Lipman D. J. 1997. A genomic perspective on protein families. Science 278:631–637 [DOI] [PubMed] [Google Scholar]
- 52. Treuner-Lange A., Kuhn A., Dürre P. 1997. The kdp system of Clostridium acetobutylicum: cloning, sequencing, and transcriptional regulation in response to potassium concentration. J. Bacteriol. 179:4501–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verstraeten N., et al. 2008. Living on a surface: swarming and biofilm formation. Trends Microbiol. 16:496–506 [DOI] [PubMed] [Google Scholar]
- 54. Walderhaug M. O., et al. 1992. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J. Bacteriol. 174:2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Q., Frye J. G., McClelland M., Harshey R. M. 2004. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52:169–187 [DOI] [PubMed] [Google Scholar]
- 56. Whiteley M., et al. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864 [DOI] [PubMed] [Google Scholar]
- 57. Yeung A. T., et al. 2009. Swarming of Pseudomonas aeruginosa is controlled by a broad spectrum of transcriptional regulators, including MetR. J. Bacteriol. 191:5592–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang R., et al. 2006. Structure of phage protein BC1872 from Bacillus cereus, a singleton with new fold. Proteins 64:280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.