Abstract
The aim of this work was to develop an approach for chromosomal engineering of the thermophile Rhodothermus marinus. A selection strategy for R. marinus had previously been developed; this strategy was based on complementing a restriction-negative trpB strain with the R. marinus trpB gene. The current work identified an additional selective marker, purA, which encodes adenylosuccinate synthase and confers adenine prototrophy. In a two-step procedure, the available Trp+ selection was used during the deletion of purA from the R. marinus chromosome. The alternative Ade+ selection was in turn used while deleting the endogenous trpB gene. Since both deletions are unmarked, the purA and trpB markers may be reused. Through the double deletant SB-62 (ΔtrpB ΔpurA), the difficulties that are associated with spontaneous revertants and unintended chromosomal integration of marker-containing molecules are circumvented. The selection efficiency in R. marinus strain SB-62 (ΔtrpB ΔpurA) was demonstrated by targeting putative carotenoid biosynthesis genes, crtBI, using a linear molecule containing a marked deletion with 717 and 810 bp of 5′ and 3′ homologous sequences, respectively. The resulting Trp+ transformants were colorless rather than orange-red. The correct replacement of an internal crtBI fragment with the trpB marker was confirmed by Southern hybridization analysis of the transformants. Thus, it appears that target genes in the R. marinus chromosome can be readily replaced with linear molecules in a single step by double-crossover recombination.
INTRODUCTION
Rhodothermus marinus is an aerobic, thermophilic bacterium that was originally isolated from an intertidal hot spring in Isafjardardjup in northwestern Iceland (1). This species is of interest because of its phylogenetic position, adaptation to its natural environment, and diverse inherent enzyme activity (4). Of particular interest is its ability to degrade biomass, including cellulose and hemicellulose polymers. This makes R. marinus an attractive candidate for being metabolically engineered as a biocatalyst for the production of valuable chemicals from polysaccharides.
A challenging aspect of our efforts to develop genetic manipulation methods for R. marinus was to find a suitable selective marker. This species belongs to the proposed family “Rhodothermaceae” of the phylum Bacteroidetes (13) and has no close relatives for which genetic methodologies have been developed. The thermoadapted kanamycin resistance determinant frequently used for selection in thermophiles (11, 24) is unsuitable for R. marinus because of its natural resistance to aminoglycosides (1). Therefore, an endogenous marker was chosen, the R. marinus trpB gene. While it was being expressed from the R. marinus groESL control region, this gene complemented a point mutation in the recipient strain SB-1 (trpB1), a derivative of the restriction-negative isolate PRI 493 (5). This selection strategy not only permitted the development of an optimized transformation protocol, which yielded up to 1 × 107 Trp+ transformants per μg of DNA; it also permitted the construction of cloning vectors and studies of expression from R. marinus promoters in vivo (3, 5). Neither the spontaneous reversion of the trpB1 mutation nor recombination between the trpB marker and the mutated allele on the chromosome significantly affected the cloning experiments. This was due to high transformation efficiency and segregational stability of the vectors. However, a background of Trp+ colonies would present a major drawback when attempting to detect rare events, such as gene replacements.
The aim of this work was to develop an approach for chromosomal engineering, which would along with the recent completion of sequencing the R. marinus genome (17) open the prospect of performing functional genetic studies in vivo. First, we wanted to identify an additional marker that could be used along with Trp+ selection. Second, we wanted to improve genetic selection in R. marinus by deleting sequences corresponding to the markers from the chromosome. Unmarked deletions can be introduced into prokaryotic chromosomes by a two-step procedure (12, 14, 19). In this procedure, a nonreplicating plasmid, which contains sequences flanking a deletion, is introduced into the host, and a plasmid-encoded marker allows direct selection for its integration into the chromosome. This single-crossover event produces chromosomal duplications, which are segregated by further recombination events that leave either the wild-type gene or a deleted copy of the target gene on the chromosome. Subsequently, segregants can be identified by screening for the loss of the plasmid-encoded marker (7, 8). A single selective marker thus suffices for constructing an unmarked deletion, although often a second marker, usually a counterselectable one like the Bacillus subtilis sacB gene, is included in the deletion plasmid (14, 20). Since our attempts to establish a counterselectable marker system for R. marinus had failed, our strategy was to use the trpB gene while deleting a second selectable marker gene from the R. marinus chromosome. The second marker, purA, was in turn used for isolating trpB deletants. Eventually, the improved selection was used for targeting deletions in other R. marinus genes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. The R. marinus strains were grown at 65°C in medium 162 (6), which contained 1% NaCl and 0.053% NH4Cl. The medium also contained either 0.25% tryptone and 0.25% yeast extract (nonselective medium) or 0.2% soluble starch, 0.2% Casamino Acids, and a vitamin mixture (6) (selective medium). Both media were solidified with 2.5% agar (Becton Dickinson, Franklin Lakes, NJ). Tryptophan, indole, and adenine (12.5 to 25 μg/ml) were added when appropriate.
Table 1.
Bacterial strains and plasmids used in this work
| Bacterial strain or plasmid | Characteristicsa | Source and/or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli SDH-1 | Trp−, E. coli DH5α derivative (trpB) | 3 |
| R. marinus | ||
| PRI 376 | wt strain; DSM 4252T | Matís; complete genomic sequence in GenBank |
| PRI 378 | wt strain; DSM 4253 | Matís; partial genomic sequence available at Matís |
| PRI 493 | wt strain; tested negative for restriction activity | Matís; 5 |
| SB-1 | Trp−, PRI 493 derivative (trpB1) | Matís; 5 |
| SB-24 | Trp+ Ade−, SB-1 derivative (trpB1 ΔpurA::trpB) | This work |
| SB-32 | Trp− Ade−, SB-24 derivative (trpB1 ΔpurA) | This work |
| SB-41 | Trp+ Ade−, SB-32 derivative (ΔpurA) | This work |
| SB-51 | Trp− Ade+, SB-41 derivative (ΔpurA ΔtrpB::purA) | This work |
| SB-62 | Trp− Ade−, SB-51 derivative (ΔtrpB ΔpurA) | This work |
| SB-71 | Trp+ Ade−, colorless, SB-62 derivative (ΔtrpB ΔpurA crtBI′::trpB) | This work |
| SB-72 | Trp+ Ade−, colorless, SB-62 derivative (ΔtrpB ΔpurA crtBI′::trpB) | This work |
| Plasmids | ||
| pUC19 | Ampr; general cloning vector for E. coli | 27 |
| pKO1 | pUC19 derivative; contains R. marinus PRI 493 purA and flanking sequences (3,016 bp) | This work |
| pKO2 | pKO1 derivative, with bp 16 to 1276 of purA deleted; contains 808 and 947 bp of 5′ and 3′ flanking sequences, respectively | This work |
| pKO4 | pKO2 derivative; trpB marker cassette inserted into the plasmid backbone | This work |
| pKO6 | pUC19 derivative; contains R. marinus PRI 493 trpB and flanking sequences (2,972 bp) | This work |
| pKO7 | pKO6 derivative, with the entire trpB ORF and trpBA intergenic sequence deleted; contains 500 and 1,127 bp of 5′ and 3′ flanking sequences, respectively | This work |
| pKO9 | pKO7 derivative; purA marker inserted into the plasmid backbone | This work |
| pKOA | pUC19 derivative; contains the purA marker (with 97 bp of purA upstream sequence) | This work |
| pKOB | pUC19 derivative; contains the trpB marker cassette (with 200 bp of groESL upstream sequence) | This work |
| pRM3000 | trpB+; R. marinus-E. coli shuttle vector | Matís; 3 |
| pRM3100 | trpB+purA+; pRM3000 derivative with PRI 378 purA marker under its own promoter | This work |
wt, wild type.
Escherichia coli SDH-1 (Table 1) was used for cloning and preparing plasmids. It was routinely grown at 37°C in LB medium (15) with or without ampicillin (50 to 100 μg/ml). For Trp+ selection, E. coli SDH-1 was grown on minimal medium A (15) containing 0.2% glucose, 0.2% Casamino Acids, and vitamin B1 (25 μg/ml).
DNA manipulations.
DNA manipulations and cloning were performed according to standard procedures (22). Chromosomal DNA was extracted from R. marinus using the NucleoSpin tissue kit (Macherey-Nagel, Düren, Germany). PCR was carried out with Teg polymerase (Matís, Reykjavik, Iceland) or the PCR Extender System (5 PRIME, Hamburg, Germany). DNA was purified from enzymatic reaction mixtures and agarose gels using the GFX PCR DNA and gel band purification kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Restriction endonucleases and DNA-modifying enzymes were obtained from New England BioLabs (Beverly, MA). Plasmid DNA was isolated by using the GenElute plasmid miniprep kit (Sigma, St. Louis, MO). DNA was concentrated with Microcon centrifuge filter units (Millipore, Bedford, MA). DNA sequencing was performed by using the BigDye Terminator cycle sequencing kit, version 3.1, and a 3730 sequencer (Applied Biosystems, Foster City, CA).
Generation of deletion molecules.
This work took advantage of a partial genomic sequence of R. marinus PRI 378, which was available at Matís. Primers were designed according to this sequence to retrieve target genes to be deleted from the R. marinus recipient strain PRI 493 (Table 1). The purA gene and flanking sequences were amplified (3,016 bp) using the purkoF and purkoR primers (ko stands for knockout, F stands for forward, and R stands for reverse in the primer designations) (Table 2). The product was cloned into the XbaI site of pUC19 to form pKO1 and then subcloned for sequencing. The divergent primers purdelF and purdelR were used in amplifying the pKO1 plasmid while replacing bases 809 to 2069 of the insert with a unique XhoI restriction site. The deletion corresponded to bases 16 to 1276 of purA. The product was XhoI digested, religated, and used for transforming E. coli SDH-1 (Table 1). The pKO2 plasmid, with the deletion flanked by 808 and 947 bp of 5′ and 3′ sequences, respectively, was isolated from an Ampr transformant. Finally, the trpB marker with the fused 200-bp groESL upstream sequence (trpB marker cassette) was isolated from pRM3000 by HindIII digestion. It was end filled and ligated into the SmaI site of the pKO2 polylinker. The resulting purA deletion plasmid pKO4 (Ampr trpB+) was isolated from an Ampr Trp+ transformant of E. coli SDH-1. For complementation, the purA gene with a 97-bp upstream sequence was amplified from R. marinus PRI 378, using the purApr and purAR primers. The resulting product was cloned into the AatII site of pRM3000 to construct the replicative shuttle plasmid pRM3100 (trpB+ purA+). Also, the purA gene and the trpB marker cassette amplified using the groF and trpRR primers (Table 2) were cloned into the AatII site of pUC19. This gave rise to the pKOA and pKOB plasmids (Table 1), which were used in control transformations.
Table 2.
Primers used in this work
| Primer | Sequencea (5′ to 3′) |
|---|---|
| purkoF | GGAATTCTCTAGAGACACCGTGGTCGTCTTC |
| purkoR | GGAATTCTCTAGACGATAGGCCTTCATACGCTTCG |
| purdelF | GGAATTCCTCGAGGCGCCGTCATCCCGGCCTC |
| purdelR | GGAATTCCTCGAGAACCGTTACCGGCATTGCTGG |
| purApr | GGAATTCGACGTCACTTCATGGCGAACCTCCCTTACGG |
| purAR | GGAATTCGACGTCTCACGCCGAGGCCGGGATG |
| trpkoF | TTCGGAAGAATTCCCCCGGTGATCAAAGCCATCCATGTG |
| trpkoR | TTCGGAAAAGCTTTCCTGAGTGCGCGGTTCGATGG |
| trpdelF | GGAATTCCTCGAGATGATCGATCGCCTGCAATCGATG |
| trpdelR | GGAATTCCTCGAGGGTCTGTGTCCCCTGCAGAATTTTAC |
| crtBIF | TTCCCAAGCTTGAAGGACTTTCGCCGGGAAGTTG |
| crtBIR | TTCCCAAGCTTGCACGCCCGTCTATGCCACC |
| groF | CACCGACGTCTTTAAAGCTACACATTCTGGCCGT |
| groF2 | AACGCGGGACGATTGTAACG |
| trpRF | ATGTCGACCGCCGAGACCG |
| trpRR | CACCGACGTCTTACATATACCGTGCAATGG |
| purprobeF | GCCAATGCAGGTCATACG |
| purprobeR | TGACCTTGCCGTCGTACC |
| oriF | GCCGCGTTGCTGGCGTTTTTCCAT |
| oriR | AGACCCCGTAGAAAAGATCA |
| crtBIdelF | TCGCGCCTACACGACGCTC |
| crtBIdelR | GGGCGCATGCAGATAGAGGC |
The restriction sites used for cloning are underlined.
The R. marinus PRI 493 trpB and flanking sequences were amplified (2,972 bp) using the trpkoF and trpkoR primers (Table 2). The resulting product was cloned into EcoRI/HindIII-digested pUC19 to form pKO6 and subsequently sequenced. The trpdelF and trpdelR primers (del stands for deletion) were used in amplifying pKO6 while replacing bases 501 to 1845 of the insert, which corresponded to the entire trpB open reading frame (ORF) and the trpBA intergenic region, with a unique XhoI restriction site. The product, containing 500 and 1,127 bp of 5′ and 3′ flanking sequences, respectively, was XhoI digested, religated, and then used to transform E. coli SDH-1. A plasmid was subsequently isolated from an Ampr transformant and designated pKO7. The purA marker was isolated from pRM3100 by AatII digestion and inserted into the AatII site in the pKO7 backbone to form the deletion plasmid pKO9 (Ampr purA+).
A 3,950-bp genomic sequence was amplified from R. marinus PRI 493 using the crtBIF and crtBIR primers (Table 2). The resulting product was cloned into the HindIII site of pUC19 and subcloned for sequencing. The sequence contained the 3′ end of an ORF homologous to phytoene synthase genes, crtB (bases 1 to 814) and ORFs homologous to phytoene desaturase genes, crtI (bases 2069 to 3583) and putative peptidase genes (bases 3921 to 3544). A MluI fragment corresponding to bases 716 to 3142 was deleted from the insert, and the plasmid was end filled and ligated to the blunted trpB marker cassette. The linear insert of 2,952 bp containing 717 and 810 bp of 5′ and 3′ homologous sequences, respectively, was isolated to transform R. marinus. No difference was observed in transformation frequencies whether the insert was isolated by HindIII digestion or amplified using primers crtBIF and crtBIR before agarose gel purification.
Transformation of R. marinus.
Electrocompetent R. marinus cells were prepared as previously described (5). Electrotransformation was performed using the Gene Pulser II system (Bio-Rad, Hercules, CA), with pulse delivery at 20 to 22.5 kV/cm, 25 μF, and 200 Ω and concomitant pulse times of about 5 ms. Each transformation used 5 μg of circular DNA or 2.5 μg of linear DNA. While approximately 1 × 109 cells were subjected to each transformation, the average number of surviving cells was about 5 × 108. The transformants were grown on selective plates for 4 to 7 days. The transformation efficiency for replicative plasmids was calculated as the estimated number of transformants per microgram of DNA. For nonreplicative plasmids and linear molecules, the number of transformants per milliliter and the transformation frequencies (number of transformants per surviving cells) were calculated for 5 μg and 2.5 μg of DNA, respectively. The plasmid stability and copy numbers in strain SB-62 (ΔtrpB ΔpurA) were analyzed as previously described (3). Transformant derivatives were examined for plasmid stability, using the groF2 and trpRR primers (Table 2).
Southern hybridization analyses.
About 3 μg of digested genomic DNA was separated on 0.8% agarose gels, transferred, and cross-linked to Hybond N+ nylon membranes using the UVC500 UV cross-linker (both from GE Healthcare). Probe labeling and chemiluminescence detection were performed using the digoxigenin (DIG) high prime DNA labeling and detection kit (Roche Diagnostics, Penzberg, Germany). Procedures for hybridization and stripping followed the manufacturer's instructions. The probes used while constructing purA and trpB deletion mutants were purA, which was amplified using the purprobeF and purprobeR primers, trpB, which was amplified using the trpRF and trpRR primers, and pUC19 ori, which was amplified using the oriF and oriR primers (Table 2). The trpB probe was also applied for analyzing Trp+ transformants and crtBI mutants. The latter were also probed with the deleted crtBI MluI fragment, after its amplification using the crtBIdelF and crtBIdelR primers.
Nucleotide sequence accession numbers.
The nucleotide sequences determined during this work containing the R. marinus PRI 493 purA, trpB, and crtBI genes have been deposited in the GenBank database under accession numbers JF701602 to JF701604, respectively.
RESULTS
Retrieval of the purA marker.
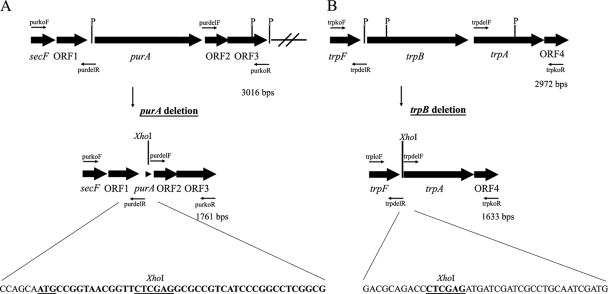
In the beginning, we wanted to isolate an additional selective marker for Rhodothermus marinus so it could be used along with the established Trp+ selection. The marker should ideally be selected on minimal medium enriched with Casamino Acids. We identified a potential marker in a known genomic sequence of 3 kb from R. marinus PRI 378. The purA marker encodes a protein with homology to adenylosuccinate synthase, which confers adenine prototrophy (2). The purA gene and flanking sequences were amplified from the R. marinus recipient strain PRI 493 (5) and cloned into pUC19, giving rise to pKO1. The fragment contained an open reading frame of 1,299 bp showing 95% identity with the R. marinus PRI 378 purA gene, as well as 793 and 924 bp of 5′ and 3′ flanking sequences, respectively. Homology searches revealed that the R. marinus purA gene was located downstream of genes encoding a protein export membrane protein and a putative anti-sigma factor antagonist (Fig. 1A). The 3′ flanking sequence of strain PRI 493 contained two ORFs, which encode products with similarities to conserved hypothetical proteins, one of which was absent from the PRI 378 sequence. No known purine biosynthesis genes were found in the vicinity of the R. marinus purA gene.
Fig. 1.
Sequential deletion of the R. marinus purA and trpB genes. (A) Gene organization flanking the R. marinus strain PRI 493 purA gene. The open reading frames secF, ORF1, and purA show homology to genes encoding a protein export membrane protein, an anti-sigma factor antagonist, and adenylosuccinate synthase, respectively. The products of ORF2 and ORF3 show homology to conserved hypothetical proteins. PstI restriction sites (P) are indicated. The purkoF and purkoR primers were used to amplify the sequence, while the purdelR and purdelF primers were used to replace bases 16 to 1276 from the purA ORF with an XhoI restriction site. The remaining purA sequence (38 bp) in the deletion plasmid pKO4 as well as in the genome of strain SB-32 is shown (in bold type with the start codon underlined) as well as six bases of the 5′ flanking sequence. (B) Gene organization surrounding the R. marinus PRI 493 trpB gene. The trpB and trpA genes encode tryptophan synthase components B and A, respectively. They are flanked by a gene showing homology to trpF and encoding phosphoribosylanthranilate isomerase and a gene encoding a hypothetical protein (ORF4). PstI recognition sites (P) are indicated. The region was amplified using primers trpkoF and trpkoR, and the entire trpB ORF and the trpBA intergenic sequence were replaced with a unique XhoI site. The sequences that flank the XhoI site in the trpB deletion plasmid pKO9 and also in the genome of SB-62 are shown.
Construction and complementation of an unmarked ΔpurA deletant.
The use of the purA gene as a selective marker in R. marinus required a strain that was defective in adenylosuccinate synthase activity. Our aim was to obtain a ΔpurA recombinant for future work, which would not lead to the chromosomal integration of molecules containing purA. The strategy we followed was to use a suicide plasmid containing the 5′ and 3′ purA flanking regions for homologous recombination and integration into the host chromosome by a single crossover. In addition, the plasmid backbone contained the trpB marker cassette to select for the integration by restoration of prototrophy in R. marinus SB-1 (trpB1). It would then be possible to screen for the resolution of cointegrates and loss of the purA and trpB markers following nonselective growth by examining adenine and tryptophan requirements. A ΔpurA mutant obtained this way would allow the reuse of both the purA and trpB markers.
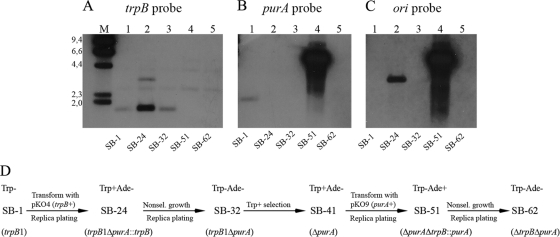
The above strategy yielded pKO4, a derivative of pKO1 in which nearly all of the purA sequence was deleted and which contained the trpB marker cassette (Table 1). Upon using pKO4 to transform R. marinus SB-1 (trpB1), Trp+ colonies were recovered with a frequency of 2.8 × 10−6 ± 1.2 × 10−6, which was approximately 10 times higher than the spontaneous reversion frequency of the trpB1 mutation obtained in parallel experiments. The Trp+ strains could therefore represent either spontaneous revertants or recombinants with the plasmid integrated at one of the four homologous sequences in the R. marinus chromosome, the 5′ or the 3′ purA flanking regions, the trpB gene, or its fused groESL control region. When the adenine requirements of the Trp+ strains were examined, 2.5% were found to be stable adenine auxotrophs. Southern hybridization failed to detect a fragment containing purA in strain SB-24, one of the Trp+ Ade− strains analyzed (Fig. 2B, lane 2), thus verifying the deletion of purA from the chromosome. However, the Trp+ phenotype indicated that a part of the suicide plasmid was still present, and this was confirmed by the hybridization of an ori probe from the plasmid to the same membrane (Fig. 2C, lane 2).
Fig. 2.
Construction of the double deletant SB-62 (ΔtrpB ΔpurA). (A to C) Southern hybridization analyses of PstI-digested genomic DNA of strains derived from R. marinus PRI 493 (lanes 1 to 5). Lane M, HindIII-digested λ DNA as a size marker. The positions (in kilobases) of molecular size markers (from 2.0 to 9.4 kb) are shown to the left of the gel shown in panel A. The membrane was probed three times with digoxigenin-labeled fragments corresponding to trpB (A), purA (B), and the ori fragment from pUC19 (C). For detection of the size marker, labeled λ DNA was added with each probe. (D) The strategy used for generating the double deletant SB-62 (ΔtrpB ΔpurA), using both the trpB and purA selective markers. The phenotypes and genotypes are indicated for each strain. Nonsel., nonselective.
Strain SB-24 was subjected to nonselective growth overnight, and outgrowth colonies were screened for additional recombination events that lost the plasmid-encoded trpB gene. Every strain examined exhibited adenine auxotrophy, with 1.3% also showing tryptophan auxotrophy. The plasmid-encoded ori sequence could not be amplified from the Trp− Ade− strains. The results of Southern hybridization analysis for one of the strains, SB-32, were consistent with the loss of the suicide plasmid (Fig. 2C, lane 3). This strain was of the correct genotype, ΔpurA, as shown both by Southern analysis (Fig. 2B, lane 3) and DNA sequencing (Fig. 1A). To demonstrate complementation of the deletant, the R. marinus PRI 378 purA gene was introduced under its own promoter (97 bp) into the replicative shuttle vector pRM3000 (3). The resulting derivative pRM3100 (trpB+ purA+), transformed strain SB-32 (trpB1 ΔpurA), and Trp+ Ade+ transformants were selected on minimal medium enriched with Casamino Acids. They appeared after 3 or 4 days when selected for tryptophan prototrophy and after 4 or 5 days when selected for adenine prototrophy. Thus, a second selection strategy had been implemented for R. marinus based on the restoration of adenine prototrophy by a ΔpurA deletant with the R. marinus purA gene. Furthermore, Ade+ selection could be applied together with Trp+ selection.
Construction of an unmarked ΔtrpB ΔpurA deletant.
An additional marker for R. marinus enabled the construction of a strain in which the endogenous trpB gene was deleted. The strategy was the same as for the purA deletion, except that chromosomal integration was selected by means of the purA marker in the ΔpurA strain. Inactivation of trpB was to be assessed by the tryptophan requirements of derivatives of the deletant SB-32 (trpB1 ΔpurA). Since the trpB1 mutation reverts with a frequency of about 5 × 10−7 (5), a spontaneous Trp+ revertant, SB-41 (ΔpurA), was first isolated to serve as a recipient. The deletion strategy was however complicated by the fact that the R. marinus trpB gene is flanked by other tryptophan biosynthesis genes (Fig. 1B). Consequently, the insertion of a suicide plasmid might interrupt the expression of other trp genes. The trpB gene and flanking sequences were amplified from the genome of the wild-type recipient strain PRI 493 (Table 1). Sequencing the 3-kb product revealed the gene organization trpFBA (Fig. 1B), which is the prominent order in bacterial tryptophan operons (26). Between the trpBA genes, a region of 139 bp was observed. To minimize the perturbation of the original gene organization and expression, the trpB deletion plasmid pKO9 was constructed to delete the entire trpB ORF and the trpBA intergenic region while leaving intact the 5′ and 3′ flanking sequences of 500 and 1,127 bp, respectively (Table 1). For selection, the plasmid backbone contained purA under its own promoter.
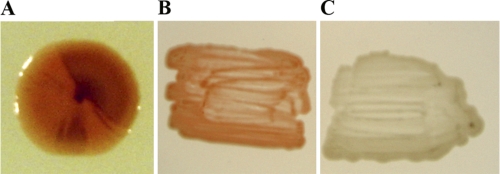
The purA deletant SB-41 was transformed with pKO9, and Ade+ colonies were recovered at a frequency of 2.9 × 10−6 ± 2.2 × 10−6 on selective medium supplemented with tryptophan. The initial characterization of the Ade+ strains revealed that about 4% grew poorly without tryptophan. However, only a single strain, SB-51, was identified to be a stable tryptophan auxotroph. It was also the only one (0.2%) of the strains examined that did not respond to the tryptophan biosynthesis intermediate indole. This suggested that strain SB-51 was defective in TrpB activity (28). Furthermore, SB-51 was the only strain from which the trpB gene could not be amplified by PCR, thus indicating that trpB had been deleted. Derivatives of strain SB-51 (Trp− Ade+) and of several Trp+ Ade+ integrants were screened for loss of the purA marker, and no stable Ade− derivatives were identified. This observation was clarified through Southern analysis of strain SB-51. Although the trpB gene proved to be absent, a part of the suicide plasmid, which included purA, was not only present in the genome but extensively amplified (Fig. 2A to C, lanes 4). A total loss of the expanded fragment was required for isolating a genetically stable Trp− Ade− recipient strain. Finally, a fortuitous observation aided the isolation. When single outgrowth colonies of strain SB-51 had been incubated on nonselective plates for a prolonged period (about 2 weeks), some of them developed dark-brown sectors, masking the usual orange-red color of R. marinus (Fig. 3A). As dark coloring had previously been observed in purA mutants, this color change was believed to be associated with the Ade− phenotype. Indeed, stable Ade− strains were isolated from the dark colony segments. One of the strains, designated SB-62 (Trp− Ade−), was shown by Southern analysis to have lost the deletion plasmid and the purA marker (Fig. 2B and C, lanes 5). Furthermore, sequencing revealed the expected context in the genome of strain SB-62 (ΔtrpB ΔpurA) following the trpB deletion (Fig. 1B). This established that the endogenous sequences corresponding to the two selective markers had been deleted from the R. marinus recipient strain PRI 493. Figure 2D summarizes how the double deletant SB-62 was constructed.
Fig. 3.
Cells of wild-type and mutant R. marinus strains. (A) Strain SB-51 (ΔpurA ΔtrpB::purA). The dark color is associated with the Ade− phenotype; it appears that the cointegrate is being resolved. Stable Ade− derivatives, including SB-62 (ΔtrpB ΔpurA) were isolated from the dark colony segments. (B) PRI-493 (wild type). (C) The colorless strain SB-71 (ΔtrpB ΔpurA crtBI′::trpB).
Vector propagation in the ΔtrpB ΔpurA deletant.
The R. marinus double deletant SB-62 (ΔtrpB ΔpurA) was transformed with the replicative shuttle vector pRM3000 (trpB+) in order to examine whether the strain was suitable for Trp+ selection and vector propagation. The transformation efficiency obtained, 1.6 × 104 ± 0.9 × 104, was considerably lower than the efficiency of 1.3 × 107 ± 0.3 × 107 that was obtained for the same vector in the R. marinus strain SB-1 (trpB1) (3). On the other hand, every Trp+ transformant of strain SB-62 examined was found to harbor the vector, indicating a more effective selection in SB-62. Several Trp+ transformants were examined by Southern analysis. The trpB gene was only found associated with plasmid bands and not integrated in the chromosome (data not shown). Upon examining growth and shuttle vector stability in three Trp+ SB-62 transformants, no difference in growth rates was observed compared to the wild-type R. marinus PRI 493. Derivatives of the transformants were obtained after up to 75 generations of nonselective growth, and all the derivatives examined contained the pRM3000 vector with the correct size and restriction pattern. Furthermore, the vector was present in the same number of copies or 22 ± 3 in the examined derivative strains during subsequent growth in selective medium. PCR analysis was conducted on the strains using primers directed to trpB and the 5′ flanking region of the groESL control sequence. Since no amplification was detected, the vector was apparently not integrated in the groESL control sequence of the chromosome. Moreover, strain SB-62 (ΔtrpB ΔpurA) was transformed with plasmids pKOA and pKOB containing either marker, purA or trpB with 112 or 200 bp, respectively, of upstream control sequences homologous to the chro-mosome. Ade+ and Trp+ strains were obtained with frequencies of 5 × 10−10 and 2 × 10−9, respectively. Taken together, the results indicated high segregational and structural stability of the shuttle vector pRM3000 in the double deletant SB-62. Furthermore, due to the effective selection, lack of spontaneous revertants, and low frequency of marker integrants, the combination of SB-62 (ΔtrpB ΔpurA) and the selective markers was considered suitable for homologous gene targeting strategies.
Inactivation of carotenoid biosynthesis genes.
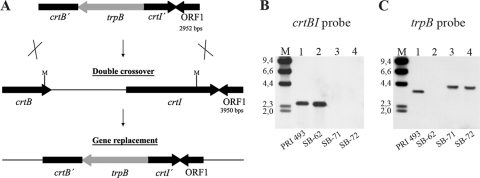
Replacement with a selective marker was examined as a general procedure for disrupting chromosomal genes in R. marinus. Double-crossover recombination using a linear molecule was attempted in order to avoid the chromosomal amplification observed when using suicide plasmids. The putative carotenoid biosynthesis genes crtB and crtI were targeted. The genes show homology to phytoene synthase and phytoene desaturase genes, and a 4-kb region surrounding the R. marinus PRI 493 genes was sequenced. A 2.4-kb MluI fragment containing the last 96 bp of crtB and the first 1,077 bp of crtI was replaced with the trpB marker cassette (Fig. 4A). R. marinus SB-62 (ΔtrpB ΔpurA) was transformed with the linear molecule, and Trp+ transformants were recovered with a frequency of 1 × 10−8. These were all colorless rather than orange-red, which indicated crtBI inactivation (Fig. 3B and C). The correct gene replacement, the absence of the crtBI MluI fragment (Fig. 4B, lanes 3 and 4), and the reappearance of trpB (Fig. 4C, lanes 3 and 4) were demonstrated by Southern analysis. Thus, several R. marinus mutants, including strains SB-71 and SB-72 (ΔtrpB ΔpurA crtBI′::trpB) were obtained from a single transformation and gene replacement by double-crossover recombination using homologous sequences of 717 and 810 bp.
Fig. 4.
Gene replacement by double-crossover recombination in R. marinus. (A) Double-crossover recombination between the linear crtBI deletion molecule and the chromosome of R. marinus SB-62 (ΔtrpB ΔpurA). The known sequence of 3,950 bp surrounding the carotenoid biosynthesis genes crtB (partial, 814 bp) and crtI (1,515 bp) in the wild-type strain PRI 493 is shown schematically. ORF1 encodes a putative peptidase (378 bp). The trpB marker cassette (1.4 kb) containing the groESL upstream sequence (200 bp) replaced a 2.4-kb MluI fragment. MluI sites (M) are indicated. (B and C) Southern analysis of MluI-digested genomic DNA. Lanes M, HindIII-digested λ DNA as a size marker (kb). Lane 1, strain PRI 493 (wt); lane 2, SB-62 (ΔtrpB ΔpurA); lanes 3 and 4, strains SB-71 and SB-72 (ΔtrpB ΔpurA crtBI′::trpB). The membrane was probed with the deleted MluI fragment (B) and trpB (C). For detection of the size marker, labeled λ DNA was added with each probe.
DISCUSSION
This work succeeded in establishing a selection strategy for R. marinus based on adenine complementation in addition to the described Trp+ selection (3, 5). Moreover, the work succeeded in deleting both the purA and trpB genes from the R. marinus chromosome. This results in more efficient selection by preventing the development of spontaneous Ade+ and Trp+ revertants and by decreasing unintended marker integration into the chromosome. Since the purA gene was not surrounded by other genes of adenine biosynthesis, in-frame deletion was not pursued. However, the trpB gene was part of an operon, and the integration of the suicide plasmid appeared to affect its expression in 4% of the Trp+ transformants. The entire trpB ORF was deleted from the R. marinus chromosome along with the downstream trpBA intergenic sequence, thereby positioning the trpA gene in place of trpB. The resulting deletion did not repress neighboring tryptophan biosynthesis genes, since it was complemented by the trpB marker cassette. Intriguingly, the transformation efficiency for the replicative vector pRM3000 (trpB+) dropped by almost 3 orders of magnitude upon the deletion of trpB compared to strain SB-1 (trpB1). Whereas this can partly be explained by the lack of both spontaneous revertants and of recombination between vector-encoded and chromosomally encoded trpB genes, it does imply some complications during transformation with pRM3000, possibly affecting the vector's establishment as a replicon rather than its uptake. The presence of homologous sequences has been shown to promote plasmid establishment during transformation of Thermus thermophilus (10). To examine the importance of the trpB sequence during the transformation of R. marinus with pRM3000 (trpB+), the mutated gene of strain SB-1 was reintroduced into the chromosome of strain SB-62 using a linear molecule. This increased the transformation efficiency only about 20-fold. The decrease in the transformation efficiency of strain SB-62 is therefore largely unexplained.
Unexpectedly, during the construction of an unmarked purA deletant, examination revealed that purA was already deleted in some (2.5%) of the selected transformants, although all the deletion mutants still contained portions of the integrated plasmid. This suggested that the deletion had not occurred by a complete cointegrate resolution. A deletion phenotype was therefore searched for and identified among transformants during the trpB deletion prior to removing the selection pressure. Both chromosomal genes had therefore deleted independently of the integrated plasmid-encoded markers, which had to be resolved separately. Using Southern analysis explained this as due to chromosomal amplification of the deletion plasmids, probably by homologous recombination between chromosomal duplications and as a result of a selective pressure. Recombination between homologous sequences would therefore delete the target genes while still leaving multiple copies of the integrated plasmids and selective marker genes, which would be resolved upon removing the selection. Chromosomal duplications are known to expand in the E. coli chromosome (18) with the expansion believed to occur via homologous recombination and to be prompted by selection. Here, chromosomal amplification was extensive when the purA marker was used for selection (Fig. 2B, lane 4) and was still observed albeit to a much lower extent when using the trpB marker cassette (Fig. 2A, lane 2). Different degrees of amplification are conceivably a response to different selective pressures, as observed in other bacteria. For example, in response to elevated concentrations of tetracycline, cointegrate sequences of a Salmonella strain containing a tetracycline resistance gene were observed to amplify up to 30-fold (7). Here, however, duplications resulted in amplification of native R. marinus genes that were positioned at heterologous chromosomal locations. The purA gene was expressed from its native promoter, while the trpB gene was transferred from the tryptophan biosynthesis operon under the control of the groESL upstream region. The amplification indicates that the genes were under selection at their new positions due to inadequate expression, although this was not investigated further.
The greater amplification when using purA instead of the trpB marker cassette is consistent with the lower efficiency of the former, which required an additional day for complementation when it was carried by a replicative vector. This contrast can account for the higher occurrence of deletants among transformants, which comprised 2.5% of transformants when using trpB, compared to 0.2% when using purA. The extensive amplification further complicated the screen for segregants that had lost the plasmid-encoded purA marker, although this was facilitated by a color change apparently associated with the lack of a PurA product. While the reason for this color change remains unexplained, the change probably reflected accumulation of an adenine biosynthesis intermediate. Dark coloring was observed in the ΔpurA strains SB-32 (trpB1 ΔpurA), SB-41 (ΔpurA), and SB-62 (ΔtrpB ΔpurA). However, the color change was reversed upon complementing the double deletant SB-62 with pRM3100 (trpB+ purA+), though not upon complementing it with pRM3000 (trpB+). The purA gene was later expressed from the groESL control region instead of from its native promoter. Ade+ transformants appeared after 3 or 4 days, about the same time as Trp+ transformants, when the trpB gene used for selection was expressed from the same control region. Such increased efficiency should reduce the chromosomal amplification of fragments containing purA. Although fragments containing trpB were also amplified, this did not affect the screen for segregants, as resolved strains were readily isolated after overnight growth in nonselective medium.
Chromosomal amplification and the separate loss of target genes and plasmid-encoded markers affect the construction of R. marinus mutants with circular suicide plasmids. In this work, the isolation of unmarked purA and trpB mutants was facilitated by the possibility of directly identifying deletion mutants among the transformants by screening for auxotrophy. Nevertheless, unmarked deletions that do not impart a detectable phenotype may be found by using a similar method. Integrants can be selected and subsequently screened for deletions through molecular methods such as PCR or colony hybridization. The deletion frequency depends on each experiment, on the genes to be deleted, the marker used, and the length of homologous sequences. Subsequently, segregants without integrated suicide plasmids may be identified by screening for marker loss. Finally, marked deletions may be isolated by using both selective markers, whereby one of them replaces the target gene and the other one is inserted into the plasmid backbone.
The most straightforward approach for generating marked deletions is selecting gene replacements directly by double crossover with a linear molecule containing a marker flanked by homologous regions of the chromosome (16). This approach is limited to bacteria with efficient recombination systems and that can be transformed with linear DNA. Its use is therefore restricted, for example, in E. coli to engineered strains that either are highly recombinogenic (16, 29) or lacking in exonuclease activity (21, 25). Gene replacement using linear molecules has been achieved in the thermophiles Thermus thermophilus (9) and Thermococcus kodakarensis (23). Here, it was achieved for the deletion of putative R. marinus carotenoid genes. As complementation was not intended, the crtBI genes were truncated simply by replacing a restriction fragment with the trpB marker cassette. Transformation with the deletion molecule yielded Trp+ double-crossover recombinants in a single step. While gene replacement with linear molecules depends on efficient transformation, it circumvents both the screening for second recombination events and the amplification of suicide plasmids in R. marinus. The approach should therefore be of value for genetic analysis in this species as an accessible general method for introducing targeted deletions.
ACKNOWLEDGMENTS
We thank Aegir Thor Thorsson for help preparing figures for the manuscript.
This work was supported by grants from the Icelandic Research Fund and the Icelandic Research Fund for Graduate Students, both operated by Rannis, the Icelandic Centre for Research.
Footnotes
Published ahead of print on 24 June 2011.
REFERENCES
- 1. Alfredsson G. A., Kristjansson J. K., Hjorleifsdottir S., Stetter K. O. 1988. Rhodothermus marinus, gen. nov., sp. nov., a thermophilic, halophilic bacterium from submarine hot springs in Iceland. J. Gen. Microbiol. 134:299–306 [Google Scholar]
- 2. Benson C. E., Love S. H., Remy C. N. 1970. Inhibition of de novo purine biosynthesis and interconversion of 6-methyl purine in Escherichia coli. J. Bacteriol. 101:872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjornsdottir S. H., Fridjonsson O. H., Kristjansson J. K., Eggertsson G. 2007. Cloning and expression of heterologous genes in Rhodothermus marinus. Extremophiles 11:283–293 [DOI] [PubMed] [Google Scholar]
- 4. Bjornsdottir S. H., et al. 2006. Rhodothermus marinus: physiology and molecular biology. Extremophiles 10:1–16 [DOI] [PubMed] [Google Scholar]
- 5. Bjornsdottir S. H., Thorbjarnardottir S. H., Eggertsson G. 2005. Establishment of a gene transfer system for Rhodothermus marinus. Appl. Microbiol. Biotechnol. 66:675–682 [DOI] [PubMed] [Google Scholar]
- 6. Degryse E., Glansdorff N., Piérard A. 1978. A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch. Microbiol. 17:189–196 [DOI] [PubMed] [Google Scholar]
- 7. Gutterson N. I., Koshland D. E. 1983. Replacement and amplification of bacterial genes with sequences altered in vitro. Proc. Natl. Acad. Sci. U. S. A. 80:4894–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamilton C. M., Aldea M., Washburn B. K., Babitzke P., Kushner S. R. 1989. New method for generating deletions and gene replacements in Escherichia coli. J. Bacteriol. 171:4617–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hashimoto Y., Yano T., Kuramitsu S., Kagamiyane H. 2001. Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistance marker. FEBS Lett. 506:231–234 [DOI] [PubMed] [Google Scholar]
- 10. Hoshino T., Maseda H., Nakahara T. 1993. Plasmid marker rescue transformation in Thermus thermophilus. J. Ferm. Bioeng. 76:276–279 [Google Scholar]
- 11. Lasa I., de Grado M., de Pedro M. A., Berenguer J. 1992. Development of Thermus-Escherichia coli shuttle vectors and their use for expression of the Clostridium thermocellum celA gene in Thermus thermophilus. J. Bacteriol. 174:6424–6431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Link A. J., Phillips D., Church G. M. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: applications to open reading frame characterization. J. Bacteriol. 179:6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ludwig W., Euzéby J., Whitman W. B. 2008. Draft taxonomic outline of the Bacteroidetes, Plantomycetes, Chlamydiae, Spirochaetes, Fibrobacteres, Fusobacteria, Acidobacteria, Verrucomicrobia, Dictyoglomi and Gemmatimonadetes. http://bergeys.org/outlines/Bergeys_Vol_4_Outline_linked.pdf
- 14. Marx C. J. 2008. Development of a broad-host range sacB-based vector for unmarked allelic exchange. BMC Res. Notes 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 16. Murphy K. C. 1998. Use of bacteriophage λ recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nolan M., et al. 2009. Complete genome sequence of Rhodothermus marinus type strain (R-10T). Stand. Genomic Sci. 1:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poteete A. R. 2009. Expansion of a chromosomal repeat in Escherichia coli: roles of replication, repair, and recombination functions. BMC Mol. Biol. 10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pritchett M. A., Zhang J. K., Metcalf W. W. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl. Environ. Microbiol. 70:1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reyrat J. M., Pelicic V., Gicquel B., Rappuoli R. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66:4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell C. B., Thaler D. S., Dahlquist F. W. 1989. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J. Bacteriol. 171:2609–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sambrook H., Russell D. W. 2001. Molecular cloning. A laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 23. Sato T., Fukai T., Atomi H., Imanaka T. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl. Environ. Microbiol. 71:3889–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber J. M., Johnson S. P., Vonstein V., Casabdan M. J., Demirjian D. C. 1995. A chromosome integration system for stable gene transfer into Thermus flavus. Biotechnology (NY) 13:271–275 [DOI] [PubMed] [Google Scholar]
- 25. Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. 1985. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J. Bacteriol. 161:1219–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie G., Keyhani N. O., Bonner C. A., Jensen R. A. 2003. Ancient origin of the tryptophan operon and the dynamics of evolutionary change. Microbiol. Mol. Biol. Rev. 67:303–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yanisch-Perron C., Vieira J., Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 28. Yanofsky C., Crawford I. P. 1959. The effects of deletions, point mutations, reversions and suppressor mutations on the two components of the tryptophan synthetase of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 45:1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu D., et al. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]