Abstract
UndAHRCR-6 was identified from the metal-reducing bacterium Shewanella sp. strain HRCR-6. Both in vivo and in vitro characterization results indicate that UndAHRCR-6 is an outer membrane endecaheme c-type cytochrome and probably has a key functional role in the extracellular reduction of iron [Fe(III)] oxides and uranium [U(VI)] by Shewanella sp. HRCR-6.
TEXT
In a recent study, 23 Shewanella strains were isolated from the Hanford Reach of the Columbia River (HRCR) (M. J. Marshall, D. W. Kennedy, A. E. Plymale, A. C. Dohnalkova, A. S. Beliaev, and J. K. Fredrickson, unpublished data). Analyses of the proteins from the metal-reducing HRCR isolates showed that they were rich in heme-containing proteins, and some of the strains had homologues of the outer membrane (OM) decaheme c-type cytochromes (c-Cyts) MtrC and OmcA of Shewanella oneidensis MR-1 (1, 10; Marshall et al., unpublished). However, little information is available regarding the functions of these c-Cyts because their encoding genes were not identified
Gene identification.
Shewanella isolates HRCR-2, -3, -4, -6, -7, -9, and -14 were selected for PCR screening of possible mtrC, omcA, and undA1 (a gene encoding putative an 11-heme c-Cyt) homologues with the primers listed in Table S1 in the supplemental material. All tested strains were positive for mtrC homologues. While HRCR-2, -4, and -7 were positive for omcA homologues, HRCR-3, -6, -9, and -14 were positive for undA1 homologues (Fig. 1). The undA1-like gene of the isolate HRCR-6 was cloned and sequenced by using a PCR-based method (5, 6) (GenBank accession number JF933770). Phylogenetic analysis of the deduced amino acid sequence of the sequenced gene revealed that it was 94% identical to UndA of S. baltica OS223 (see Fig. S1 in the supplemental material) (2, 9). Based on these results, this sequenced gene and its protein product were named undA and UndAHRCR-6, respectively.
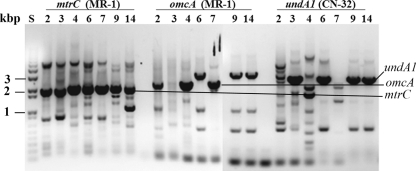
Fig. 1.
PCR amplification of the mtrC, omcA, and undA1 homologues from the genomes of 7 Shewanella HRCR isolates. Agarose gel showing the sizes of DNA standards in kilobase pairs (lane S) and PCR products amplified with the primers based on MR-1 mtrC or omcA or CN-32 undA1 from the genomic DNA of HRCR-2 (lane 2), HRCR-3 (lane 3), HRCR-4 (lane 4), HRCR-6 (lane 6), HRCR-7 (lane 7), HRCR-9 (lane 9), and HRCR-14 (lane 14). The PCR products whose sizes were similar to that of MR-1 mtrC, MR-1 omcA, or CN-32 undA1 are indicated.
FH and uranium [U(VI)] reduction by UndAHRCR-6.
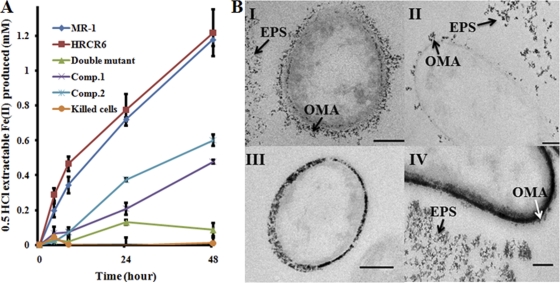
Ferrihydrite (FH) reduction was conducted in 1,4-piperazinediethanesulfonic acid-buffered M1 medium (pH 7.2) with 20 mM sodium lactate, 10 mM FH as the sole electron acceptor, and 1 × 108 cells ml−1 at 30°C with shaking (25 rpm) (4). Under this condition, MR-1 and HRCR-6 began to reduce FH within 4 h. At 48 h, MR-1 and HRCR-6 produced 1.18 ± 0.04 and 1.22 ± 0.13 mM iron [Fe(II)] (n = 3), respectively. At the same time, while the MR-1 ΔmtrC ΔomcA double deletion mutant with an empty vector produced only 0.09 ± 0.04 mM Fe(II) (n = 3), the mutant complemented with UndAHRCR-6 (Comp.1) or MtrC and OmcA (Comp.2) produced 0.48 ± 0.01 or 0.6 ± 0.03 mM Fe(II) (n = 3), which was 40% or 50% of that produced by MR-1, respectively. Under the same conditions, little Fe(II) was produced by the heat-killed MR-1 cells (Fig. 2A). These results demonstrate that when expressed in MR-1 cells, UndAHRCR-6 is a functional reductase of FH. All strains, plasmids, and primers used in this study are listed in Table S1 in the supplemental material.
Fig. 2.
Complementation of the MR-1 mutant without MtrC and OmcA by UndAHRCR-6. (A) Ferrihydrite reduction. Kinetics of Fe(II) formation are shown after reduction of 10 mM ferrihydrite by MR-1, HRCR-6, the ΔmtrC ΔomcA double mutant with empty vector, and the double mutant complemented with UndAHRCR-6 (Comp.1) or MtrC and OmcA (Comp.2). Heat-killed MR-1 cells were used as a negative control. The values reported are the means and standard deviations from triplicate biological measurements. (B) U(VI) reduction. Transmission electron microscopy examination of reduced UO2 after reduction of 250 μM U(VI) for 48 h by the MR-1 wild type (I), HRCR-6 (II), the ΔmtrC ΔomcA double mutant with empty vector (III), and the double mutant complemented with UndAHRCR-6 (IV). The outer membrane-associated (OMA) and extracellular polymeric substance (EPS)-associated UO2 are indicated by arrows. Bar = 200 nm.
Uranium [U(VI)] reduction was performed in 30 mM sodium bicarbonate at pH 7.4, 10 mM sodium lactate, and 250 μM uranyl acetate as the sole electron acceptor and 2 × 108 cells ml−1 at 30°C with shaking (25 rpm) (3). MR-1 and HRCR-6 reduced U(VI) by >90% within 48 h. Compared to that of MR-1, deletion of mtrC-omcA decreased MR-1's ability to reduce U(VI) by 25%. Complementation of the ΔmtrC ΔomcA mutant with UndAHRCR-6 did not significantly increase the rate of U(VI) reduction by the mutant (data not shown) but did impact the location of the reduction end product, UO2(s). Examination of the U cell suspensions by transmission electron microscopy revealed that in MR-1- and HRCR-6-mediated incubations, electron-dense materials were associated with the extracellular polymeric substance (EPS) and the cell envelope (Fig. 2BI and II). Previous analysis of these electron-dense particles formed under similar conditions indicated that they were UO2 (3). In the ΔmtrC ΔomcA mutant with the empty vector, UO2 was present mainly in the periplasm (Fig. 2BIII), likely due to transport of U(IV) across the OM, where it can be reduced by periplasmic c-Cyts. In comparison, in the mutant complemented with UndAHRCR-6, UO2 was present not only in the periplasm but also in association with the OM and EPS (Fig. 2BIV). Complementation of the ΔmtrC ΔomcA double deletion mutant with UndAHRCR-6, thus, increases UO2 formation on the cell surface and in the EPS. This supports the prediction that UndAHRCR-6, like OmcA and MtrC, facilitates electron transfer to U(VI) externally to the cell and hence is an OM and extracellular protein. Given that MtrC and OmcA contribute only 25% of the total U(VI) reduction under the conditions tested and that UndAHRCR-6 only partially complements the mutant in FH reduction, an increase in the U(VI) reduction by addition of UndAHRCR-6 may be too small to be detected by the U(VI) reduction assay used in this study.
Extracellular secretion of UndAHRCR-6 by the MR-1 type II secretion system.
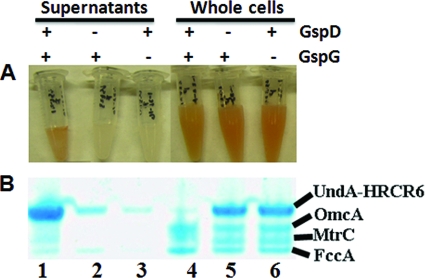
Nonacylated UndAHRCR-6 was constructed and was then heterologously expressed in MR-1 and in two MR-1 mutants without functional type II secretion systems (T2S) (i.e., ΔgspD and ΔgspG) under the culture condition in which the electron acceptor was limited and 20 mM lactate was used as the sole carbon and energy source (8). After it was concentrated, supernatant from the MR-1 with UndAHRCR-6 cultures was red, while the supernatants of the T2S mutants with UndAHRCR-6 were pale yellow (Fig. 3A). A dominant band of heme-containing protein with an apparent molecular mass of ∼90 kDa was found in the supernatant of MR-1 with UndAHRCR-6 (Fig. 3B). Western blot analysis of the same sample confirmed that this dominant heme-containing protein was UndAHRCR-6 (data not shown). Compared to the wild type (wt), much less UndAHRCR-6 was found in the culture supernatants of the ΔgspD and ΔgspG mutants. Conversely, more UndAHRCR-6 was found in the whole cells of the ΔgspD and ΔgspG mutants than in the wt, demonstrating that inactivation of the MR-1 T2S decreases extracellular transport of UndAHRCR-6 and increases its accumulation inside the cells (Fig. 3B). Thus, UndAHRCR-6 is recognized and secreted extracellularly by the MR-1 T2S.
Fig. 3.
Influence of the deletion of MR-1 gspD and gspG on the extracellular release of nonacylated UndAHRCR-6. (A) Concentrated supernatants (lanes 1 to 3) and whole cells (lanes 4 to 6) of wild-type MR-1 (lanes 1 and 4), the gspD mutant (lanes 2 and 5), and the gspG mutant (lanes 3 and 6) in which nonacylated UndAHRCR-6 was overexpressed. (B) Heme staining. A total of 1 μg of concentrated supernatant proteins (lanes 1 to 3) and 10 μg of whole-cell lysate proteins (lanes 4 to 6) from the MR-1 wild type (lanes 1 and 4), gspD mutant (lanes 2 and 5), and gspG mutant (lanes 3 and 6) were separated by SDS-PAGE and then visualized by heme staining.
Purification and characterization of recombinant UndAHRCR-6.
Recombinant UndAHRCR-6 was overexpressed in MR-1 cells. Following ultracentrifugation, UndAHRCR-6 was solubilized from membrane fractions with 3-[(3-cholamidopropyl)-dimethylammonio]-propanesulfonic acid (CHAPS) and isolated by immobilized metal-ion affinity chromatography, followed by gel filtration chromatography (7) (see Fig. S2 in the supplemental material). Given that UndAHRCR-6 (i) is purified from membrane fractions, (ii) possesses a putative lipid-binding site (removal of this site renders the remaining protein water soluble without using CHAPS as a solubilizing regent), and (iii) impacts the formation of UO2 exterior to the OM, we conclude that UndAHRCR-6 is an OM c-Cyt. Inductively coupled plasma (ICP) analysis of the purified protein showed that its Fe content was 10.8 μmol per μmol of protein. Results of heme staining confirm that purified UndAHRCR-6 is a heme-containing protein (Fig. S2). Furthermore, the UndAHRCR-6 polypeptide contains 11 heme-binding motifs. Together, these results suggest that UndAHRCR-6 is an endecaheme (i.e., 11-heme) c-Cyt. Reduction kinetics of soluble Fe(III) complexed with citrate, nitrilotriacetic acid (NTA), or EDTA by the purified protein were investigated with a stopped-flow method (11). All reactions occurred in the following two stages: a fast stage that was completed in less than 1 s and a slow stage that followed the fast stage (see Fig. S3 in the supplemental material). The second-order rate constants calculated ranged from 12.336 μM−1 s−1 for the fast reduction of Fe(III)-EDTA to 0.013 μM−1 s−1 for the slow reduction of Fe(III)-citrate (Table 1). The in vitro rate of oxidation of fully reduced UndAHRCR-6 by Fe(III)-EDTA was more than 1 order of magnitude higher than the oxidation of either MtrC or OmcA. Consistent with UndAHRCR-6 being a facile reductant of complexed Fe(III), its rate of oxidation by Fe(III)-NTA was 2 to 16 times higher than those of MtrC and OmcA. Interestingly, the initial rate of oxidation of UndAHRCR-6 by Fe(III)-citrate was 24% of that of MtrC but nearly twice as high as the oxidation of OmcA (11). The different rates among UndAHRCR-6, MtrC, and OmcA in reducing these ferric complexes may be attributed to the structural difference among these c-Cyts, such as the extra heme group found in the UndAHRCR-6 polypeptide.
Table 1.
Kinetic rate constants for the reduction of Fe(III) complexes by UndAHRCR-6 at 25°C
| Complex | Rate constant (μM−1 s−1)a |
|
|---|---|---|
| First component | Second component | |
| Fe(III)-citrate | 0.0736 ± 0.0002 (60) | 0.0134 ± 0.0001 (40) |
| Fe(III)-NTA | 0.8078 ± 0.0180 (75) | 0.3453 ± 0.0021 (25) |
| Fe(III)-EDTA | 12.336 ± 0.365 (90) | 2.5752 ± 0.0269 (10) |
The final concentrations were 66.7 μM for the Fe(III) complexes and 3.3 μM for UndAHRCR-6. The numbers in parentheses represents the percent reductions.
Conclusions.
UndAHRCR-6, an OM endecaheme c-Cyt of Shewanella sp. HRCR-6, was identified and characterized. In MR-1, UndAHRCR-6 partially complemented a ΔmtrC ΔomcA mutant in an FH reduction assay, increased formation of UO2 associated with the OM and EPS in a U(VI) reduction assay, and is translocated across the OM to the extracellular environment by the T2S. Reduced UndAHRCR-6 was also rapidly oxidized by soluble Fe(III) complexes at rates comparable to or higher than those previously reported for MtrC and OmcA. Collectively, these results show that UndAHRCR-6 can serve as an extracellular metal reductase in MR-1 and is probably involved in extracellular reduction of Fe(III) oxides and U(VI) by Shewanella sp. HRCR-6.
Supplementary Material
Acknowledgments
This research was supported by the Subsurface Biogeochemical Research program (SBR)/Office of Biological and Environmental Research (BER), U.S. Department of Energy (DOE). A portion of the research was performed using EMSL, a national scientific user facility sponsored by the DOE-BER and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated for the DOE by Battelle under contract DE-AC05-76RLO 1830.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 7 June 2011.
REFERENCES
- 1. Cao B., et al. 2011. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 13:1018–1031 [DOI] [PubMed] [Google Scholar]
- 2. Fredrickson J. K., et al. 2008. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 6:592–603 [DOI] [PubMed] [Google Scholar]
- 3. Marshall M. J., et al. 2006. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 4:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reardon C. L., et al. 2010. Role of outer-membrane cytochromes MtrC and OmcA in the biomineralization of ferrihydrite by Shewanella oneidensis MR-1. Geobiology 8:56–68 [DOI] [PubMed] [Google Scholar]
- 5. Shi L., Carmichael W. W. 1997. pp1-cyano2, a protein serine/threonine phosphatase 1 gene from the cyanobacterium Microcystis aeruginosa UTEX 2063. Arch. Microbiol. 168:528–531 [DOI] [PubMed] [Google Scholar]
- 6. Shi L., Carmichael W. W., Kennelly P. J. 1999. Cyanobacterial PPP family protein phosphatases possess multifunctional capabilities and are resistant to microcystin-LR. J. Biol. Chem. 274:10039–10046 [DOI] [PubMed] [Google Scholar]
- 7. Shi L., et al. 2006. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J. Bacteriol. 188:4705–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi L., et al. 2008. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J. Bacteriol. 190:5512–5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi L., Zhang W. 2004. Comparative analysis of eukaryotic-type protein phosphatases in two streptomycete genomes. Microbiology 150:2247–2256 [DOI] [PubMed] [Google Scholar]
- 10. Turse J. E., Marshall M. J., Fredrickson J. K., Lipton M. S., Callister S. J. 2010. An empirical strategy for characterizing bacterial proteomes across species in the absence of genomic sequences. PLoS One 5:e13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z., et al. 2008. Reduction kinetics of Fe(III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 74:6746–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.