Abstract
Although biofilms represent a common bacterial lifestyle in clinically and environmentally important habitats, there is scant information on the extent of gene transfer in these spatially structured populations. The objective of this study was to gain insight into factors that affect transfer of the promiscuous multidrug resistance plasmid pB10 in Escherichia coli biofilms. Biofilms were grown in different experimental settings, and plasmid transfer was monitored using laser scanning confocal microscopy and plate counting. In closed flow cells, plasmid transfer in surface-attached submerged biofilms was negligible. In contrast, a high plasmid transfer efficiency was observed in a biofilm floating at the air-liquid interface in an open flow cell with low flow rates. A vertical flow cell and a batch culture biofilm reactor were then used to detect plasmid transfer at different depths away from the air-liquid interface. Extensive plasmid transfer occurred only in a narrow zone near that interface. The much lower transfer frequencies in the lower zones coincided with rapidly decreasing oxygen concentrations. However, when an E. coli csrA mutant was used as the recipient, a thick biofilm was obtained at all depths, and plasmid transfer occurred at similar frequencies throughout. These results and data from separate aerobic and anaerobic matings suggest that oxygen can affect IncP-1 plasmid transfer efficiency, not only directly but also indirectly, through influencing population densities and therefore colocalization of donors and recipients. In conclusion, the air-liquid interface can be a hot spot for plasmid-mediated gene transfer due to high densities of juxtaposed donor and recipient cells.
INTRODUCTION
Plasmid-mediated horizontal gene transfer is one of the key mechanisms of adaptive evolution in bacteria. Since many self-transmissible plasmids confer resistance to antibiotics and heavy metals, catabolic pathways, or virulence and colonization factors (37, 68), horizontal gene transfer by plasmid-mediated conjugation plays an important role in adaptation of bacteria to various conditions in natural and clinical environments (34). Despite the importance of these mobile elements in the rapid rise of multidrug-resistant bacterial pathogens (36), we still have a limited understanding of the environmental factors that affect rates of plasmid spread.
In many natural environments, bacteria colonize different surfaces, where they form microcolonies, often embedded in a polymeric matrix. These bacterial populations are spatially structured, thereby creating environmental gradients and exhibiting behaviors that are different from those in well-mixed liquid environments (16, 67). Such structured bacterial communities attached to a surface or to each other are called biofilms (16). The presence of transferrable plasmids and conjugation events has been shown to positively affect biofilm formation (10, 21, 29, 50, 54, 57, 69). Since bacterial cells in biofilms stay in close contact, it is believed that gene exchange by conjugation is favored by this “biofilm mode” of growth. However, several studies on the efficiency of plasmid transfer in laboratory-type bacterial biofilms have shown mixed results, which seem to be dependent on experimental conditions and transfer detection methods. For example, an early study by Angles and coworkers showed an increased transfer of plasmid RP1 in biofilms formed by a Vibrio sp. on glass beads in a bioreactor compared to that for cells in the aqueous phase by using a standard plating method (3). The application of fluorescent protein reporters in combination with confocal laser scanning microscopy (CLSM) has facilitated the in situ tracking of plasmid transfer in bacterial biofilms to the individual cell level. Hausner and Wüertz found that the plasmid transfer rate quantified by in situ image analysis was 1,000-fold higher than that determined by classical plating techniques (31), and similar results were obtained in other studies (39, 65). A combination of standard plating and fluorescence microscopy has been used to monitor transfer of the IncP-9 plasmid pWW0 (12, 14, 30, 53, 62), the IncP-1 plasmids pRK415 (31) and pJP4 (5), the IncX1 plasmid pMAS2027 (54), and the novel unclassified plasmids pQBR11 (47) and pBF1 (19) in biofilms grown under different experimental conditions.
Interestingly, plasmid transfer has often been shown to be limited to the surfaces of colonies or biofilms, and complete plasmid invasion has not been observed (12, 14, 30). Possible inhibition of plasmid transfer by unfavorable cell contact mechanics (50, 62) and a lack of nutrient availability in the deeper cell layers (27) have been suggested as potential explanations. Nutrients have been shown to positively affect plasmid transfer efficiency in some cases but not in others (27, 31). In summary, most studies have shown that plasmid transfer in bacterial biofilms is not easy to detect and depends on many different factors, such as plasmid type, host strain, medium, and biofilm growth conditions.
The IncP-1 plasmids are among the most promiscuous self-transmissible plasmids in proteobacteria and often code for resistance to multiple antibiotics and mercury or for factors involved in the degradation of xenobiotics (1). They code for short rigid sex pili and have been observed to transfer at higher rates between cells growing on solid surfaces than between those in liquids (9), in part due to shear forces in liquids that hinder the formation and stability of mating pairs (70). Few studies have monitored the spread of IncP-1 plasmids in biofilms (3, 5, 31, 48), and to the best of our knowledge, virtually nothing is known about the factors that affect their transfer efficiency in this important environment.
To improve our insights into the efficiency of IncP-1 plasmid transfer in biofilms and to determine what parameters affect the transfer frequency, we monitored the transfer of plasmid pB10 in Escherichia coli biofilms. The multidrug resistance broad-host-range IncP-1β plasmid was isolated from a wastewater treatment plant (25), and its genome sequence is known (61). Several studies have determined its ability to transfer to different hosts under a variety of experimental conditions (8, 24, 27, 70). E. coli was chosen as the host because it is a well-known model organism with widely available tools and well-characterized plasmid transfer mechanisms. Also, many E. coli isolates are known to form biofilms, and several threaten human health by colonizing medical devices and causing recurrent urogenital infections (6, 40). Here we show that plasmid pB10 transfer between E. coli K-12 cells occurs at a much higher efficiency in biofilms formed at the air-liquid interface (also called pellicles) than in submerged, substrate-attached biofilms and that this is due, at least in part, to differences in oxygen concentration and in the spatial location of donor and recipient cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this work and their relevant characteristics are listed in Table 1. The strains were grown in Luria-Bertani broth (LB) (LB-Miller; Fisher Scientific, Pittsburgh, PA) or M9 mineral salts medium with 0.2 g/liter glucose (called M9 here) (60), and when required, antibiotics were added at final concentrations of 10 μg ml−1 for tetracycline and gentamicin, 25 μg ml−1 for chloramphenicol, 100 μg ml−1 for ampicillin, and 50 μg ml−1 for rifampin, nalidixic acid, and kanamycin.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant phenotype or genotypea | Reference or source |
|---|---|---|
| E. coli strains | ||
| S17-1 λpir | Tmpr SmrrecAthi pro hsd(r− m+) RP4:2-Tc:Mu:Km::Tn7 λpir | 33 |
| K-12 MG1655 | Wild type; ATCC 700926 | ATCC |
| K-12 Rifr | MG1655 Rifr | 27 |
| K-12 Nalr | MG1655 Nalr | 27 |
| K-12 Nal::gfp | K-12 Nalr containing mini-Tn5-PA1–04/03::gfpmut3 cassette; Nalr Kmr | 27 |
| MG1655 csrA | csrA::mini-Tn5 Kmr | 35 |
| Plasmids | ||
| pB10 | IncP-1β Tcr Smr Amxr Sulr Hgr | 61 |
| pB10::rfp | pB10 with mini-Tn5-Km-PA1-04/03::rfp | 27 |
| pB10::T7cat | pB10 with mini-MaT7cat | This work |
| pMaT7cat | Mini-mariner transposon containing T7 RNA polymerase; Cmr Gmr | 42 |
| pB2a-Evoglow | pGem3Zf+ with pT7-Evoglow lacp-dsRedcat cassette in PvuII site; reporter plasmid; Ampr Cmr | This work |
| pG-RFP | pGem3Zf+ with pT7-dsRedcat in SmaI site; reporter plasmid; Ampr Cmr | This work |
| pG-GFP | pGem3Zf+ with pT7-gfpmut3 in SmaI site; reporter plasmid; Ampr | This work |
Tmpr, Smr, Ampr, Cmr, Gmr, Tcr, Amxr, Sulr, Kmr, Rifr, Nalr, and Hgr, trimethoprim, streptomycin, ampicillin, chloramphenicol, gentamicin, tetracycline, amoxicillin, sulfonamide, kanamycin, rifampin, nalidixic acid, and mercury ion resistance phenotypes, respectively.
Plasmid marking and localization of MaT7cat transposon insertion sites.
As an alternative plasmid detection system to be used in batch culture biofilms besides the DsRed-based plasmid pB10::rfp, pB10 was also marked with a T7 polymerase/cat gene cassette (MaT7cat), using a previously described approach (42). The donor of this plasmid was used in combination with the recipient strains E. coli MG1655 and MG1655 csrA carrying one of three constructed reporter plasmids (pB2a-Evoglow, pG-GFP, or pG-RFP) (Table 1). The vectors were constructed as previously described (42). To determine the MaT7cat transposon insertion sites in five selected clones of pB10::T7cat, DNA was isolated by a standard alkaline lysis-phenol-chloroform extraction method, digested with EcoRI or SalI, and cloned into the pGem3Zf+ vector (Promega, Madison, WI). Recombinant clones were selected on LB agar (LBA) with ampicillin and gentamicin or ampicillin and chloramphenicol, as the EcoRI and SalI sites are located between the gentamicin and chloramphenicol resistance genes in the MaT7cat transposon. This allowed us to determine the flanking sequences from both transposon ends, using primers BT20out2 and MaT7seq1 (42) for the gentamicin- and chloramphenicol-resistant clones, respectively, with a Big Dye Terminator v.3.1 cycle sequencing kit and a model 3730 DNA analyzer (Applied Biosystems, Carlsbad, CA). Sequence similarity searches were done using BLAST (2). The five marked pB10::T7cat plasmids, varying only in the insertion site of the transposon tag, were transferred back into rifampin-resistant E. coli K-12 (K-12 Rifr), and the resulting transconjugants were used as donors in conjugation experiments.
Conjugation experiments.
A filter mating protocol was performed as described elsewhere (27). The transfer frequency of plasmid pB10 after the specified incubation period is presented as the ratio of the number of transconjugant cells (T) to the number of donor (D) or recipient (R) cells at the end of the mating. For conjugation under anaerobic conditions, media and saline supplemented with resazurine (0.0001%) after sterilization were purged with nitrogen (20 min) and stored in an anaerobic (5.21% CO2, 5.18% H2, and 89.61% N2) chamber (AS-580 gloveless anaerobic chamber; Anaerobe Systems, Morgan Hill, CA). Cells from aerobically or anaerobically grown precultures (1 ml) were spun down (4 min at 2,000 × g), resuspended in 50 μl of LB medium, mixed together, and incubated in Eppendorf tubes or on 0.45-μm cellulose nitrate filters (Whatman Inc., Piscataway, NJ) under aerobic or anaerobic conditions for 18 h at 37°C. Student's t test was used on log10-transformed data to compare transfer efficiencies under different conditions, as previously reported (66).
Biofilm growth conditions.
Four different biofilm growth systems were used to monitor pB10 plasmid transfer. The first one used a closed horizontal flow cell, either a three-channel flow cell (4 mm × 40 mm × 1-mm channels) or a convertible flow cell (24 mm × 40 mm × 8-mm channel) (Stovall Life Science Inc., Greensboro, NC). The medium used was M9 or 0.1× LB broth; separate experiments were done with both systems unless otherwise stated. Flow cells were inoculated for 1 h with a mixture of donor and recipient cells (1:1 ratio) (105 CFU/ml; 200 μl/channel or 2 ml/cell) under static conditions, and then medium flow was applied (0.1 ml/min for the three-channel flow cell and 0.5 ml/min for the convertible flow cell). Flow cells were placed in the typical horizontal position and incubated at 37°C. The flow rates ensured that any planktonic cells were flushed out. Biofilms were analyzed as described below at various time points for up to 6 days.
The second system was an open horizontal flow cell, which consisted of only the bottom part of the convertible flow cell (the top was removed). The cell was placed horizontally on a heating plate to maintain a temperature of 37°C and inoculated with 1 ml of a donor-recipient mixture (1:1 ratio; 105 CFU/ml); after 1 h of static incubation, medium flow was applied at 0.11 ml/min. The biofilm floating at the air-liquid interface was collected on a microscope coverslip and analyzed as described below.
The third system consisted of a vertical flow cell (see Fig. 2A). The convertible flow cell was inoculated with 1 ml of donor-recipient mixture (1:1 ratio; 105 CFU/ml) and kept in a horizontal position for 1 h with no medium flow. Subsequently, the inoculum was removed, the flow cell was raised to a vertical position, and then medium flow was applied from the bottom at 0.330 ml/min. The flow cell was incubated vertically at 37°C. The liquid-air level was maintained ∼2 cm from the top of the flow cell by removing the liquid medium from the top using a blunted needle connected to a peristaltic pump (1.1 ml/min) (see Fig. 2A). After 3 days, the needle was removed, the flow cell was filled with medium, and the biofilm was analyzed as described below.
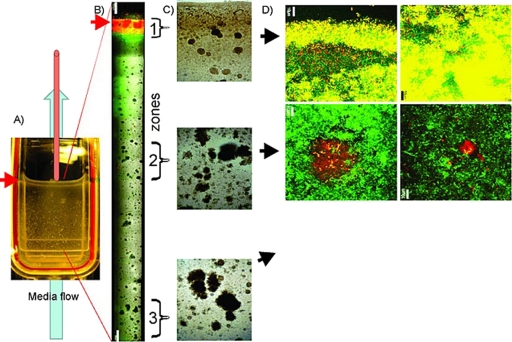
Fig. 2.
Vertical flow cell biofilm 72 h after inoculation of the donor strain MG1655(pB10::rfp) and the recipient strain MG1655::gfp. Red arrows indicate the air-liquid interface. (A) Photograph of the vertical flow cell. The red bar extending out of the top represents the needle used to maintain the liquid level. (B) Composite of biofilm images along the flow cell, indicating high levels of transconjugants (represented by yellow cells) (GFP, RFP, and bright light channels combined). Magnification, ×10. Bar, 0.5 mm. (C) Bright-field microscope images of three zones. Magnification, ×4. (D) Microphotographs of the biofilm sampled from zone 1 (upper panels) and of donor colonies surrounded by recipient cells and very few transconjugants in zones 2 and 3 (GFP and RFP channels). Magnification, ×60. Bars, 10 μm.
Finally, the fourth system was a simple batch culture biofilm reactor (see Fig. 4A). A sterile microscope slide was submerged in 25 ml of medium (M9 or 0.1× LB) in a 50-ml conical tube and incubated at 37°C in an orbital shaker at 50 rpm. To grow the biofilm, the bioreactor was inoculated with 50 μl of overnight culture; the slides were transferred daily to tubes with fresh medium. For plasmid infection studies, slides with established (24 to 48 h) recipient biofilms were washed by being dipped in 50 ml saline to remove nonattached cells and then were transferred into 25 ml of an overnight culture of the plasmid donor (approximately 108 CFU/ml) and incubated for 4 h at 37°C (50 rpm). The nonattached cells were washed off by submersion in saline, and the slide was transferred into fresh medium. After 24 or 48 h, the slide biofilms were washed, submerged in saline in a petri plate, and analyzed by confocal microscopy.
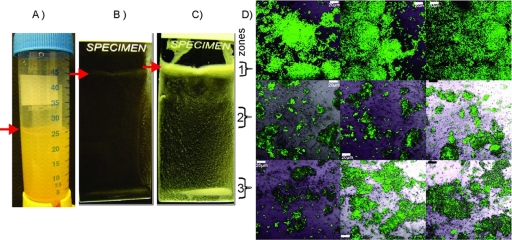
Fig. 4.
Batch culture biofilm reactor (A) and glass slide biofilms (24 h) of E. coli MG1655 (B) and E. coli MG1655 csrA (C) 24 h after infection with E. coli MG1655(pB10::MaT7cat). (D) Microphotographs (using GFP and bright-field channels) of the MG1655 csrA(pG-GFP) biofilm infected with E. coli MG1655(pB10::MaT7cat), sampled from three zones: zone 1 (air-liquid interface) (red arrows) and zones 2 and 3. Green cells represent transconjugants. Magnification, ×60. Bars, 20 μm.
A previously described E. coli K-12 Nal::gfp strain and plasmid pB10::rfp (27), marked with constitutively expressed gfp and rfp reporter genes (13), respectively, were used in the first three biofilm setups, while in the batch culture biofilm, plasmid pB10::T7cat was used in combination with recipient strains with one of three reporter plasmids (pB2a-Evoglow, pG-GFP, or pG-RFP).
Microscopy techniques for biofilm analysis.
Biofilms were observed at different time points with a standard bright-field Olympus BX41 microscope with a 5× lens (Plan-Neofluar) or with an Olympus Fluoview FV1000 confocal microscope (Olympus, Center Valley, PA) using 10× (UPLanFLN) or 60× (LUMPlanFI/IR) lenses and settings for green fluorescent protein (GFP), red fluorescent protein (RFP), or cyan fluorescent protein (CFP) (Evoglow) detection. The images were processed using FV10-ASW 1.6 (Olympus, Center Valley, PA) and Canon PhotoStitch software. For quantitative biofilm analyses, such as areal porosity measurements, ISA2 software was used, and at least 10 images from each zone were analyzed using standard settings (7).
Quantification of plasmid transfer in biofilms.
To quantify pB10 plasmid transfer in biofilms by means of plate counting, a batch culture biofilm protocol with E. coli MG1655 as the plasmid donor and MG1655 csrA as the recipient strain was used. After 4 h of incubation with the donor strain, the slides were washed with saline; the bacteria were resuspended in 25 ml of saline by a combination of scraping and vortexing (2 min), and serial dilutions were plated on selective medium. The efficiency of transfer at different depths from the air-liquid interface was analyzed as follows. Biofilm samples (∼1 cm2) were collected with sterile swabs immediately after the 4-h inoculation period, cells were resuspended in 1 ml of saline, and serial dilutions were plated onto selective medium. The transfer frequency was calculated as described above. For statistical analysis, we first took log(T/R) or log(T/D) and then performed a t test: for each replicate, we took log(T/R) or log(T/D) from zone 1 as x and log(T/R) or log(T/D) from zone 2 as y and performed a t test for (x, y).
Oxygen measurements.
The oxygen concentration was measured in the liquid column of the vertical flow cell and along the microscope slide for batch culture biofilms. Measurements were done immediately or within minutes after stopping the medium flow or shaking. An amperometric dissolved oxygen microelectrode (DOM) was used for oxygen profile measurements as described previously (45). Briefly, the tip of a tapered Pt wire was plated with gold and used as the cathode. The outer case was made of a tapered Pasteur pipette (Fisher). A homemade silver-silver chloride reference electrode was then inserted, and the DOM was filled with the electrolyte (0.3 M K2CO3, 0.2 M KHCO3, and 1 M KCl). For this specific application, the tip of the microelectrode was around 50 μm, and the length of the tip was 15 cm. The tip was covered with a silicone rubber membrane that allows diffusion of gases. The DOM was calibrated with an oxygen concentration of zero (in saturated Na2SO3 solution) and air-saturated water. The response time was around 1 to 3 s. An HP 4140B pA meter/direct current (DC) voltage source device was used to polarize the gold cathode and to measure the current. The DOM was moved into the solution in 10-μm steps, using a Mercury-Step stepper motor controller (PI M-230.10S; Physik Instrumente, Auburn, MA). The microelectrode movement and data collection were controlled by custom-made software called Microprofiler.
RESULTS
Low frequency of plasmid transfer in submerged biofilms.
Several experiments wherein transfer of pB10::rfp in E. coli MG1655 biofilms was monitored in closed horizontal flow cells showed very small numbers of transconjugant cells in submerged biofilms. This was observed under many conditions, such as in two different closed flow cells with different media and flow rates as well as different combinations of parental cells (premixed donor and recipient cells, pregrown donor biofilm inoculated with recipients, and vice versa). If any transconjugants were observed, they were represented by single cells and did not form multicell clusters (data not shown). These results suggested that only a first round of conjugation occurred, with no secondary transfer events.
To examine this low occurrence of plasmid transfer, we monitored submerged biofilms formed on the glass bottom of an open horizontal flow cell by using an upright CLSM with water immersion lenses. In these experiments, we observed spatial separation of donor and recipient cells. When the cells were first mixed and then allowed to form a biofilm, the donor cells formed a biofilm attached to the glass, while the recipient cells formed a discrete layer above the donor biofilm. The spatial separation was even more striking when an already established donor biofilm was inoculated with recipients (Fig. 1A) or when an established recipient biofilm was infected by donor cells. In the latter case, the donor cells seemed to have migrated through the recipient biofilm and settled down onto the slide surface, somehow pushing up the layer of recipient cells (Fig. 1B). The same phenomenon was observed when the experiment was repeated, and it persisted over a prolonged incubation (data not shown). At the interface between donors and recipients, few transconjugant cells were observed, and clusters indicative of multiple rounds of transfer were never observed. These results suggest that the spatial separation of plasmid-bearing and plasmid-free cells was largely responsible for the inefficient spread of an otherwise highly transferable IncP-1 plasmid.
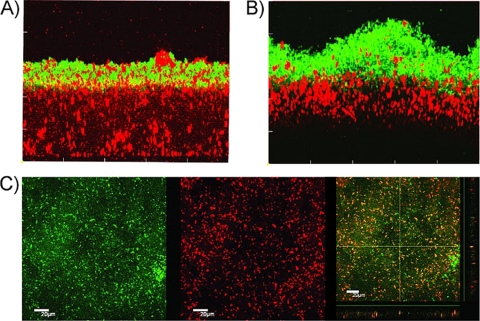
Fig. 1.
Biofilms formed in closed (A and B) and open (C) horizontal flow cells, showing donors (red), recipients (green), and transconjugants (yellow). (A) Side view of a submerged 68.5-h-old recipient MG1655::gfp biofilm observed 2.5 h after donor MG1655(pB10::rfp) was inoculated (1 h of static conditions and 1.5 h with medium flow). (B) Side view of a submerged 75.5-h-old donor MG1655(pB10::rfp) biofilm observed 4.5 h after recipient MG1655::gfp was inoculated (1 h of static conditions and 3.5 h of medium flow). Similar results were obtained when the donor and recipient cells were inoculated together and incubated for 90 h (data not shown). (C) Air-liquid interface biofilm formed 90 h after inoculation of the same donor and recipient (applied together) and transferred onto a microscope coverslip. (Left) GFP channel; (center) RFP channel; (right) both channels.
Increased plasmid transfer at the air-liquid interface.
In the open horizontal flow cell under conditions of low or no liquid flow, an additional pellicle floating at the air-liquid interface was observed. After carefully transferring this floating layer of cells from the interface to a microscope slide, we observed a large number of transconjugants (Fig. 1C). Moreover, it appeared that more transconjugant cells were located in the upper layer (facing the air) than in the lower part that faced the liquid (data not shown). This finding suggests that plasmid pB10 transfers much more efficiently at the air-liquid interface than in a submerged biofilm attached to a glass substrate.
To further study plasmid transfer in a biofilm at the air-liquid interface in more detail and under different conditions, we designed a vertical flow cell as shown in Fig. 2A. In this setup, nutrients and oxygen were supplied continuously from the bottom of the cell through the incoming growth medium flow, and additional oxygen was available at the top through gas exchange at the air-medium interface. That interface was kept at the same level during the course of the experiment. The biofilm formed by wild-type E. coli K-12 in this vertical flow cell showed a high substrate coverage and biovolume along the glass surface (data not shown). We observed structural differences between the three zones. In zone 1 (submerged, close to the air-liquid interface), bacteria were closely packed, and only a few small isolated microcolonies were observed (Fig. 2B to D), while the biofilm further away from the interface tended to consist of more isolated microcolonies of increasing size. The degree of coverage of the glass surface by the biofilm was quantified by the areal porosity parameter, defined as the ratio of the combined areas of the voids to the total area (7, 45). Areal porosity increased with distance from the air-liquid interface (zones 2 and 3), from 0.57 ± 0.03 for zone 1 to 0.74 ± 0.03 for zone 2 and 0.75 ± 0.05 for zone 3 (P < 0.003). Since the flow cell was initially inoculated with approximately 105 CFU of donor E. coli K-12 Rifr(pB10::rfp) and recipient K-12 Nal::gfp (1:1 ratio), we assumed that, as in submerged biofilms, plasmid-bearing cells would attach to the glass surface more efficiently. However, green recipient cells formed the main part of the biofilm. Only in zone 1 did we observe a large number of red donor cells as well as yellow transconjugants (Fig. 2B and D). This zone extended down about 0.7 mm from the air-medium interface. In the lower zones, we observed several microcolonies formed by donor cells surrounded by recipients. In these cases, even if the cells were in close contact along the colony border, only a limited number of transconjugants, located mostly on the top of the microcolony, were detected (Fig. 2D). These results are consistent with those for the open horizontal flow cell, i.e., there was more invasion of the IncP-1 plasmid pB10 in E. coli K-12 biofilms at the air-liquid interface than in submerged biofilms.
Oxygen concentration at different depths in vertical flow cell.
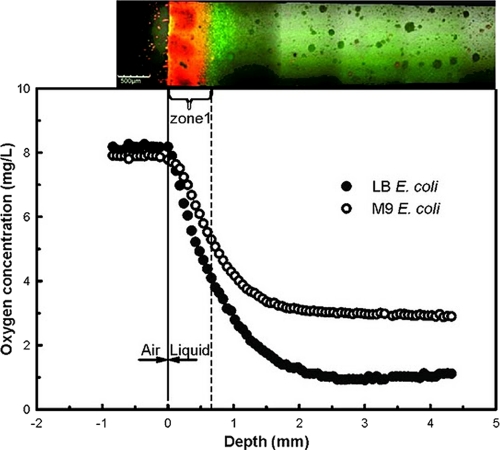
The simplest explanation for the intensive plasmid transfer at the air-medium interface could be the high oxygen level in this area. It is well known that oxygen concentrations drop rapidly both inside biofilms and in the liquid column (23). To test the oxygen level at different depths in the vertical flow cell, a 15-cm-long oxygen microelectrode was used. A rapid drop in oxygen concentration was observed immediately away from the surface (Fig. 3); 1.5 to 2 mm from the surface, the oxygen concentration had dropped from 8 mg/liter to the lowest level. The minimal O2 level depended on the medium used. In M9 medium, where the total number of cells reached ∼106 to 107 CFU/ml, the lowest oxygen concentration was 3 mg/liter. In the rich LB medium, where the number of bacteria was at least 10 times higher, the oxygen concentration reached 1 mg/liter.
Fig. 3.
Oxygen profiles along the liquid column in the vertical flow cell (see Fig. 2A) 24 h after inoculation with donor and recipient in 0.1× LB or M9 medium. Oxygen concentrations were measured using a custom-made microelectrode. Similar profiles were observed 48 h and 72 h after inoculation. The picture above the graph shows part of the vertical flow cell from Fig. 2B, indicating zone 1 (zones 2 and 3 are beyond the 5 mm shown in this figure).
Effect of oxygen on transfer of plasmid pB10.
To determine if the presence of oxygen directly affects the conjugation efficiency of plasmid pB10, four combinations of filter matings were performed. Using aerobically or anaerobically grown donor and recipient precultures, matings were performed under both aerobic and anaerobic conditions (for 18 h at 37°C), followed by enumeration of donor, recipient, and transconjugant cells on selective medium. The average ratios of transconjugants to recipients (T/R) under aerobic and anaerobic conditions for aerobically grown precultures were 0.40 (±0.13) and 0.32 (±0.14), respectively, and were not significantly different (P = 0.2; standard t test). However, when anaerobically grown donor and recipient precultures were used, significant differences in transfer frequencies were observed between aerobic and anaerobic matings (P < 0.001), with ratios of 0.60 (±0.25) and 0.022 (±0.006), respectively. When results from aerobic precultivation and mating conditions were compared with those from fully anaerobic conditions, the difference was also highly significant (P < 0.001).
Because spatial separation of donor and recipient cells could affect the conjugation efficiency in filter matings, we performed additional liquid matings under the same four conditions of aerobic and anaerobic precultivation and matings. Here we observed an even more striking difference in T/R ratios for anaerobically grown precultures, with ratios of 1.79 × 10−2 and 4.68 × 10−6 for aerobic and anaerobic matings, respectively (P < 0.001). However, just like in the filter matings, the difference was not significant when donor and recipient cells were pregrown aerobically. The low frequencies under completely anaerobic conditions were not due to plasmid loss in the donor strain, based on counts of plasmid-bearing donor cells (data not shown). In conclusion, anaerobic conditions negatively affected the conjugation efficiency of the IncP-1 plasmid pB10, but trace amounts of oxygen, as expected in biofilm flow cells, should not.
Effect of cell density on plasmid transfer in a batch culture biofilm.
To confirm that there was a higher degree of plasmid invasion in E. coli populations at the air-liquid interface than in biofilms submerged in liquid, the transfer of pB10 was monitored in a fourth system, i.e., biofilms grown on vertically placed microscope slides in batch culture reactors (Fig. 4A). In this case, a different marker system was used to visualize transconjugants. An E. coli donor carrying pB10::T7cat was used in combination with recipients carrying different reporter plasmids (pG-GFP, pG-RFP, and pB2a-Evoglow, encoding the oxygen-independent fluorescent protein Evoglow) (Table 1). Since each of these recipients required the presence of T7 polymerase to express the fluorescent reporter protein, the only fluorescent cells were recipients that acquired the plasmid. The observed plasmid transfer patterns were similar to those observed for the vertical flow cell, i.e., efficient plasmid transfer in zone 1 and smaller numbers of transconjugants in the lower zones. This coincided again with the very uneven distribution patterns of bacteria on the slide and with an oxygen profile similar to that in the vertical flow cell (data not shown). Juxtaposition of donor and recipients was observed only in zone 1 at the air-liquid interface, where most of the biofilm was formed (Fig. 4B). Only a thin layer of single cells or small microcolonies were formed by plasmid-bearing donor cells in the deeper zones (data not shown). These findings strongly suggest that here, too, spatial separation of donor and recipient cells can explain the lack of gene transfer in the lower submerged zones.
To test the hypothesis that the low frequency of plasmid transfer in submerged biofilms was due to spatial separation of donors and recipients, we monitored plasmid transfer into an E. coli MG1655 csrA mutant that has an increased biofilm formation ability (35). This strain not only formed a substantial biofilm at the air-medium interface in the batch culture reactor but also efficiently covered the microscope slide in deeper zones (Fig. 4C). With this strain, fluorescent transconjugants were observed all the way down the slide in experiments with pG-GFP (Fig. 4D) as well as pG-RFP and pB2a-Evoglow (data not shown) as reporter plasmids. This strongly suggests that the very low frequency of plasmid transfer in wild-type E. coli MG1655 submerged biofilms was due largely to the poor biofilm formation ability of this strain causing spatial separation of donor and recipient cells.
To quantify the efficiency of plasmid transfer in the three different zones of the batch reactor biofilm of E. coli MG1655 csrA, parental and transconjugant cells were enumerated by the classical dilution/plating method (Table 2). We found a larger absolute number of transconjugants in zone 1, but the differences in T/R ratios in all zones were not statistically significant (P = 0.23 between zones 1 and 2). This was because lower transconjugant densities in the lower zones were correlated with lower recipient densities. On the other hand, the T/D ratios in all zones were statistically different (P < 0.01 between zones 1 and 2) because the donor densities did not decrease as drastically with depth. Thus, for a given number of invading donor cells, transconjugant densities were highest at the air-liquid interface because of the higher recipient densities at that location. Again, the air-liquid interface seemed to promote biofilm growth of the csrA recipient strain and therefore allowed more extensive plasmid invasion.
Table 2.
Efficiency of pB10 transfer in batch culture biofilms of E. coli MG1655 csrA at different depths from the air-liquid interface
| Environment | No. of cells (CFU/ml) |
T/D ratio | T/R ratio | ||
|---|---|---|---|---|---|
| Donors (D) | Recipients (R) | Transconjugants (T) | |||
| Biofilm (total)a | 2.29 × 106 | 4.73 × 107 | 7.37 × 103 | 3.21 × 10−3 | 1.56 × 10−4 |
| Zone 1b | 4.43 × 106 | 2.84 × 108 | 2.86 × 104 | 6.46 × 10−3 | 1.01 × 10−4 |
| Zone 2b | 3.35 × 106 | 4.34 × 107 | 8.11 × 103 | 2.42 × 10−3 | 1.87 × 10−4 |
| Zone 3b | 2.61 × 106 | 2.62 × 107 | 2.67 × 103 | 1.02 × 10−3 | 1.02 × 10−4 |
Total biofilm data are mean values for 6 independent experiments. Cell counts represent cells attached to the glass after rinsing (see Materials and Methods).
Zone data are mean values for 5 independent experiments.
DISCUSSION
Due to the close cell-to-cell contact and minimal shear force in biofilms, the hypothesis has been raised that exchange of genetic information mediated by conjugative plasmid transfer should be highly efficient in this ubiquitous form of bacterial growth. To test this postulate, different experimental settings for growing biofilms, different bacterial hosts and plasmids, and different plasmid transfer detection methods have been used in previous studies (3, 12, 14, 26, 31, 46, 47, 49–51, 53, 54). Since the detection of plasmid transfer in situ in spatially structured bacterial populations has many technical challenges (65), direct evidence of extensive conjugative gene transfer in biofilms is not convincing so far. In this study, we developed and used several biofilm flow cell and reporter systems to determine some of the basic parameters that govern transfer of the IncP-1 plasmid pB10 in E. coli biofilms. Our results indicate that plasmid transfer occurs at very low frequencies in typical thick biofilms submerged in liquid due to the intricate spatial architecture of biofilms sometimes causing physical separation between clonal populations. However, the broad definition of biofilms also includes pellicles floating at the air-liquid interface (16). We found that plasmid pB10 could efficiently invade floating pellicles as well as surface-attached biofilms close to this interface. Recently, Nguyen et al. showed that transfer of chromosomal markers among Mycobacterium smegmatis strains also occurred predominantly at the air-liquid interface (52).
Our findings strongly suggest that many bacterial communities that are naturally floating at the air-liquid interface may be hot spots for plasmid-mediated gene transfer. Examples of such natural hot spots are the scum layers on standing bodies of water and the surface microlayers (SML) in lakes and oceans (17, 28). Our postulate is corroborated by various studies that observed plasmid transfer or detected plasmids in such natural ecosystems (4, 19, 20, 32, 38, 44). There are multiple possible explanations for the observed increased plasmid transfer at the air-liquid interface compared to that in submerged biofilms, and they likely involve a combination of the physical properties of the environment as well as the physiological state of the cells. Here we discuss only a few possible scenarios, which can be separated into the colocalization hypothesis and the oxygen-effect-on-conjugation hypothesis.
First, the colocalization hypothesis states that higher densities of well-mixed donor and recipient populations at the air-liquid interface, as shown in Fig. 2 and 4, promote gene transfer by conjugation. It is known that in spatially structured populations, initial cell densities of both the donor and recipient, as well as their relative positions in the matrix, strongly determine the success of plasmid invasion, a phenomenon not observed in mixed liquids (27, 43, 64). Juxtaposition of donor and recipient cells is absolutely required for cells to exchange DNA by conjugation. In our horizontal flow cells, the formation of a surface-attached E. coli K-12 biofilm required the presence of plasmid pB10, and therefore donor and recipient populations were separated out rather than being well mixed. This is in agreement with previously published observations that E. coli K-12 strains barely form biofilms in various flow cells (56, 57) and that their ability to adhere to surfaces is increased by the presence of conjugative plasmids (10, 29, 50, 54, 56, 57, 69). In the batch culture system, E. coli MG1655 without plasmid formed a biofilm mostly at the air-liquid interface (Fig. 4B). Previous studies have also shown an increased ability of E. coli strains to form biofilms at the air-liquid interface, even in the absence of conjugative plasmids (11, 15, 22). Thus, the low transfer frequencies in submerged biofilms of E. coli K-12 must be due in part to the poor ability of the plasmid-free strain to adhere and form a biofilm.
To test our hypothesis that the absence of dense mixed populations of donor and recipient cells in the lower zones of the vertical biofilms is the basis of poor plasmid transfer, we used an E. coli csrA mutant known to form thick biofilms as the recipient strain (35). While this strain also formed the densest biofilm at the air-liquid interface, unlike E. coli MG1655, it also covered deeper zones on the microscope slide in the batch culture biofilm reactor (Fig. 4C). Because of the gradient in recipient densities along the slide, there again were more transconjugants at the air-liquid interface per donor cell attached (Table 2). However, similar transconjugant/recipient ratios were found at different depths, suggesting that the recipient density was the limiting factor, not the conjugation efficiency itself. While the csrA mutation may have unknown effects on plasmid transferability, comparison of the results for wild-type E. coli and the csrA mutant suggests that the air-liquid interface is a hot spot for plasmid invasion because it facilitates the formation of dense, well-mixed biofilms of both plasmid donors and recipients.
One of the factors causing the higher densities of donor and recipient cells at the air-liquid interface is likely the higher oxygen concentration in this zone. Steep oxygen gradients were observed in the vertical flow cell (Fig. 3) and the batch culture reactor (data not shown) and correlated with decreasing cell densities in the biofilms along the glass surface. While the physicochemical conditions of the air-liquid interface may also directly promote adherence of E. coli cells to each other and to the glass surface, the mere presence of more cells in that zone due to aerobic conditions may well explain the larger proportion of adhering cells and therefore the increased cell-to-cell contact. However, previous studies have shown that E. coli does not form biofilms under anaerobic or anoxic conditions, suggesting that there may be a specific biofilm inhibition mechanism in the absence of oxygen (11, 15). Future studies will have to clarify the role of aerobiosis in E. coli biofilm formation and determine if characteristics of the air-liquid interface other than the oxygen level are responsible for enhanced plasmid transfer.
The observation that single donor and recipient cells grew out into separate donor and recipient layers (Fig. 1) or microcolonies (Fig. 2C) in submerged biofilms, thus limiting gene exchange, is consistent with a recent observation made in Pseudomonas aeruginosa biofilms. Klayman et al. showed that a biofilm initiated with isogenic strains of P. aeruginosa carrying reporter plasmids (expressing CFP or yellow fluorescent protein [YFP]) was composed of separate clusters of cells containing a single fluorescent protein rather than clusters containing mixed populations (41). Tolker-Nielsen and Molin (67) also observed that Pseudomonas putida biofilms were formed as separate clusters of cells but that a few single cells were able to move between these microcolonies. The movement of recipient cells into a microcolony of donors or the rare mating in the donor-recipient contact zone could result in conjugation and probably explains the presence of single fluorescent transconjugant cells in our submerged horizontal and vertical flow cell biofilms.
The second hypothesis is that conjugation itself is affected by the oxygen concentration gradient in the biofilms, either directly through an oxygen-regulated mechanism or indirectly through its effect on cell physiology. Conjugation is known to require energy needed for DNA replication and protein biosynthesis in both donor and recipient cells (18, 58). Christensen et al. showed that the presence of transconjugants at the surfaces of biofilm microcolonies coincided with a greater metabolic activity of those cells (12). In addition, Salyers and coworkers concluded previously that efficient transfer of IncP-1 plasmids requires oxygen (59, 63). Our observations support these conclusions, since plasmid transfer frequencies in both filter and liquid mating experiments were significantly lower under anaerobic conditions than under aerobic conditions. However, aerobic precultivation obliterated this difference. The cells in our submerged biofilms were likely exposed to traces of oxygen (Fig. 3). Moreover, the presence of transconjugants in the deeply submerged zones of the batch culture E. coli csrA biofilms at similar T/R ratios to those at the air-liquid interface (Fig. 4D) indicates that oxygen was not the limiting factor. We therefore conclude that our biofilm results cannot be explained by a direct effect of oxygen on plasmid transfer frequencies.
While it is known that DsRed and GFP require oxygen for proper folding and fluorescence (55), we do not think that a lack of fluorescence was the cause of the low densities of transconjugants observed in submerged biofilms. First, single or small clusters of fluorescent transconjugants were observed in those locations (e.g., Fig. 2B and D and 4D), indicating that the fluorescent proteins were expressed and folded correctly. Second, the last experiments in batch culture reactors with the wild type and the csrA mutant showed similar transconjugant/recipient ratios along the slide for GFP (Fig. 4), DsRed, and the oxygen-independent Evoglow protein.
Based on previous reports and our own observations, we conclude that conjugative transfer of IncP-1 plasmids was hindered in the submerged E. coli K-12 biofilms largely by spatial separation of donor and recipient cells and was promoted at the air-liquid interface by the high densities of juxtaposed recipient and donor cells. However, plasmid transfer was not limited to the air-liquid interface: when the plasmid-receiving E. coli strain was a good biofilm former, which is the case for many pathogenic E. coli strains (6, 40), conjugation also occurred extensively in submerged biofilms. Given the high medical, environmental, and industrial importance of bacterial biofilms and of the promiscuous multidrug resistance and catabolic plasmids of the IncP-1 group, greater insight is needed into the conditions and plasmid-host combinations that affect the rate of gene spread through these ubiquitous bacterial ecosystems. The experimental systems described here can be used further to study the effects of various parameters on plasmid transfer in biofilms.
ACKNOWLEDGMENTS
This work was supported by NIH grant 1R01 GM73821 from the National Institute for General Medical Sciences (NIGMS). Funding for the Olympus Fluoview FV1000 multiphoton confocal microscope was obtained from the M. J. Murdock Charitable Trust (grant 2006-127JVZ022207) and from the NIH (small instrumentation grant 1S10RR02260601).
We thank L. Forney, H.-Y. La, and X. Zhong for helpful suggestions, A. Paszczynski for use of his anaerobic chamber, and K. Turner and L. Sherick for technical assistance. We are also grateful to T. Romeo (University of Florida, Gainesville, FL) for providing E. coli MG1655 csrA.
J.E.K. designed the dual reporter system, constructed all strains and vectors, designed vertical flow cell and batch culture biofilm experimental settings, performed the plasmid transfer experiments, interpreted data, and wrote the manuscript draft. H.D.N. and H.B. measured and analyzed oxygen profiles. L.M.R. provided technical assistance and helped with manuscript writing. E.M.T. and S.M.K. oversaw the project, provided help with the experimental design and data interpretation, and assisted in writing the manuscript.
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Adamczyk M., Jagura-Burdzy G. 2003. Spread and survival of promiscuous IncP-1 plasmids. Acta Biochim. Pol. 50:425–453 [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angles M. L., Marshall K. C., Goodman A. E. 1993. Plasmid transfer between marine bacteria in the aqueous phase and biofilms in reactor microcosms. Appl. Environ. Microbiol. 59:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashelford K. E., et al. 1997. Using microcosms to study gene transfer in aquatic habitats. FEMS Microbiol. Ecol. 23:81–94 [Google Scholar]
- 5. Aspray T. J., Hansen S. K., Burns R. G. 2005. A soil-based microbial biofilm exposed to 2,4-D: bacterial community development and establishment of conjugative plasmid pJP4. FEMS Microbiol. Ecol. 54:317–327 [DOI] [PubMed] [Google Scholar]
- 6. Beloin C., Roux A., Ghigo J. M. 2008. Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 322:249–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beyenal H., Donovan C., Lewandowski Z., Harkin G. 2004. Three-dimensional biofilm structure quantification. J. Microbiol. Methods 59:395–413 [DOI] [PubMed] [Google Scholar]
- 8. Bonot S., Merlin C. 2010. Monitoring the dissemination of the broad-host-range plasmid pB10 in sediment microcosms by quantitative PCR. Appl. Environ. Microbiol. 76:378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley D. E., Taylor D. E., Cohen D. R. 1980. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J. Bacteriol. 143:1466–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burmølle M., Bahl M. I., Jensen L. B., Sørensen S. J., Hansen L. H. 2008. Type 3 fimbriae, encoded by the conjugative plasmid pOLA52, enhance biofilm formation and transfer frequencies in Enterobacteriaceae strains. Microbiology 154:187–195 [DOI] [PubMed] [Google Scholar]
- 11. Cabellos-Avelar T., Souza V., Membrillo-Hernandez J. 2006. Spent media from cultures of environmental isolates of Escherichia coli can suppress the deficiency of biofilm formation under anoxic conditions of laboratory E. coli strains. FEMS Microbiol. Ecol. 58:414–424 [DOI] [PubMed] [Google Scholar]
- 12. Christensen B. B., et al. 1998. Establishment of new genetic traits in a microbial biofilm community. Appl. Environ. Microbiol. 64:2247–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christensen B. B., Sternberg C., Andersen J. B., Molin O. S. 1998. In situ detection of gene transfer in a model biofilm engaged in degradation of benzyl alcohol. APMIS Suppl. 84:25–28 [DOI] [PubMed] [Google Scholar]
- 14. Christensen B. B., Sternberg C., Molin S. 1996. Bacterial plasmid conjugation on semi-solid surfaces monitored with the green fluorescent protein (GFP) from Aequorea victoria as a marker. Gene 173:59–65 [DOI] [PubMed] [Google Scholar]
- 15. Colon-Gonzalez M., Mendez-Ortiz M. M., Membrillo-Hernandez J. 2004. Anaerobic growth does not support biofilm formation in Escherichia coli K-12. Res. Microbiol. 155:514–521 [DOI] [PubMed] [Google Scholar]
- 16. Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 17. Cunliffe M., Murrell J. C. 2009. The sea-surface microlayer is a gelatinous biofilm. ISME J. 3:1001–1003 [DOI] [PubMed] [Google Scholar]
- 18. Curtiss R., III, Charamella L. J., Stallions D. R., Mays J. A. 1968. Parental functions during conjugation in Escherichia coli K-12. Microbiol. Mol. Biol. Rev. 32:320–348 [PMC free article] [PubMed] [Google Scholar]
- 19. Dahlberg C., Bergström M., Hermansson M. 1998. In situ detection of high levels of horizontal plasmid transfer in marine bacterial communities. Appl. Environ. Microbiol. 64:2670–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahlberg C., Linberg C., Torsvik V., Hermansson M. 1997. Conjugative plasmids isolated from bacteria in marine environments show various degrees of homology to each other and are not closely related to well-characterized plasmids. Appl. Environ. Microbiol. 63:4692–4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Alvise P. W., et al. 2010. TOL plasmid carriage enhances biofilm formation and increases extracellular DNA content in Pseudomonas putida KT2440. FEMS Microbiol. Lett. 312:84–92 [DOI] [PubMed] [Google Scholar]
- 22. Danese P. N., Pratt L. A., Kolter R. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Beer D., Stoodley P., Roe F., Lewandowski Z. 1994. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 43:1131–1138 [DOI] [PubMed] [Google Scholar]
- 24. De Gelder L., Vandecasteele F. P., Brown C. J., Forney L. J., Top E. M. 2005. Plasmid donor affects host range of promiscuous IncP-1β plasmid pB10 in an activated-sludge microbial community. Appl. Environ. Microbiol. 71:5309–5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dröge M., Pühler A., Selbitschka W. 2000. Phenotypic and molecular characterization of conjugative antibiotic resistance plasmids isolated from bacterial communities of activated sludge. Mol. Gen. Genet. 263:471–482 [DOI] [PubMed] [Google Scholar]
- 26. Ehlers L. J., Bouwer E. J. 1999. RP4 plasmid transfer among species of Pseudomonas in a biofilm reactor. Water Sci. Technol. 39:163–171 [Google Scholar]
- 27. Fox R. E., Zhong X., Krone S. M., Top E. M. 2008. Spatial structure and nutrients promote invasion of IncP-1 plasmids in bacterial populations. ISME J. 2:1024–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franklin M. P., et al. 2005. Bacterial diversity in the bacterioneuston (sea surface microlayer): the bacterioneuston through the looking glass. Environ. Microbiol. 7:723–736 [DOI] [PubMed] [Google Scholar]
- 29. Ghigo J. M. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442–445 [DOI] [PubMed] [Google Scholar]
- 30. Haagensen J. A., Hansen S., Johansen T., Molin S. 2002. In situ detection of horizontal transfer of mobile genetic elements. FEMS Microbiol. Ecol. 42:261–268 [DOI] [PubMed] [Google Scholar]
- 31. Hausner M., Wüertz S. 1999. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 65:3710–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hermansson M., Linberg C. 1994. Gene transfer in the marine environment. FEMS Microbiol. Ecol. 15:47–54 [Google Scholar]
- 33. Herrero M., de Lorenzo V., Timmis K. N. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heuer H., Smalla K. 2007. Horizontal gene transfer between bacteria. Environ. Biosafety Res. 6:3–13 [DOI] [PubMed] [Google Scholar]
- 35. Jackson D. W., et al. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jacoby G. A., Gacharna N., Black T. A., Miller G. H., Hooper D. C. 2009. Temporal appearance of plasmid-mediated quinolone resistance genes. Antimicrob. Agents Chemother. 53:1665–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson T. J., Nolan L. K. 2009. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 73:750–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones G. W., Baines L., Genthner F. J. 1991. Heterotrophic bacteria of the freshwater neuston and their ability to act as plasmid recipients under nutrient deprived conditions. Microb. Ecol. 22:15–25 [DOI] [PubMed] [Google Scholar]
- 39. Jorquera M., Yamaguchi N., Tani K., Nasu M. 2006. A combination of direct viable counting, fluorescence in situ hybridization, and green fluorescent protein gene expression for estimating plasmid transfer at the single cell level. Microbes Environ. 21:101–111 [Google Scholar]
- 40. Kaper J. B., Nataro J. P., Mobley H. L. T. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 41. Klayman B. J., Klapper I., Stewart P. S., Camper A. K. 2008. Measurements of accumulation and displacement at the single cell cluster level in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 10:2344–2354 [DOI] [PubMed] [Google Scholar]
- 42. Król J. E., Rogers L. M., Krone S. M., Top E. M. 2010. Dual reporter system for in situ detection of plasmid transfer under aerobic and anaerobic conditions. Appl. Environ. Microbiol. 76:4553–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krone S. M., Lu R., Fox R., Suzuki H., Top E. M. 2007. Modelling the spatial dynamics of plasmid transfer and persistence. Microbiology 153:2803–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leitet C., Riemann L., Hagström Ä. 2006. Plasmids and prophages in Baltic Sea bacterioplankton isolates. J. Mar. Biol. Assoc. U.K. 86:567–575 [Google Scholar]
- 45. Lewandowski Z., Beyenal H. 2007. Fundamentals of biofilm research. CRC Press, New York, NY [Google Scholar]
- 46. Licht T. R., Christensen B. B., Krogfelt K. A., Molin S. 1999. Plasmid transfer in the animal intestine and other dynamic bacterial populations: the role of community structure and environment. Microbiology 145:2615–2622 [DOI] [PubMed] [Google Scholar]
- 47. Lilley A. K., Bailey M. J. 2002. The transfer dynamics of Pseudomonas sp. plasmid pQBR11 in biofilms. FEMS Microbiol. Ecol. 42:243–250 [DOI] [PubMed] [Google Scholar]
- 48. Mølbak L., Licht T. R., Kvist T., Kroer N., Andersen S. R. 2003. Plasmid transfer from Pseudomonas putida to the indigenous bacteria on alfalfa sprouts: characterization, direct quantification, and in situ location of transconjugant cells. Appl. Environ. Microbiol. 69:5536–5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mølbak L., Molin S., Kroer N. 2007. Root growth and exudate production define the frequency of horizontal plasmid transfer in the rhizosphere. FEMS Microbiol. Ecol. 59:167–176 [DOI] [PubMed] [Google Scholar]
- 50. Molin S., Tolker-Nielsen T. 2003. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr. Opin. Biotechnol. 14:255–261 [DOI] [PubMed] [Google Scholar]
- 51. Nancharaiah Y. V., et al. 2003. Dual labeling of Pseudomonas putida with fluorescent proteins for in situ monitoring of conjugal transfer of the TOL plasmid. Appl. Environ. Microbiol. 69:4846–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nguyen K. T., Piastro K., Gray T. A., Derbyshire K. M. 2010. Mycobacterial biofilms facilitate horizontal DNA transfer between strains of Mycobacterium smegmatis. J. Bacteriol. 192:5134–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Normander B., Christensen B. B., Molin S., Kroer N. 1998. Effect of bacterial distribution and activity on conjugal gene transfer on the phylloplane of the bush bean (Phaseolus vulgaris). Appl. Environ. Microbiol. 64:1902–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ong C. L., Beatson S. A., McEwan A. G., Schembri M. A. 2009. Conjugative plasmid transfer and adhesion dynamics in an Escherichia coli biofilm. Appl. Environ. Microbiol. 75:6783–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reid B. G., Flynn G. C. 1997. Chromophore formation in green fluorescent protein. Biochemistry 36:6786–6791 [DOI] [PubMed] [Google Scholar]
- 56. Reisner A., Haagensen J. A. J., Schembri M. A., Zechner E. L., Molin S. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933–946 [DOI] [PubMed] [Google Scholar]
- 57. Reisner A., Höller B. M., Molin S., Zechner E. L. 2006. Synergistic effects in mixed Escherichia coli biofilms: conjugative plasmid transfer drives biofilm expansion. J. Bacteriol. 188:3582–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rittmann B. E., Smets B. F., MacDonald J. A., Stahl D. A. 1995. Plasmid transfer for enhancing degradation capabilities. Environ. Health Perspect. 103:113–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salyers A. A., Bonheyo G., Shoemaker N. B. 2000. Starting a new genetic system: lessons from Bacteroides. Methods 20:35–46 [DOI] [PubMed] [Google Scholar]
- 60. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 61. Schlüter A., et al. 2003. The 64 508 bp IncP-1β antibiotic multiresistance plasmid pB10 isolated from a waste-water treatment plant provides evidence for recombination between members of different branches of the IncP-1β group. Microbiology 149:3139–3153 [DOI] [PubMed] [Google Scholar]
- 62. Seoane J., et al. 2010. An individual-based approach to explain plasmid invasion in bacterial populations. FEMS Microbiol. Ecol. 75:17–27 [DOI] [PubMed] [Google Scholar]
- 63. Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. 1986. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J. Bacteriol. 165:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simonsen L. 1990. Dynamics of plasmid transfer on surfaces. J. Gen. Microbiol. 136:1001–1007 [DOI] [PubMed] [Google Scholar]
- 65. Sørensen S. J., Bailey M., Hansen L. H., Kroer N., Wüertz S. 2005. Studying plasmid horizontal transfer in situ: a critical review. Nat. Rev. Microbiol. 3:700–710 [DOI] [PubMed] [Google Scholar]
- 66. Sørensen S. J., Jensen L. E. 1998. Transfer of plasmid RP4 in the spermosphere and rhizosphere of barley seedling. Antonie Van Leeuwenhoek 73:69–77 [DOI] [PubMed] [Google Scholar]
- 67. Tolker-Nielsen T., Molin S. 2000. Spatial organization of microbial biofilm communities. Microb. Ecol. 40:75–84 [DOI] [PubMed] [Google Scholar]
- 68. Top E. M., Springael D. 2003. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr. Opin. Biotechnol. 14:262–269 [DOI] [PubMed] [Google Scholar]
- 69. Yang X., Ma Q., Wood T. K. 2008. The R1 conjugative plasmid increases Escherichia coli biofilm formation through an envelope stress response. Appl. Environ. Microbiol. 74:2690–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhong X., Król J. E., Top E. M., Krone S. M. 2009. Accounting for mating pair formation in plasmid population dynamics. J. Theor. Biol. 262:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]