Abstract
Exposure to an aerial environment or severe nutrient limitation induces asexual differentiation in filamentous fungi. Submerged cultivation of Aspergillus niger in carbon- and energy-limited retentostat cultures both induces and fuels conidiation. Physiological and transcriptomic analyses have revealed that this differentiation strongly affects product formation. Since conidiation is inherent in the aerial environment, we hypothesized that product formation near zero growth can be influenced by affecting differentiation or development of aerial hyphae in general. To investigate this idea, three developmental mutants (ΔfwnA, scl-1, and scl-2 mutants) that have no apparent vegetative growth defects were cultured in maltose-limited retentostat cultures. The secondary-metabolite profile of the wild-type strain defined flavasperone, aurasperone B, tensidol B, and two so far uncharacterized compounds as associated with conidium formation, while fumonisins B2, B4, and B6 were characteristic of early response to nutrient limitation by the vegetative mycelium. The developmental mutants responded differently to the severe substrate limitation, which resulted in distinct profiles of growth and product formation. fwnA encodes the polyketide synthase responsible for melanin biosynthesis during aerial differentiation, and we show that conidial melanin synthesis in submerged retentostat cultures and aurasperone B production are fwnA dependent. The scl-1 and scl-2 strains are two UV mutants generated in the ΔfwnA background that displayed reduced asexual conidiation and formed sclerotium-like structures on agar plates. The reduced conidiation phenotypes of the scl-1 and scl-2 strains are reflected in the retentostat cultivation and are accompanied by elimination or severely reduced accumulation of secondary metabolites and distinctly enhanced accumulation of extracellular protein. This investigation shows that submerged conidiation and product formation of a mitosporic fungus cultured at low specific growth rates can be fundamentally affected by interfering with the genetic program for differentiation of aerial hyphae, opening new perspectives for tailoring industrial performance.
INTRODUCTION
The black-spored ascomycete Aspergillus niger is a ubiquitous saprophyte in nature and occurs as a common spoilage mold on stored food and feed (15, 26, 30). Its successful occupation of these ecological niches results at least partially from an ability to secrete a wide array of substrate-degrading enzymes, which, like organic acid biosynthesis, is exploited in industrial production (32, 34). Also contributing to its economic importance are less desirable traits, such as conditional production of toxic secondary metabolites or mycotoxins, like ochratoxin A and fumonisins (30, 36). Secondary-metabolite accumulation in the genus Aspergillus and its relatives is most diverse and abundant under subaerial growth conditions on solid media that allow differentiation or development of vegetative hyphae into conidia, sclerotia, or cleistothecia (2, 45).
Despite a prominent role in the mold life cycle, aerial development has received little attention with respect to innovative exploitation or improvement of existing industrial applications. However, reproductive differentiation and submerged productivity become intimately related under certain physiological circumstances that may be relevant to submerged industrial processes. A low growth rate is a common consequence of substrate-limited growth during prolonged fed-batch cultivation and poor mixing in large volumes of dense culture broth. Severe limitation of nutrients and low specific growth rates (μ) trigger submerged conidiation and secondary metabolism in both fed and starved cultures of aspergilli (6, 8, 37, 40). Retentostat cultures are continuous cultures with cell retention, which, instead of maintaining a fixed specific growth rate, forces μ into a transient decline to approach zero. We have recently shown that A. niger becomes committed to conidiation in carbon- and energy-limited retentostat cultures (22). This is apparent from transcriptomic and physiological analyses, which strongly indicate that sporulation-associated molecules become major products of the submerged process.
The development of specialized reproductive hyphae, conidiophores, the terminal vesicle, sterigmata, and conidia has traditionally been described as a phenomenon inherent in the subaerial environment (14). The conidiophores of aspergilli are formed inversely to fluffy aerial hyphae and sexual reproductive structures, cleistothecia, or vestiges of these, sclerotia (7, 16, 19, 44). A. niger (strict definition) is reported to form neither of the last two structures (35, 42) and is characterized by abundant asexual sporulation on subaerial colonies. The intimate relationship of fungal development and the production of secondary metabolites has been well established (9, 46).
Based on present evidence, we hypothesized that the submerged response of A. niger to low specific growth rates can be fundamentally affected by interfering with aerial development. To test this hypothesis, we compared carbon- and energy-limited retentostat cultures of three mutants displaying abnormal aerial differentiation, but no apparent vegetative phenotypes, with previously generated profiles of a reference strain with normal development. The differences in aerial development were reflected in the nutrient-limited submerged cultures and growth and product formation affected, which resulted in distinct profiles of secondary-metabolite and solute protein accumulation.
MATERIALS AND METHODS
Strains.
A. niger N402 (cspA1) (5) was used as a reference strain. The MA93.1 strain is a fawn-colored mutant of N402, generated by directed knockout of the conidial melanin polyketide synthase (PKS) An09g05730 (ΔfwnA fwnA::hygB in N402) (23). A uridine-auxotrophic ΔfwnA strain (MA100.1; cspA1 kusA::amdS fwnA::hygB pyrG378) (23) was used as a template for random generation of developmental mutants. UV mutagenesis of MA100.1 was carried out as described by Damveld et al. (11). Conidia (the survival rate after mutagenesis was about 70%) were plated out on complete medium (CM) plates containing 0.05% Triton X-100 to restrict radial growth. After 7 days of growth at 30°C, the plates were inspected visually for colonies with aberrant aerial development. Two mutants that were affected in aerial development and that did not display any apparent vegetative growth defects (see Results) were selected for further analysis. Prototrophy was restored by transformation of the pyrG gene (pAB4.1) to the two strains TJ1.1 and TJ1.2. Both UV-generated mutants, besides being affected in asexual development, had also gained the ability to form sclerotial structures on CM plates and hence were termed scl-1 (JP5.1) and scl-2 (MA146.1).
Inoculum.
The four A. niger strains N402, MA93.1, JP5.1, and MA146.1 were propagated on solidified CM to produce a conidial inoculum for submerged cultivation. The CM agar plates contained (per liter) 10.0 g glucose, 6.0 g NaNO3, 1.5 g KH2PO4, 0.5 g KCl, 0.5 g MgSO4·7H2O, 1.0 g Casamino Acids, 5.0 g yeast extract, 20 g agar, and 1 ml trace metal solution. The trace metal solution contained (per liter) 10 g EDTA, 4.4 g ZnSO4·7H2O, 1.01 g MnCl2·4H2O, 0.32 g CoCl2·6H2O, 0.315 g CuSO4·5H2O, 0.22 g (NH4)6Mo7O24·4H2O, 1.47 g CaCl2·2H2O, and 1 g FeSO4·7H2O (modified from the composition given by Vishniac and Santer [43]). The pH of the medium was adjusted to 5.8 with NaOH prior to autoclaving. The solid-medium cultures were incubated for at least 6 days at 30°C to allow adequate conidium formation. Spore plates were stored for no more than 3 months at 4°C. For the preparation of inoculum, conidia were harvested and washed with a sterile detergent solution containing 0.05% (wt/vol) Tween 80 and 0.9% (wt/vol) NaCl.
Culture conditions.
Maltose-limited batch and retentostat cultivations were performed as described previously for A. niger N402 (22).The developmental mutants were cultured in duplicate (JP5.1 and MA146.1) or triplicate (MA93.1).
(i) Batch cultures.
BioFlo3000 bioreactors (6.6 liters; New Brunswick Scientific, NJ) holding 5 liters (kg) minimal medium (MM) was inoculated with the conidial suspension to give 109 conidia liter−1. The MM contained (per liter) 4.5 g NH4Cl, 1.5 g KH2PO4, 0.5 KCl, 0.5 MgSO4·7H2O, and 1 ml trace metal solution (described above). The pH was adjusted to 3. The final-cell-density-limiting (growth-limiting) substrate was 4 g maltose (monohydrate) per liter. The temperature was 30°C at pH 3, kept constant by computer-controlled addition of 2 M NaOH. Sterile air was supplied at 1 liter min−1 through a ring sparger. The dissolved-oxygen tension was above 40% of air saturation at any time, ensuring oxygen-sufficient growth. Germination of the dormant conidia was induced by addition of 0.003% (wt/wt) yeast extract before inoculation. During the first 6 h of cultivation, the culture was aerated through the headspace of the reactor, and the stirrer speed was kept low at 250 rpm. These precautions minimized the gas-liquid interface, thereby reducing loss of hydrophobic conidia to the exhaust gas. After 6 h and germination of most conidia (now hydrophilic), air was sparged into the culture broth and mixing was intensified (750 rpm). At this stage, 0.01% (vol/vol) polypropylene glycol (PPG) was added as an antifoaming agent. The culture mass was monitored with a Mettler Toledo KCC150s scale. The glass surface of the headspace was cooled to minimize wall growth.
(ii) Retentostat cultures.
Continuous cultivation commenced in the late exponential growth phase of a batch culture, when 90% of the maltose had been consumed, at a biomass concentration of about 2.1 g (dry weight) kg−1. The exact time for starting the flow was based on alkali addition (20, 21). Carbon-limited MM, containing 8 g maltose (monohydrate) per liter and 0.01% (vol/vol) PPG, was fed to the culture from two interconnected 20-liter reservoirs. The flow rate was 0.125 liter h−1, which corresponded to a dilution rate (D) of 0.025 h−1 and a substrate feed rate of 0.2 g maltose h−1 kg−1. The cell retention device (22) was inserted aseptically just before the medium flow was started (to avoid growth of spores in the cylinder), and the level was adjusted to maintain a culture mass of 5 kg. The corrected airflow rate during retentostat cultivation was 1.3 liters min−1. Samples were withdrawn regularly to ensure good resolution of transient variables. Samples of the dilute effluent from the retention device were also taken.
Analysis of culture broth.
The dry-weight biomass concentration was determined by weighing lyophilized mycelium separated from a known mass of culture broth. The culture broth was filtered through GF/C glass microfiber filters (Whatman). The filtrate was collected and frozen for use in solute analyses. The mycelium was washed with demineralized water, rapidly frozen in liquid nitrogen, and stored at −80°C until lyophilization. The dry cell composition, i.e., (i) melanin content, (ii) total RNA, and (iii) protein, was determined using the protocols listed by Jørgensen et al. (22). In brief, total RNA was extracted with perchloric acid according to the method of Herbert et al. (18) and determined by the orcinol method (38) for pentoses, using yeast RNA as a standard. Biomass protein was extracted with hot alkali (8) and determined using the alkali-compatible DC Protein Assay (Bio-Rad) with bovine serum albumin (BSA) as a standard. Melanin was extracted with hot alkali and purified according to the method of de Cássia et al. (12). The total solute carbon in the supernatant was measured with a Total Organic Carbon Analyzer (TOC-Vcsn; Shimadzu, Japan), using glucose as a standard. Extracellular protein was determined with the Quick Start Bradford Protein Assay (Bio-Rad) with BSA as a reference. The growth yield (Y), referring to the substrate consumed, was calculated based on the known reservoir concentration of maltose and depletion of maltose and the derived glucose during cultivation; this was confirmed by gas chromatography-mass spectrometry (GC-MS) (see below).
Secondary-metabolite profiling.
Culture filtrate samples were analyzed by high-performance liquid chromatography (HPLC)–UV/visible light (Vis)–time of flight (TOF)-MS on an Agilent 1100 HPLC system equipped with a diode array detector (DAD). Separation was done on a 50- by 2-mm inside diameter (i.d.), 3 μm, Luna C18 II column (Phenomenex, Torrance, CA) using a water-acetonitrile (MeCN) gradient at a flow of 0.3 ml/min with 15% MeCN to 100% in 20 min, keeping this level for 5 min. The solvents were buffered with 20 mM formic acid. The LC system was coupled to a Waters LCT orthogonal time-of-flight mass spectrometer (Waters-Micromass, Manchester, United Kingdom) operating in the ESI+ mode (29). The target ions, usually [M+H]+, [M+NH4]+, [M-H2O+Ha]+, and [M+Na]+, were extracted with an interval of the theoretical mass ± m/z 0.02 and were used for identification of all compounds (29). The peak areas from the extracted ion chromatograms were determined using QuanLynx 4.1 (Waters-Micromass, Manchester, United Kingdom) and compared between samples. Peaks not matching the metabolite list of Nielsen and Smedsgaard (29) were matched against an internal database of approximately 850 reference standards, as well as the 35,500 structures in Antibase2010 (John Wiley and Sons, Inc.).
Solutions of ochratoxin A; fumonisins B1, B2, and B3 (Biopure, Tulln, Austria); and fumonisins B4 and B6 (27) were coanalyzed.
Analysis of extracellular metabolites.
Culture filtrate samples (100 μl) were freeze-dried and methoximated with O-methylhydroxylamine hydrochloride and subsequently trimethylsilylated with N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) containing 1% trimethylchlorosilane as described in detail by Kind et al. (25). Samples were analyzed on a DSQ2 single-quadrupole GC-MS system (Thermo Scientific, Runcorn, United Kingdom) equipped with a Phenomenex ZB-5 column (30 m; 0.25 mm i.d.; 0.25-μm film; 5% diphenyl polysiloxane) using the settings of Kind et al. The data were analyzed with AMDIS v. 2.66 (39) using the Fiehn v.1.0 library for identification (Agilent Technologies, CA). Reference standard solutions of citrate, glucose, malate, oxalate, phosphate, and glycerol were coanalyzed to characterize retention time differences from the Fiehn library.
Pictures.
Overview pictures of subaerial colonies were taken with an Olympus Camedia digital camera (C-5050 zoom), and more detailed micrographs illustrating aerial development were obtained using a Leica EZ4D stereo microscope (8× to 35×). Submerged development was studied under the microscope. Culture broth samples rapidly frozen in liquid nitrogen and stored at −80°C were thawed on ice for microscope analyses. Diluted samples were fixed, stained, and mounted in Lactophenol blue solution (Fluka) according to the manufacturer's protocol. Differential interference contrast (DIC) micrographs of mycelium morphology were taken with an Axiocam MRc5 using a Zeiss Axioplan 2 microscope.
RESULTS
A. niger mutants with abnormal aerial development.
Three mutants were selected for submerged characterization in retentostat cultures based on their phenotypes associated with aerial development and vegetative growth under subaerial conditions (Fig. 1). Although the aerial development of each mutant was distinct from that of the reference strain, A. niger N402, the mutants maintained radial extension rates equal to that of N402. Furthermore, all mutants were, like N402, capable of producing elaborate conidiophores with abundant conidiogenous cells and chains of viable conidia.
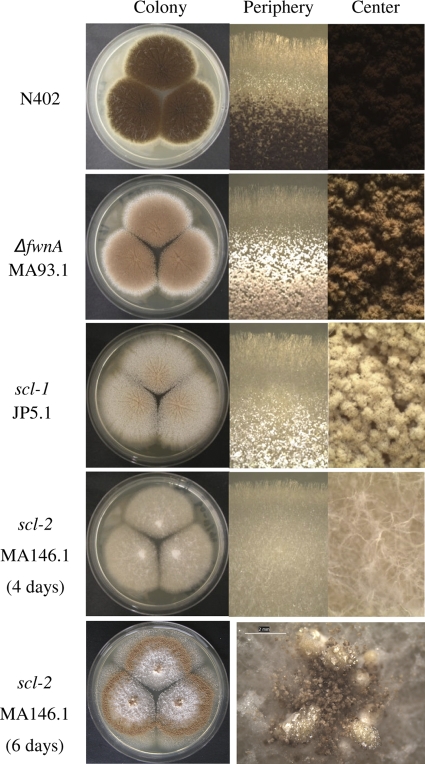
Fig. 1.
Subaerial development and pigmentation of reference (N402), ΔfwnA (MA93.1), scl-1 (JP5.1), and scl-2 (MA146.1) strains after 4 days growth on complete medium. (Left) Full colony overview pictures. (Center) Micrographs of the periphery of a colony. (Right) Micrographs of conidial heads in the center of a colony. A colony overview and a close-up of sclerotia near the colony center are shown for the scl-2 strain at day 6.
The first strain (MA93.1; ΔfwnA) is a defined mutant in which the fwnA gene (An09g05730), encoding the major polyketide synthase involved in formation of conidial melanin, was deleted (23). The phenotypic effects of ΔfwnA were apparently limited to conidial pigmentation under subaerial growth conditions (Fig. 1). The other two strains, JP5.1 (scl-1) and MA146.1 (scl-2), were derived from mutants generated by random UV mutagenesis in a ΔfwnA background. Both mutants displayed reduced conidiation (Fig. 1). The peripheral zone of subaerial colonies of the scl-1 mutant revealed a characteristic phenotype in aerial development in which conidiophore emergence, elaboration of conidiogenous cells, and conidial maturation were affected (Fig. 1). The whitish appearance of the scl-1 conidia likely reflects delayed or reduced maturation, since the residual pigment displayed spectral properties identical to those of pigment extracted from the parental ΔfwnA strain (MA100.1), and the pigmentation intensified during prolonged incubation (results not shown). An additional characteristic of the developmental phenotype of scl-1 is the occasional appearance of sterile fruiting bodies or sclerotia near the colony center. Sclerotium formation is much more consistent and extensive in the scl-2 strains (MA146.1 and TJ1.2), which initially produce abundant aerial hyphae and sclerotium initials near the colony center (Fig. 1). The aerial mycelium forms hyphal tufts and appears to be coated with a matrix. After about 4 days of incubation on CM agar, there is a transition in development, and fully elaborated conidiophores are formed in emerging ring zones of single-point-inoculated colonies (Fig. 1). Conidiation is early and predominant if the conidial inoculum is spread in a dense carpet. Fluffy aerial hyphae are abundant on MM, while sclerotium formation is very limited compared to that on CM. The scl-1 and scl-2 mutants are the first described sclerotium-forming strains in a well-defined A. niger background, and further studies of sclerotogenesis in A. niger are in progress. Further genetic characterization of the scl-1 and scl-2 mutants is in progress, but attempts to identify the loci affected in the scl-1 and scl-2 strains have so far been unsuccessful.
Developmental phenotypes did not affect vegetative growth in batch cultures.
The initial propagation in batch cultures allowed exponential vegetative growth near the conditional maximal specific growth rate (μmax). The μmax and the growth yield (Y) on maltose monohydrate were 0.22 to 0.24 h−1 and 0.58 g (dry weight) g−1, respectively, for all three strains in duplicate or triplicate cultures and the same as previously determined for N402 (22). The evaluation of physiological parameters, such as μmax and Y, revealed that the developmental mutants had no apparent vegetative phenotypes, which is in line with the observations from subaerial growth. At the end of the exponential growth phase of the batch cultures, continuous cultivation in retentostat mode was initiated. The feed rate of the growth-limiting substrate supported an initial μ of 0.05 h−1 at exit from exponential growth. The μ quickly decreased to approach zero and was below 0.01 h−1 after about 2 days of retentostat cultivation for all strains (not shown).
Disruption of fwnA involved in conidium maturation does not affect development.
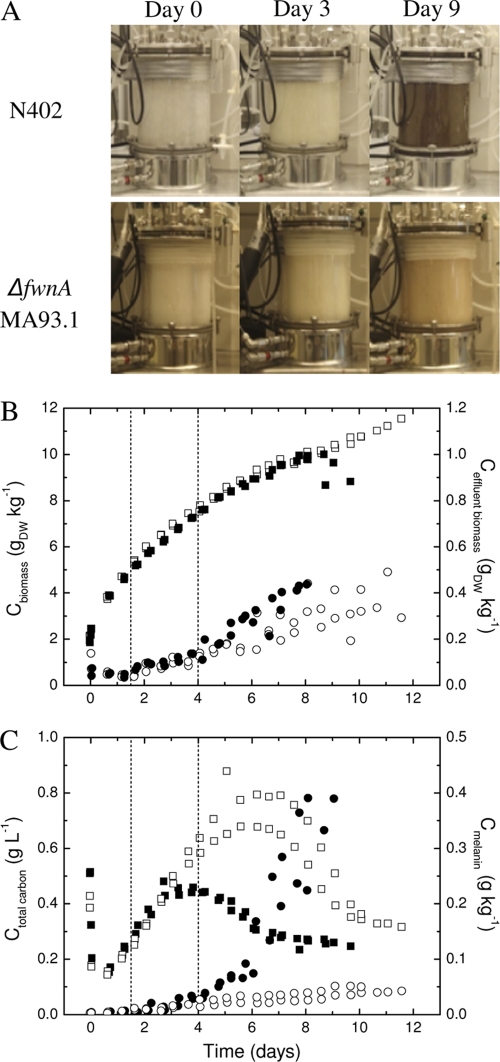
Conidial melanin is a significant sink for the limited carbon and energy sources in retentostat cultures of N402, where it constitutes at least 20% of the biomass produced during prolific conidiation (22). Disruption of the major PKS gene involved in spore pigmentation, fwnA, should free carbon and energy sources for other purposes and possibly affect development or product formation. It was already apparent during retentostat cultivation that the ΔfwnA mutant (MA93.1) produced less pigment than N402 when μ approached zero (Fig. 2A). Uncontrollable foaming was a consistent phenomenon of the sporulating reference cultures of N402 after 8 to 10 days. The foaming caused biomass deposition in the headspace and expulsion with the exhaust gas, and both were reflected in a drop in the biomass concentration (Fig. 2B). These foaming problems were completely eliminated in the ΔfwnA strain, which lacks the hydrophobic melanin. However, the timing and extent of submerged conidiation in the ΔfwnA strain were very similar to those of N402. There were two growth phenotypes associated with the ΔfwnA strain at low μ: the growth yield was consistently higher from 12 to 36 h into retentostat cultivation, while biomass accumulation in the spore-enriched effluent was 31% lower during sporulation-committed growth than for N402 (Fig. 2B). Extraction and quantification of alkali-soluble melanin proved that only a minor residue was still formed in the ΔfwnA strain (Fig. 2C). In the period corresponding to melaninization of N402 (days 3 to 10), the level of solute carbon was greatly increased in the ΔfwnA strain (Fig. 2C), while the respiratory quotient was lower (not shown), which indicated enhanced organic acid production by the mutant. GC-MS revealed that oxalic acid was the major source of the increased solute carbon measured for the ΔfwnA strain (results not shown).
Fig. 2.
Comparison of N402 (wt) and the ΔfwnA strain (MA93.1) in duplicate maltose-limited retentostat cultures. (A) Pictures of the bioreactor vessel showing the progressive pigmentation of the culture broth of both strains. (B) Biomass accumulation in cultures of N402 (▪) and the ΔfwnA strain (□) and in the effluent of N402 (•) and the ΔfwnA strain (○). (C) Accumulation of total solute carbon in N402 (▪) and the ΔfwnA strain (□) and melanin accumulation in N402 (•) and the ΔfwnA strain (○) in the culture broth. The cultivation results for MA93.1 are compared to N402 from previous data obtained under identical culture conditions (22). The two vertical dashed lines indicate the first observation of conidiophores and the subsequent commitment to conidiation in cultures of N402.
The aerial developmental phenotypes of the scl-1 and scl-2 strains have profound effects on submerged conidiation and accumulation of extracellular protein.
The mutants affected in early processes of aerial development, the scl-1 and scl-2 strains, displayed limited submerged conidiation compared to the ΔfwnA strain (and N402). Near the end of the cultures, the dominant morphology of the scl-1 and scl-2 strains was filamentous, consisting of thin, infrequently branched hyphae with a tendency to form fluffy aggregates (Fig. 3A). The reduced conidiation of both mutants was also apparent from the low biomass accumulation in the effluent (Fig. 3B). Since growth was shifted from conidium to hypha formation, more biomass was retained in the culture; however, the biomass accumulation profiles of the scl-1 and scl-2 strains were distinctly different from each other, as scl-2 biomass formed at a higher yield as μ approached zero. Just like N402 (22), the three mutants all reached a minimum growth yield (Ymin) around day 3 or 4. Ymin was 19 to 21 g (dry weight) g−1 for the ΔfwnA (MA93.1) and scl-1 (JP5.1) strains and N402, while it was 0.26 g (dry weight) g−1 for the scl-2 strain (MA146.1), indicating fundamental differences in the biomass or hyphae accumulating in the later stages of cultivation (after day 4).
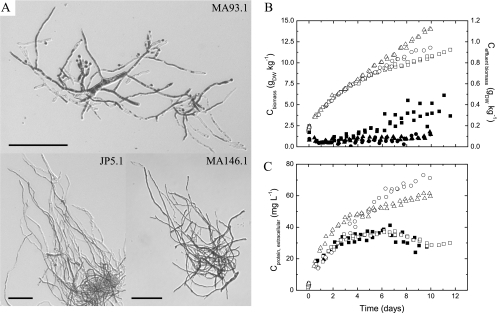
Fig. 3.
scl-1 (JP5.1) and scl-2 (MA146.1) strains in duplicate maltose-limited retentostat cultures. (A) Micrographs showing typical morphologies encountered after 9 days of retentostat cultivation (bars = 50 μm). (B) Biomass accumulation in cultures of MA93.1 (□), JP5.1 (○), and MA146.1 (▵) and in the effluents of MA93.1 (▪), JP5.1 (•), and MA146.1 (▴) during retentostat cultivation. (C) Profiles of extracellular protein accumulation in cultures of JP5.1 (○) and MA146.1 (▵) compared to MA93.1 (□) and N402 (▪) (22).
Extracellular accumulation of protein was enhanced in scl-1 and scl-2 cultures compared to N402 and the ΔfwnA strain (Fig. 3C); the last strain displayed profiles of solute protein identical to those determined for N402. scl-1 protein accumulation followed the profile of the ΔfwnA strain and N402 until day 3, at which point the reference strains conidiated abundantly and protein secretion was reduced. Again, scl-2 was very different from the other three strains, showing enhanced protein secretion early in the retentostat cultures, from the start of continuous cultivation to day 3, after which accumulation stabilized at a lower level. Thus, the scl-1 strain displayed enhanced protein release as μ approached zero, where conidiation normally takes place, while it was enhanced in the scl-2 strain early at moderately low specific growth rates (μ ≤ 0.05 h−1) (Fig. 3C).
Secondary metabolism reflects development and is distinctly impaired in each mutant.
The recent transcriptomic analysis of maltose-limited retentostat cultures of N402 (22) revealed induction of several coexpressed gene clusters with known or suspected roles in secondary metabolism. This observation, together with melanin synthesis, was a strong indication that secondary metabolism is a significant feature of the retentostat cultures. We consequently established semiquantitative temporal profiles of secondary-metabolite accumulation in the culture medium (Fig. 4) to correlate these with asexual development in the reference strain (N402) and to evaluate the consequences of altered development in the mutants.
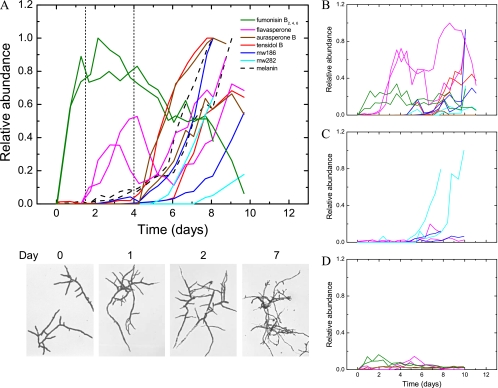
Fig. 4.
Temporal profiles of secondary-metabolite accumulation in the culture media of duplicate retentostat cultures. The metabolite concentrations are relative to the largest area determined for each metabolite. (A) Reference metabolite profile of N402 with pictures showing morphological differentiation over time (analyses of samples from reference 21) (below). (B to D) Profiles for the ΔfwnA strain (MA93.1) (B), the scl-1 strain (JP5.1) (C), and the scl-2 strain (MA146.1) (D). The two vertical dashed lines indicate the first observation of conidiophores and subsequent commitment to conidiation in cultures of N402 (22).
The secondary-metabolite profiles of N402 (Fig. 4A) contained at least eight secondary metabolites, six of which are known to be polyketide derived (reviewed by Nielsen et al. [30]). The secondary metabolites can be divided into three groups based on their accumulation profiles. The first group of metabolites, fumonisins B2, B4, and B6, are formed throughout retentostat cultivation, but primarily as an initial response of the vegetative mycelium to the severe substrate limitation. Another polyketide, flavasperone, defines its own group by accelerating in concentration twice, first at the onset of conidiation and then again later during prolific conidiation. The third group of metabolites was closely associated with conidium formation or maturation, as their appearance coincided with melanization and the commitment to conidiation in N402. The group consists of the dimeric polyketide aurasperone B and the alkaloid tensidol B, the biosynthesis of which is still obscure. Furthermore, two uncharacterized A. niger metabolites (mw186 and mw282) with monoisotopic masses of 186 and 282 Da, respectively, were identified. Both of them did not match any of the 35,500 secondary metabolites in Antibase2010 or the KEGG database (http://www.genome.jp/kegg/).
Retentostat cultivation of MA93.1 (ΔfwnA) revealed that accumulation of aurasperone B was fwnA dependent (Fig. 4B), while the scl-1 and scl-2 mutations had more drastic yet distinct effects on secondary metabolism (Fig. 4C and D). Very low levels of fumonisin and tensidol B were detected in the cultures of the scl-1 strain, and mw282 was the only metabolite formed in relatively high abundance during conidiation. This was different for the scl-2 strain, which maintained early but moderate fumonisin accumulation. As the cultivation progressed, fewer metabolites were formed, and the conidium-related metabolites were absent, except for aurasperone B, which curiously reappeared at low levels early in the cultures of the scl-2 mutant, although the mutant was made in a ΔfwnA background.
DISCUSSION
The present retentostat study shows that defects apparently restricted to aerial development of A. niger are reflected in submerged culture during severe carbon- and energy-limited growth. This finding strongly suggests the existence of basal mechanistic homologies between aerial differentiation and starvation-induced submerged development beyond that of BrlA-induced conidiation (the role of brlA in conidiogenesis has been reviewed by Adams et al. [1] and Fischer [14]). Additional homologies between submerged nutrient limitation and aerial development were found in our recent transcriptomic study of A. niger N402 in retentostat cultures (22). This study revealed that, in addition to fluffy genes (flbC and flbD) involved in conidiophore development upstream of BrlA, a number of homologs of genes with roles in sexual development (10, 17, 24) or affecting the balance between sexual and asexual development (7) in other aspergilli become induced in response to severe carbon limitation. Among these genes we find the velvet gene (veA in A. nidulans) homolog (An08g05100), the nsdC and nsdD homologs (An13g00370 and An02g09610), and lipoxygenase genes homologous to ppoA (An04g05880), ppoC (An02g07930), and ppoD (An12g01320) in A. flavus. In the end, these cultures are dominated by conidiation, and brlA, downstream regulator, and structural conidiation genes become highly expressed. Similarly, Twumasi-Boateng et al. (40) found genes (e.g., nsdD) with roles in sexual development to be induced in submerged cultures of A. fumigatus conidiating in response to transfer to nutrient-poor media.
Comparison of the two conidiation-reduced mutants, the scl-1 and scl-2 mutants, which are fundamentally affected in aerial development, shows that the effects on submerged behavior are not a simple question of conidiation or no conidiation. The profiles of extracellular protein, secondary metabolites, and growth were very different in the two strains. Fumonisin production in the scl-1 mutant was nearly eliminated compared to that in other strains. This mycotoxin is most abundantly produced by the starved vegetative mycelium before conidiation occurs, stressing that scl-1 has an early role in determining developmental fate. The fact that the distinct phenotypes of the scl-1 and scl-2 strains became apparent only during carbon limitation suggests that nutrient limitation may be a common trigger for submerged, as well as aerial, reproductive development. Both strains are sclerotogenic in subaerial settings, and the trait is very consistently expressed in the scl-2 strain; however, such structures were not observed in the retentostat cultures. The lack of sclerotia in the submerged cultures could be explained by the constant mixing of the culture broth, which prevented maintenance of the proximity of neighboring mycelia required for morphogenesis. The second plausible cause is suboptimal medium composition for sclerotogenesis during bioreactor cultivation, as the scl-2 strain produces very few sclerotium initials on MM compared to CM in a subaerial environment.
The cultivation of developmental mutants also revealed that simple maintenance of energy relations (33, 41) cannot be applied to retentostat cultures of A. niger, as growth of all strains stabilized at Ymin. It is interesting that Ymin was obtained in all cultures around day 4, but it appears that although conidiation is not causing the minimum growth, it may affect the magnitude of Ymin in the approach to zero growth.
A previous transcriptomics-based study on the retentostat cultures of A. niger N402 (22) hinted that several secondary metabolites were synthesized at some time, as more than 14 putative secondary-metabolite gene clusters were transcriptionally activated. Although we did identify a number of secondary metabolites, it is difficult to assign them to specific gene clusters, as more metabolites and gene clusters share expression profiles. Additional expression analyses at more frequent time intervals or gene disruption studies are required to link metabolites and genes. Exceptions are the fumonisins and the associated gene cluster (An01g06790-An01g06930) and the fwnA (An09g05370)-dependent melanin and aurasperone B. Low levels of aurasperone B were found in the scl-2 culture, even though the scl-2 mutant was made in the ΔfwnA background, which suggests partial redundancy to synthesize aurasperone B. In submerged culture, flavasperone production did not show the same dependence on FwnA as was recently described for subaerial culture (23); this contradiction could be explained by induction of a complementary polyketide synthase or an efficient metabolism of flavasperone in subaerial settings. Another intriguing observation which shows that the environment plays a role in pigment maturation and naphtho-γ-pyrone formation is that the heptaketide funalone, which is dependent on the putative oxidase BrnA and associated with conidial pigmentation in a subaerial environment (23), was not found in the submerged cultures of A. niger N402. Furthermore, the wild-type (wt) melanin extracted from submerged culture was brownish and lacked the green spectral component that is characteristic of subaerial pigmentation (results not shown).
Chemical modification of chromatin structure to induce expression of silent gene clusters has been used to unravel the diversity of secondary metabolism in filamentous fungi (4, 13). Although such methods have yielded valuable insights into the chemical diversity encoded by fungal genomes, they lack a biological context for understanding the roles and regulation of the metabolites. The retentostat approach can be used to induce secondary metabolism and to define the individual metabolites in relation to stages of development, thus concomitantly supplying a biological context for their synthesis. The method would also present a defined basis for comparing metabolite synthesis and development in other filamentous fungi.
Exploitation of whole regulated pathways is an attractive approach to metabolic engineering (28). The disruption of fwnA gives interesting perspectives in this direction. Although not burdened by melanin synthesis, the ΔfwnA strain followed wt differentiation and was locked in a development program where the liberated carbon and energy was redirected into other excreted metabolites and not used for growth, which is sometimes a problem when manipulating metabolic fluxes (31). This may provide a context for enhanced synthesis of metabolites where developmental destiny is exploited to direct the metabolic fluxes into the desired pathways.
Other, more specific application possibilities are apparent from studying the developmental mutants in retentostat culture. The lack of melanin accumulation in sporulating ΔfwnA cultures eliminated the technical problems associated with foaming and represents a technical advance similar to the disruption of hydrophobin expression in Trichoderma reesei to avoid foaming during continuous cultivation (3). Affecting development also reduced the formation of potentially undesired secondary metabolites and appeared to enhance protein secretion in some periods of cultivation, depending on the mutant phenotype.
It is possible that some of these effects, like enhanced protein secretion, observed at very low specific growth rates may apply to moderately low specific growth rates, thereby widening the perspectives for application of developmental mutants. There are probably many ways to affect submerged behavior at a low specific growth rate, since the developmental processes induced by nutrient limitation are highly complex, involving numerous regulatory and structural elements. To address these issues, it will be necessary to conduct further investigations of the links between subaerial development and submerged behavior. Cultivation under nutrient-limiting conditions could be used to identify mutants with beneficial properties with respect to submerged performance.
This study has shown that aerial developmental phenotypes become expressed under severe carbon and energy limitation in submerged culture and that development affects synthesis of several secondary metabolites and accumulation of extracellular protein. We suggest that local nutrient limitation in subaerial or submerged hyphae is a basal trigger for reproductive development.
ACKNOWLEDGMENTS
We thank Peter J. Punt for reading the manuscript. T.R.J. thanks the Zero Growth project group of the Kluyver Centre (The Netherlands) for support and valuable discussion.
This project was carried out within the research program of the Kluyver Centre for Genomics of Industrial Fermentation, which is part of The Netherlands Genomics Initiative/Netherlands Organization for Scientific Research. K.F.N. was funded by the Danish Food Industry Agency (grant 3304-FVEP-07-730-01).
Footnotes
Published ahead of print on 7 June 2011.
REFERENCES
- 1. Adams T. H., Wieser J. K., Yu J. H. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62:35–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atoui A., Mitchell D., Mathieu F., Magan N., Lebrihi A. 2007. Partitioning of ochratoxin A in mycelium and conidia of Aspergillus carbonarius and the impact on toxin contamination of grapes and wine. J. Appl. Microbiol. 103:961–968 [DOI] [PubMed] [Google Scholar]
- 3. Bailey M. J., et al. 2002. Process technological effects of deletion and amplification of hydrophobins I and II in transformants of Trichoderma reesei. Appl. Microbiol. Biotechnol. 58:721–727 [DOI] [PubMed] [Google Scholar]
- 4. Bok J. W., et al. 2009. Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol. 5:462–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bos C. J., et al. 1988. Genetic analysis and the construction of master strains for assignment of genes to six linkage groups in Aspergillus niger. Curr. Genet. 14:437–443 [DOI] [PubMed] [Google Scholar]
- 6. Broderick A. J., Greenshields R. N. 1981. Sporulation of Aspergillus niger and Aspergillus ochraceus in continuous submerged liquid culture. J. Gen. Microbiol. 126:193–202 [Google Scholar]
- 7. Brown S. H., et al. 2009. Oxygenase coordination is required for morphological transition and the host fungus interaction of Aspergillus flavus. Mol. Plant Microbe Interact. 22:882–894 [DOI] [PubMed] [Google Scholar]
- 8. Bushell M. E., Bull A. T. 1999. Sporulation at minimum specific growth rate in Aspergillus nidulans chemostat culture predicted using protein synthesis efficiency estimations. J. Basic Microbiol. 39:293–298 [DOI] [PubMed] [Google Scholar]
- 9. Calvo A. M., Wilson R. A., Bok J. W., Keller N. P. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447–459, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calvo A. M. 2008. The veA gene regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45:1053–1061 [DOI] [PubMed] [Google Scholar]
- 11. Damveld R. A., et al. 2008. A novel screening method for cell wall mutants in Aspergillus niger identifies UDP-galactopyranose mutase as an important protein in fungal cell wall biosynthesis. Genetics 178:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Cássia R., Goncalves R., Pombeiro-Sponchiado S. R. 2005. Antioxidant activity of the melanin-pigment extracted from Aspergillus nidulans. Biol. Pharm. Bull. 28:1129–1131 [DOI] [PubMed] [Google Scholar]
- 13. Fisch K. M., et al. 2009. Chemical induction of silent biosynthetic pathway transcription in Aspergillus niger. J. Ind. Microbiol. Biotechnol. 36:1199–1213 [DOI] [PubMed] [Google Scholar]
- 14. Fischer R. 2002. Conidiation in Aspergillus nidulans, p. 59–88 In Osiewacz H. (ed.), Molecular development of fungal biology. Marcel Dekker, New York, NY [Google Scholar]
- 15. Frisvad J. C., Smedsgaard J., Samson R. A., Larsen T. O., Thrane U. 2007. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 55:9727–9732 [DOI] [PubMed] [Google Scholar]
- 16. Geiser D. M., Timberlake W. E., Arnold M. L. 1996. Loss of meiosis in Aspergillus. Mol. Biol. Evol. 13:809–917 [DOI] [PubMed] [Google Scholar]
- 17. Han K. H., et al. 2001. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 41:299–309 [DOI] [PubMed] [Google Scholar]
- 18. Herbert D., Phipps P. J., Strange R. E. 1969. Chemical analysis of microbial cells, p. 209–344 In Norris J. R., Ribbons D. W.(ed.), Methods in microbiology, vol. 5B Academic Press, London, United Kingdom [Google Scholar]
- 19. Horn B. W., Ramirez-Prado J. H., Carbone I. 2009. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet. Biol. 46:169–175 [DOI] [PubMed] [Google Scholar]
- 20. Iversen J. J. L., Thomsen J. K., Cox R. P. 1994. On-line growth measurements in bioreactors by titrating metabolic proton exchange. Appl. Microbiol. Biotechnol. 42:256–262 [Google Scholar]
- 21. Jørgensen T. R., Goosen T., A. van den Hondel C., Ram A. F. J., Iversen J. J. L. 2009. Transcriptomic comparison of Aspergillus niger growing on two different sugars reveals coordinated regulation of the secretory pathway. BMC Genomics 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jørgensen T. R., et al. 2010. Transcriptomic insights into the physiology of Aspergillus niger approaching a specific growth rate of zero. Appl. Environ. Microbiol. 76:5344–5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jørgensen T. R., et al. 2011. The molecular and genetic basis of conidial pigmentation in Aspergillus niger. Fungal Genet. Biol. 48:544–553 [DOI] [PubMed] [Google Scholar]
- 24. Kim H. R., Chae K. S., Han K. H., Han D. M. 2009. The nsdC gene encoding aputative C2H2-type transcription factor is a key activator of sexual development in Aspergillus nidulans. Genetics 182:771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kind T., et al. 2009. Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 81:10038–10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kozakiewicz Z. 1989. Aspergillus species on stored products. CAB International, Wallingford, United Kingdom [Google Scholar]
- 27. Månsson M., et al. 2010. Isolation and characterization of fumonisin B6, a new fumonisin from Aspergillus niger. J. Agric. Food Chem. 58:949–953 [DOI] [PubMed] [Google Scholar]
- 28. Nielsen J. 2001. Metabolic engineering. Appl. Microbiol. Biotechnol. 55:263–283 [DOI] [PubMed] [Google Scholar]
- 29. Nielsen K. F., Smedsgaard J. 2003. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A 1002:111–136 [DOI] [PubMed] [Google Scholar]
- 30. Nielsen K. F., Mogensen J. M., Johansen M., Larsen T. O., Frisvad J. C. 2009. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 395:1225–1242 [DOI] [PubMed] [Google Scholar]
- 31. Panagiotou G., et al. 2009. Studies on the production of fungal polyketides in Aspergillus nidulans using systems biology tools. Appl. Environ. Microbiol. 75:2212–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pel H. J., et al. 2007. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25:221–231 [DOI] [PubMed] [Google Scholar]
- 33. Pirt S. J. 1965. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B Biol. Sci. 163:224–231 [DOI] [PubMed] [Google Scholar]
- 34. Ruijter G. J. G., Kubicek C. P., Visser J. 2002. Production of organic acids by fungi, p. 213–230 In Osiewacz H. D. (ed.), The Mycota X. Springer-Verlag, Berlin, Germany [Google Scholar]
- 35. Samson R. A., et al. 2007. Diagnostic tools to identify black aspergilli. Stud. Mycol. 59:129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuster E., Dunn-Coleman N., Frisvad J. C., van Dijck P. W. M. 2002. On the safety of Aspergillus niger—a review. Appl. Microbiol. Biotechnol. 59:426–435 [DOI] [PubMed] [Google Scholar]
- 37. Skromne I., Sánchez O., Aquirre J. 1995. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology 141:21–28 [DOI] [PubMed] [Google Scholar]
- 38. Standing C. N., Fredrickson A. G., Tsuchiya H. M. 1972. Batch- and continuous-culture transients for two substrate systems. Appl. Microbiol. 23:354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stein S. J. 1999. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. Am. Soc. Mass Spectrom. 10:770–781 [Google Scholar]
- 40. Twumasi-Boateng K., et al. 2009. Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 8:104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Verseveld H. W., et al. 1986. Modelling of microbial substrate conversion, growth and product formation in a recycling fermentor. Antonie Van Leeuwenhoek 52:325–342 [DOI] [PubMed] [Google Scholar]
- 42. Varga J., et al. 2007. Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution. Int. J. Syst. Evol. Microbiol. 57:1925–1932 [DOI] [PubMed] [Google Scholar]
- 43. Vishniac W., Santer M. 1957. The thiobacilli. Bacteriol. Rev. 21:195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wicklow D. T. 1983. Taxonomic features and ecological significance of sclerotia. South. Coop. Ser. Bull. 279:6–12 [Google Scholar]
- 45. Wicklow D. T., Shotwell O. L. 1983. Intrafungal distribution of aflatoxins among conidia and sclerotia of Aspergillus flavus and Aspergillus parasiticus. Can. J. Microbiol. 29:1–5 [DOI] [PubMed] [Google Scholar]
- 46. Yu J. H., Keller N. P. 2005. Regulation of secondary metabolism in filamentous fungi. Annu. Rev. Phytopathol. 43:437–458 [DOI] [PubMed] [Google Scholar]