Abstract
Alkaline phosphatases (APases) are important enzymes in organophosphate utilization. Three prokaryotic APase gene families, PhoA, PhoX, and PhoD, are known; however, their functional characterization in cyanobacteria largely remains to be clarified. In this study, we cloned the phoD gene from a halotolerant cyanobacterium, Aphanothece halophytica (phoDAp). The deduced protein, PhoDAp, contains Tat consensus motifs and a peptidase cleavage site at the N terminus. The PhoDAp enzyme was activated by Ca2+ and exhibited APase and phosphodiesterase (APDase) activities. Subcellular localization experiments revealed the secretion and processing of PhoDAp in a transformed cyanobacterium. Expression of the phoDAp gene in A. halophytica cells was upregulated not only by phosphorus (P) starvation but also under salt stress conditions. Our results suggest that A. halophytica cells possess a PhoD that participates in the assimilation of P under salinity stress.
INTRODUCTION
Phosphorus (P) is an essential nonmetal nutrient for all living cells. Despite its relative abundance in the ecosystem, P is sometimes a limiting factor for organisms in terrestrial, oceanic, and freshwater environments (1, 11). This is because while all organisms can utilize soluble inorganic phosphate (Pi), the P-containing organic compounds generally found in nature are complex insoluble forms that are not readily available to cells (1, 11). Alkaline phosphatases (APases) release free Pi from organic compounds. To date, three prokaryotic APase gene families—PhoA, PhoX, and PhoD—have been documented (7). In addition to different levels of homology among these APases, dissimilar metal requirements for their activities have been reported (7). A recent metagenomics analysis revealed that PhoX, a recently characterized phosphatase, is more abundant in marine bacteria than the previously considered classical PhoA (12). However, it has also been shown that PhoD is more abundant in marine bacteria than PhoA or PhoX (7), suggesting an important role for PhoD in marine bacteria. PhoD encompasses a family of phosphatase/phosphodiesterases (APase/APDase). Except for Bacillus subtilis PhoD (PhoDBs) (3), little is known on other PhoD proteins. PhoDBs was shown to be secreted into extracellular medium by the Tat pathway, which recognizes targeted proteins by their N-terminal twin-arginine signal peptides containing the Tat consensus (SRRXFLK) motif (3, 9).
Cyanobacteria inhabit a broad range of ecosystems and play a vital role in the global cycling of nutrients, including P. Hitherto, extensive studies on the regulation of gene expression during phosphate stress have been carried out (14). In contrast, functional properties of cyanobacterial APases have been reported only on classical PhoA (8), atypical APase (11), PhoV (21), and PhoX (5), and there is no report on the cyanobacterial PhoD. Moreover, little is known on the role of APase under high-salinity conditions, and this encouraged us to examine the role of cyanobacterial PhoD under high-salinity conditions.
Aphanothece halophytica is a halotolerant cyanobacterium isolated from the Dead Sea and can grow in a wide range of salinity conditions (0.25 to 3.0 M NaCl), as well as at alkaline pH (17). Previous studies have shown that A. halophytica has a unique biosynthetic pathway of the osmoprotectant betaine, three-step methylation of glycine (19) and novel Na+/H+ antiporters NhaP1Ap, NapA1-1Ap, NapA1-2Ap, and MrpAp (4, 18, 23). In the present study we investigated the presence of novel APases in A. halophytica and report a gene homologous to PhoDBs, termed PhoDAp. Our data reveal that PhoDAp is a secreted APase/APDase and is activated by Ca2+. Finally, we demonstrate the salt-inducible expression of phoDAp, suggesting that PhoDAp is involved in P assimilation under salinity stress.
MATERIALS AND METHODS
Culture conditions for cyanobacteria.
The cyanobacteria Synechococcus elongatus PCC7942 and A. halophytica were grown photoautotrophically in BG11 alone and in BG11 plus Turk Island salt solution containing 0.5 M NaCl, respectively, as described previously (20), under continuous cool white fluorescent light intensity of 70 μE m−2 s−1 at 30°C. For P starvation, A. halophytica cells collected by centrifugation were resuspended into the medium in which KH2PO4 was replaced with KCl (0.18 mM). For salinity stress, collected cells were resuspended into the medium containing 2.5 M NaCl.
Fractionation of the cyanobacterial cells.
In order to study the subcellular localization of PhoDAp, extracellular, cell-bound, periplasmic, and total soluble fractions from A. halophytica cells were prepared. To obtain the total soluble fraction, cyanobacterial cells (50 ml, A730 = 0.8) were harvested and resuspended in 1 ml of cold extraction buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2 [pH 10]). The suspended cells were lysed by sonication and centrifuged at 2,290 × g for 10 min at 4°C, followed by centrifugation twice at 22,000 × g for 30 min at 4°C. The resulting supernatant was used as soluble fraction, from which 20-μl aliquots were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 10% polyacrylamide gel. Proteins transferred onto polyvinylidene difluoride membranes and subjected to Western blot analysis. Pellets representing the total membrane fraction were also subjected to Western blot analysis; pellets were dissolved in 500 μl of SDS sample buffer, and then 10-μl aliquots were separated by SDS-PAGE. The periplasmic fraction of these cyanobacterial cells was obtained as described earlier (1), except that the pH of the buffer was maintained at 10. Approximately 1 ml of periplasmic fractions was obtained; 20 μl of the aliquot was subjected to Western blot analysis.
For analyzing the proteins present in extracellular medium, 50 ml of the cyanobacterial cell culture was centrifuged at 2,290 × g for 5 min at 4°C. The cell-free supernatant was filtered through a 0.22-μm-pore-size membrane (Millipore, Billerica, MA), and the resulting solution was concentrated by using YM-3 membrane (Millipore) to ∼1 ml, 30 μl of which was used for measurement of the APase/APDase activity or for Western blot analysis.
Expression of PhoDAp in Escherichia coli and its purification.
The coding region of phoDAp was amplified from the genomic DNA of A. halophytica by PCR using specific primers (see Table S1 in the supplemental material). PCR products were subcloned into vector pCR2.1 (Invitrogen, California) and digested with NcoI and SalI. The resulting fragments were ligated into the digested NcoI and SalI sites of pTrcHis2C (Invitrogen). The generated plasmid, pTrcHis2C-ApphoD, was transferred to E. coli BL21. Transformed cells were grown in 3 ml of LB medium at 37°C for 4.5 h, and the culture was transferred to 1 liter of LB medium and subcultured at 37°C for 3 h. IPTG (isopropyl-β-d-thiogalactopyranoside) was added at a final concentration of 0.5 mM, and the culture was allowed to grow for a further 3 h. The cells were harvested and subjected to affinity purification by using an Ni-NTA spin kit column (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The eluate containing the protein of interest was subjected to Superdex 75 gel filtration column chromatography using a 50 mM Tris-HCl (pH 8.0) buffer containing 100 mM NaCl and 0.1 mM CaCl2. PhoDAp containing fractions were concentrated using an YM-3 membrane and subjected to anion-exchange chromatography (Resource Q; Amersham, Little Chalfont, United Kingdom) using a buffer containing 50 mM Tris-HCl (pH 10.0) and 0.1 mM CaCl2. PhoDAp was eluted using a 0 to 100% NaCl gradient. All purification steps were carried out at 4°C. Gel filtration and anion-exchange chromatography was carried out using an AKTA prime liquid chromatography system (GE Healthcare Life Science, Little Chalfont, United Kingdom).
Measurement of APase/APDase activities.
The bacterial APases identified thus far are metalloenzymes requiring specific metal ions, i.e., Mg2+, Zn2+, and Ca2+, for their activities (7). We therefore examined the metal requirement for the APase/APDase activity of PhoDAp-His6. Portions (20 μl) of fractionated solutions from cyanobacterial cells or purified recombinant PhoDAp was mixed with 40 μl of 50 mM Tris-HCl (pH 10.0) containing various concentrations of metal and p-nitrophenyl (pNP) phosphate (pNPP) or bis-pNPP at a final concentration of 13.5 μM. The mixture was incubated at 37°C for 20 min. The reaction was stopped by the addition of 240 μl of 13% KH2PO4, and the absorbance of pNP was measured at 400 nm. Enzyme activity (expressed as nmol min−1 mg of protein−1) was calculated by using the molar extinction coefficient (ε), i.e., 18,900 M−1 cm−1 for pNP at 400 nm (2). The total protein concentration in the sample solution was measured by the Bradford method (19).
For kinetic studies of recombinant PhoDAp purified from E. coli, the same methods were used except that various amounts of substrates in the reaction mixtures were used (i.e., 0.43, 0.85, 1.7, 3.4, 6.8, and 13.5 μM). The concentration of the PhoDAp protein in the reaction mixture was kept constant at 8.3 μg/ml.
Reverse transcriptase PCR (RT-PCR) analysis.
A. halophytica cells (25 ml) were cultured to achieve a turbidity (A730) of 0.8. The cells were harvested and resuspended in 500 μl of extraction buffer (100 mM Tris-HCl, 10 mM EDTA, 100 mM LiCl, 1% SDS [pH 8.0]). After the addition of 500 μl of Tris-buffered phenol, the mixture was incubated on ice for 5 h, followed by centrifugation at 10,000 × g for 30 min at 4°C. To the aqueous fraction, 250 μl of 8 M LiCl was added, followed by incubation on ice overnight. The supernatant was removed after centrifugation at 10,000 × g for 1 h at 4°C. The resulting RNA precipitation was solubilized in sterile distilled water and re-extracted with phenol-chloroform.
The cDNA was synthesized from 1 μg of total RNA by using RT M-MLV (Takara, Otsu, Japan). Portions (20 μl) of synthesized cDNA were diluted two times with double-distilled H2O, and 2-μl aliquots were subjected to PCR in a reaction volume of 50 μl. Specific primer sets (see Table S1 in the supplemental material) were at a final concentration of 0.4 μM in the PCR mixture. RT-PCR was initiated with AmpliTaq Gold (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. The PCR conditions used were as follows: denaturation at 95°C for 3 min and then 27 amplification cycles of denaturation for 15 s at 95°C, annealing for 30 s at 55°C, and extension for 45 s at 72°C. The resulting reaction mixtures were subjected to 1% agarose gel electrophoresis and visualized by staining with ethidium bromide. The intensity of the PCE product as a clear band on the gel was quantified by ImageJ software and was normalized by setting the signals from nonstressed cells to 1.0.
Expression of PhoDAp in the cyanobacterium Synechococcus elongatus.
The region containing both the trc promoter and the coding region of phoDAp was amplified from pTrcHis2C-ApphoD by PCR using a specific primer set (see Table S1 in the supplemental material). PCR products were subcloned into vector pCR2.1 and digested with XhoI. The resulting fragments were ligated into XhoI-digested pUC303, which can replicate independently in S. elongatus cells (6). Ten micrograms of generated plasmid was added to 1 ml of S. elongatus culture (optical density at 730 nm of 0.5), followed by culture with shaking for 12 h in the dark. Transformants were selected using streptomycin (25 μg/ml) on 1.5% agar BG11 medium.
Measurement of cellular P contents.
A. halophytica cells grown at 0.5 M NaCl were harvested by centrifugation and suspended in medium containing 0.5 or 2.5 M NaCl. After 6 h growth, the cells were collected and washed twice with water. The cells were dried by vacuum evaporation at 65°C, followed digestion in a boiling mixture of nitric acid (HNO3) and sulfuric acid (H2SO4) (5:1), and incubated at ambient temperature for 10 min with H2SO4, antimony potassium tartrate, ammonium molybdate, and ascorbic acid at final concentrations of 0.47 M, 75 μM, 1.7 mM, and 11 mM, respectively. The mixture was centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was used for measurement of the cellular P contents in A. halophytica cells using the absorption at 880 nm.
Other methods.
The nucleotide sequences were determined by using an ABI310 genetic analyzer (Applied Biosystems, Foster City, CA). The protein concentration was measured by using the Bradford method. SDS-PAGE and Western blot analysis were carried out as described previously (23). Antibody-raised against a His6 (hexahistidine) tag was obtained from R&D Systems (Minneapolis, MN). For the phylogenetic analysis, CLUSTAL W (15) was used to generate a phylogenetic tree of amino acid sequences of bacterial APases/ADPases.
Nucleotide sequence accession numbers.
Nucleotide sequence data for phoA1Ap, phoA2Ap, and phoDAp from A. halophytica are available in the DDJB database under accession numbers AB602344, AB602345, and AB602343, respectively.
RESULTS
APase/APDase activity in A. halophytica.
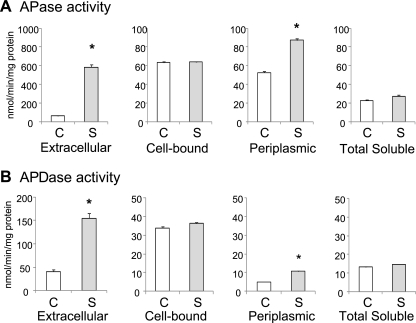
In a previous study (10), we reported that under conditions of high salinity, the intracellular P content and P uptake in Anabaena doliolum cells decreased, whereas the extracellular APase activity increased. Similar results were obtained with A. halophytica cells (data not shown). Therefore, we first investigated the effect of salinity stress on APase and APDase activities of A. halophytica cells (Fig. 1). To do so, we prepared extracellular, cell-bound, periplasmic, and total soluble fractions from A. halophytica cells exposed to salinity stress for 6 h and measured the APase and APDase activities by using pNPP and bis-pNPP, respectively, as substrates. Exposure of A. halophytica to salinity stress enhanced the extracellular and periplasmic APase activities by 9- and 1.7-fold, respectively (Fig. 1A). Salinity stress also enhanced the extracellular and periplasmic APDase activities by 3.8- and 2.1-fold, respectively (Fig. 1B). These results indicated that the extracellular APase and APDase activities of A. halophytica cells increased under high-salinity conditions.
Fig. 1.
APase (A) and APDase (B) activity in A. halophytica cells. A. halophytica cells grown in nutrient solution containing 0.5 M NaCl were transferred to medium containing 0.5 M NaCl (C columns) or 2.5 M NaCl (S columns) and incubated under standard growth conditions for 6 h. The APase and APDase activities of concentrated extracellular medium, whole cells (cell-bound), periplasmic fractions, and soluble protein extract were determined. Each value is an average of three independent measurements. Means ± the standard errors of the mean (SEM) are shown (n = 3). An asterisk indicates significant difference (P < 0.05) from the control (C).
Phylogenetic analysis of A. halophytica APases.
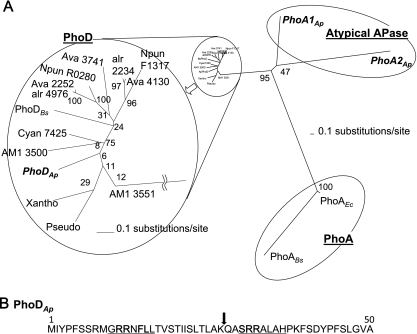
From shotgun sequencing data, several putative APase genes were found in the A. halophytica genome. In Fig. 2A, three genes, phoA1Ap, phoA2Ap, and phoDAp, are shown. PhoA1Ap and PhoA2Ap do not belong to the PhoA family and thus could be classified as atypical APases. However, we found that phoDAp encodes a protein homologous to the Bacillus PhoD protein. phoDAp encodes 526 amino acid residues. The genes homologous to phoDAp were found in several cyanobacteria, including Bacillus, Pseudomonas, and Xanthomonas spp., but not in many whole-genome-sequenced cyanobacteria such as Synechocystis sp. strain PCC6803, Synechococcus elongatus PCC7942, and Prochlorococcus spp. Among the 14 PhoD proteins shown in Fig. 2A, PhoDAp exhibited the highest homology with Acaryochloris marina MBIC11017 (AM1_3551, 56% identity), Cyanothece sp. strain PCC7425 (53% identity), Acaryochloris marina MBIC11017 (AM1_3550, 52% identity), and then other cyanobacteria (47 to 49% identity). The identities to PhoD from Bacillus, Pseudomonas, and Xanthomonas spp. ranged between 44 and 45%. Interestingly, three putative PhoD genes were found in Anabaena variabilis ATCC 29413, but only two genes were found in Anabaena sp. strain PCC7120, Acaryochloris marina MBIC11017, and Nostoc punctiforme PCC73102. One putative PhoD gene was found in A. halophytica, Cyanothece sp. strain PCC7425, Pseudomonas syringae pv. glycinea strain B076, Xanthomonas campestris, and Bacillus licheniformis ATCC 14580. Among the 14 PhoD proteins shown in Fig. 2A, 12 PhoD proteins contain 520 to 540 amino acid residues, whereas Acaryochloris PhoD (AM1_3551) contains 509 amino acid residues (due to the missing N-terminal region), and Bacillus PhoD contains 583 amino acids (due to the extra C-terminal amino acid residues). These data indicate that the homology of PhoD is highly conserved among several bacterial species. Although the distribution of PhoD in cyanobacteria is restricted to certain species, there is no tendency of distribution among them (e.g., freshwater and ocean, unicellular and filamentous, or diazotrophic and nondiazotrophic).
Fig. 2.
(A) Phylogenetic analysis of APases. A radial tree of the APases shows PhoA1Ap (accession number, AB602344), PhoA2Ap (AB602345), and PhoDAp (AB602343) from A. halophytica; AM1 3550 (AM1_3550) and AM1 3551(AM1_3551) from Acaryochloris marina MBIC11017, Cyan7425 (Cyan7425_1386) from Cyanothece sp. strain PCC 7425, Ava 2252 (Ava_2252), Ava 3741 (Ava_3741), and Ava 4130 (Ava_4130) from Anabaena variabilis ATCC 29413, alr 2234 (alr2234) and alr 4976 (alr4976) from Anabaena sp. strain PCC 7120, Npun R0280 (Npun_R0280) and Npun F1317 (Npun_F1317) from Nostoc punctiforme PCC 73102, Pseudo (EFW82021) from Pseudomonas syringae pv. glycinea strain B076, Xantho (YP_001905643) from Xanthomonas campestris, PhoABs (BSU09410) and PhoDBs (BSU02620) from Bacillus subtilis, and PhoAEc (b0383 JW0374) from Escherichia coli. The bars represent evolutionally distance. The scale bar is 0.1 expected changes per amino acid site. The reliability of the tree was measured by bootstrap analysis with 1,000 replicates. The branches of the PhoD proteins were magnified for clarity. (B) Predicted signal peptides for PhoDAp. Underlined text denotes Tat consensus motifs. An arrow at the position between 26 and 27 denotes the peptide cleavage site as predicted by SignalP, version 3.0.
All of the PhoD proteins shown in Fig. 2A, except for Acaryochloris PhoD (AM1_3551), contain the predicted Tat consensus motif and peptidase cleavage site in the N-terminal region (data not shown). We speculate that the PhoD proteins are secreted proteins. Interestingly, PhoDAp contains two Tat consensus motifs (Fig. 2B). However, the physiological role of two Tat consensus motifs remains to be clarified. In contrast, PhoA1Ap and PhoA2Ap do not contain the deduced signal peptide (data not shown). These findings prompted us to further investigate molecular mechanisms in the regulation of phoDAp.
Enzymatic characterization of PhoDAp.
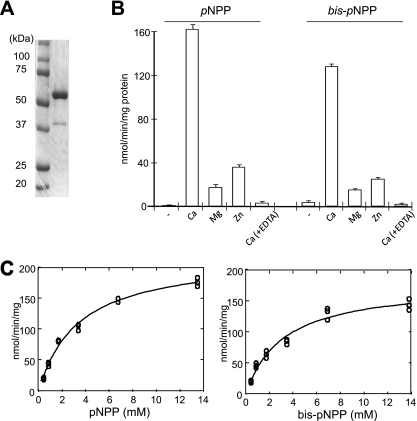
To characterize the enzymatic properties of PhoDAp, we purified recombinant PhoDAp with a His6 tag fused to its C-terminal tail. SDS-PAGE analysis revealed a protein band of ∼60 kDa, which matched well the calculated molecular mass, 61.21 kDa, of PhoDAp-His6 (Fig. 3A).
Fig. 3.
Characterization of PhoDAp. (A) Purified PhoDAp-His6 was subjected to SDS-PAGE and stained with Coomassie brilliant blue. (B) Effect of metal ions on APase/APDase activity of PhoDAp-His6. Activity was measured with each metal ion and EDTA at a final concentration of 5 mM. Each value represents an average of three independent measurements. Means ± the SEM are shown. (C) Determination of kinetic values for APase and APDase activities of PhoDAp. The values of kinetic constants were computed using the freeware program SIMFIT (http://www.simfit.man.ac.uk).
Next, we examined the metal requirement for APase/ APDase activity of PhoDAp-His6 (Fig. 3B). The results revealed that Ca2+ was required to obtain the maximal activity. The addition of EDTA strongly inhibited the enzyme activity probably by chelating Ca2+. A requirement for Ca2+ has been reported for the activity of PhoD from Bacillus (3), PhoX from Pasteurella multocida strain X73 (22), and cyanobacteria (5), but not for PhoA. In the presence of Ca2+ and at pH 10.0, the Km values for pNPP and bis-pNPP were 3.38 and 3.13 mM, respectively, while the Vmax values were 219 and 178 nmol min−1 mg of protein−1 (Fig. 3C). These results indicated that PhoDAp is a Ca2+-activated APase/APDase.
Expression of A. halophytica APases is upregulated upon salinity stress.
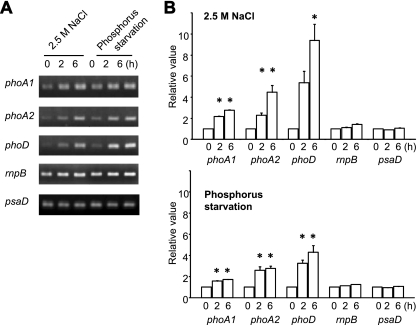
As a next step, we examined the effects of salt on APases gene expression by RT-PCR analysis. As shown in Fig. 4, the expression of phoA1Ap, phoA2Ap, and phoDAp was induced by 2.8-, 4.5-, and 9.4-fold, respectively, after a 6-h exposure of A. halophytica to salinity stress. On the other hand, the mRNA expression level of rnpB (encoding ribonucleoprotein) and psaA (photosystem I) did not change. We also observed that phosphate starvation of A. halophytica cells for 6 h upregulated the expression of phoA1Ap, phoA2Ap, and phoDAp to 1.7-, 2.8-, and 4.2-fold, respectively. These results indicated that the levels of mRNA for putative APases, i.e., phoA1Ap, phoA2Ap, and phoDAp, increase upon salt stress, as well as upon phosphate depletion. It should also be mentioned that the induction of phoDAp gene expression was much higher those that of phoA1Ap and phoA2Ap.
Fig. 4.
Expression of APase genes in A. halophytica cells. Cells were collected at 0, 2, and 6 h of exposure to salinity stress or P starvation. RT-PCR analysis was performed as described in Materials and Methods. (A) PCR products were subjected to electrophoresis. (B) Relative values of the amount of DNA fragments. RNase P (rnpB) and photosystem I subunit II (psaD) genes were used as controls whose mRNA abundance remained unchanged under salinity stress or P starvation. The values at time zero of each gene were set to 1. Each value represents an average of three independent measurements. Means ± the SEM are shown. Asterisks indicate significant difference (P < 0.05) from the values at time zero.
Secretion ability of PhoDAp in cyanobacteria.
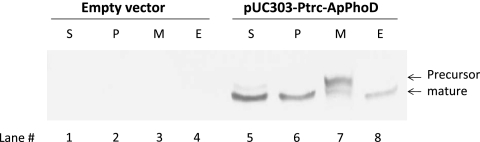
It has been reported that in B. subtilis, PhoDBs is secreted into the extracellular medium via the Tat pathway (9). Therefore, we also examined whether PhoDAp is secreted to the extracellular medium. Since transformation of A. halophytica was unsuccessful, a transformant of S. elongatus that overexpresses PhoDAp-His6 was prepared. Transformed cells were fractionated, and the proteins were analyzed by Western blotting (Fig. 5). The transformed S. elongates cells possessing empty vector (pUC303) did not show any specific band (lanes 1 to 4). In contrast, two forms of PhoDAp were observed (lanes 5 and 7) in total soluble and membrane fractions of transformed cells overexpressing PhoDAp. The presence of two bands might be explained as a full-length form (upper, precursor) and a truncated mature form that lacked the signal peptide (lower, mature). Conversely, PhoDAp was detected as a single mature form in the periplasmic and extracellular portions (lanes 6 and 8). Thus, in S. elongatus cells, PhoDAp is presumably translocated from the cytoplasm to the periplasm and extracellular medium via the inner membrane using the Tat pathway.
Fig. 5.
Secretion of PhoDAp. PhoDAp-expressing cells grown with 1 mM IPTG for 24 h were separated into total soluble (S), periplasmic (P), total membrane (M), and extracellular (E) fractions and subjected to Western blot analysis (lanes 5 to 8). Arrows indicate predicted precursor and mature PhoDAp. S. elongatus with empty vector, pUC303, was used as a control (lanes 1 to 4).
DISCUSSION
Hitherto, functional characterization of PhoD has been reported only on PhoDBs. This study is the first report on cyanobacterial PhoD. Our results have revealed that A. halophytica contains a gene encoding a protein homologous to PhoDBs (Fig. 2A). PhoDAp exhibited hydrolysis activity not only for a monoester (APase activity) but also for a diester (APDase activity), and Ca2+ was required for their activities (Fig. 3). Moreover, it was found that PhoDAp contains the Tat motif at the N-terminal region (Fig. 2B). The results of transformed S. elongatus cells supported the secretion ability of PhoDAp in A. halophytica cells (Fig. 5). We cannot rule out the possibility that PhoDAp might have been detected in extracellular medium after cell lysis. However, we could not detect chlorophyll a derived from S. elongatus cell lysis in the extracellular medium (data not shown). Moreover, the cross-reacting band from proteins in the extracellular medium in Fig. 5 support the secretion ability of PhoDAp in cyanobacteria because this signal was obtained as a clear single predicted mature protein band of PhoDAp lacking the signal peptide (see Fig. 5, lane 8). If this signal was derived from cell lysis, both mature and precursor signals should have appeared in this lane. All of these data indicate that A. halophytica contains the PhoD protein that is secreted via the Tat pathway and exhibits the Ca2+-dependent APase and ADPase activities.
One of the most interesting findings of the present study is that the expression of phoA1Ap, phoA2Ap, and phoDAp genes was induced under salinity stress conditions (Fig. 4). The induction of these genes under salinity stress was much higher than those of P starvation. Moreover, induction of phoDAp was higher than that of phoA1Ap and phoA2Ap, suggesting an important role for PhoDAp in the periplasmic and extracellular space of A. halophytica cells under salt stress (Fig. 1).
As was the case in Anabaena doliolum cells, the cellular P content in A. halophytica cells also decreased under salt stress conditions. In this context, we make the following argument. Salt stress triggers the decrease of the intracellular P level, which causes the upregulation of many genes under Pho regulation, such as phoA1Ap, phoA2Ap, and phoDAp, and phosphate uptake. Indeed, we identified two putative Pho boxes (TTACCC and TTAAGC) in the promoter region of phoDAp (data not shown) (13). Upregulation of phoDAp (Fig. 4) could promote the utilization of extracellular organic compounds as a P source. However, the direct effects of salt stress on the induction of Pho-regulated genes cannot be eliminated such as in the case of isiA, in which both low Fe and high salt levels induce the transcription of the isiA gene (16).
The detailed molecular mechanisms of PhoDAp secretion and its regulatory mechanism would be very interesting subjects for future investigations.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by the Salt Science Research Foundation; Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan; and the High-Tech Research Center of Meijo University.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Block M. A., Grossman A. R. 1988. Identification and purification of a derepressible alkaline phosphatase from Anacystis nidulans R2. Plant Physiol. 86:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coiffier A., Coradin T., Roux C., Bouvet O. M. M., Livage J. 2001. Sol-gel encapsulation of bacteria: a comparison between alkoxide and aqueous routes. J. Mater. Chem. 11:2039–2044 [Google Scholar]
- 3. Eder S., Shi L., Jensen K., Yamane K., Hulett F. M. 1996. A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology 142:2041–2047 [DOI] [PubMed] [Google Scholar]
- 4. Fukaya F., et al. 2009. An Mrp-like cluster in the halotolerant cyanobacterium Aphanothece halophytica functions as an Na+/H+ antiporter. Appl. Environ. Microbiol. 75:6626–6629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kathuria S., Martiny A. C. 2011. Prevalence of a calcium-based alkaline phosphatase associated with the marine cyanobacterium Prochlorococcus and other ocean bacteria. Environ. Microbiol. 13:74–83 [DOI] [PubMed] [Google Scholar]
- 6. Kuhlemeier C. J., et al. 1983. A host-vector system for gene cloning in the cyanobacterium Anacystis nidulans R2. Plasmid 10:156–163 [DOI] [PubMed] [Google Scholar]
- 7. Luo H., Benner R., Long R. A., Hu J. 2009. Subcellular localization of marine bacterial alkaline phosphatases. Proc. Natl. Acad. Sci. U. S. A. 106:21219–21223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo M., et al. 2010. Characterization of a monomeric heat-labile classical alkaline phosphatase from Anabaena sp. PCC7120. Biochemistry (Mosc.) 75:655–664 [DOI] [PubMed] [Google Scholar]
- 9. Pop O., Martin U., Abel C., Müller J. P. 2002. The twin-arginine signal peptide of PhoD and the TatAd/Cd proteins of Bacillus subtilis form an autonomous Tat translocation system. J. Biol. Chem. 277:3268–3273 [DOI] [PubMed] [Google Scholar]
- 10. Rai A. K., Sharma N. K. 2006. Phosphate metabolism in the cyanobacterium Anabaena doliolum under salt stress. Curr. Microbiol. 52:6–12 [DOI] [PubMed] [Google Scholar]
- 11. Ray J. M., Bhaya D., Block M. A., Grossman A. R. 1991. Isolation, transcription, and inactivation of the gene for an atypical alkaline phosphatase of Synechococcus sp. strain PCC 7942. J. Bacteriol. 173:4297–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sebastian M., Ammerman J. W. 2009. The alkaline phosphatase PhoX is more widely distributed in marine bacteria than the classical PhoA. ISME J. 3:563–572 [DOI] [PubMed] [Google Scholar]
- 13. Su Z., Olman V., Xu Y. 2007. Computational prediction of Pho regulons in cyanobacteria. BMC Genomics 8:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tetu S. G., et al. 2009. Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. ISME J. 3:835–849 [DOI] [PubMed] [Google Scholar]
- 15. Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vinnemeier J., Kunert A., Hagemann M. 1998. Transcriptional analysis of the isiAB operon in salt-stressed cells of the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Let. 169:323–330 [DOI] [PubMed] [Google Scholar]
- 17. Waditee R., et al. 2001. Halotolerant cyanobacterium Aphanothece halophytica contains an Na+/H+ antiporter, homologous to eukaryotic ones, with novel ion specificity affected by C-terminal tail. J. Biol. Chem. 276:36931–36998 [DOI] [PubMed] [Google Scholar]
- 18. Waditee R., Hibino T., Nakamura T., Incharoensakdi A., Takabe T. 2002. Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proc. Natl. Acad. Sci. U. S. A. 99:4109–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waditee R., et al. 2003. Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica. J. Biol. Chem. 278:4932–4942 [DOI] [PubMed] [Google Scholar]
- 20. Waditee R., et al. 2005. Genes for direct methylation of glycine provide high levels of glycine betaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 102:1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner K. U., Masepohl B., Pistorius E. K. 1995. The cyanobacterium Synechococcus sp. strain PCC7942 contains a second alkaline phosphatase encoded by phoV. Microbiology 141:3049–3058 [DOI] [PubMed] [Google Scholar]
- 22. Wu J. R., et al. 2007. Cloning of the gene and characterization of the enzymatic properties of the monomeric alkaline phosphatase (PhoX) from Pasteurella multocida strain X-73. FEMS Microbiol. Lett. 267:113–120 [DOI] [PubMed] [Google Scholar]
- 23. Wutipraditkul N., et al. 2005. Halotolerant cyanobacterium Aphanothece halophytica contains NapA-type Na+/H+ antiporters with novel ion specificity that are involved in salt tolerance at alkaline pH. Appl. Environ. Microbiol. 71:4176–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.