Abstract
The role of structure and molecular weight in fermentation selectivity in linear α-1,6 dextrans and dextrans with α-1,2 branching was investigated. Fermentation by gut bacteria was determined in anaerobic, pH-controlled fecal batch cultures after 36 h. Inulin (1%, wt/vol), which is a known prebiotic, was used as a control. Samples were obtained at 0, 10, 24, and 36 h of fermentation for bacterial enumeration by fluorescent in situ hybridization and short-chain fatty acid analyses. The gas production of the substrate fermentation was investigated in non-pH-controlled, fecal batch culture tubes after 36 h. Linear and branched 1-kDa dextrans produced significant increases in Bifidobacterium populations. The degree of α-1,2 branching did not influence the Bifidobacterium populations; however, α-1,2 branching increased the dietary fiber content, implying a decrease in digestibility. Other measured bacteria were unaffected by the test substrates except for the Bacteroides-Prevotella group, the growth levels of which were increased on inulin and 6- and 70-kDa dextrans, and the Faecalibacterium prausnitzii group, the growth levels of which were decreased on inulin and 1-kDa dextrans. A considerable increase in short-chain fatty acid concentration was measured following the fermentation of all dextrans and inulin. Gas production rates were similar among all dextrans tested but were significantly slower than that for inulin. The linear 1-kDa dextran produced lower total gas and shorter time to attain maximal gas production compared to those of the 70-kDa dextran (branched) and inulin. These findings indicate that dextrans induce a selective effect on the gut flora, short-chain fatty acids, and gas production depending on their length.
INTRODUCTION
The composition of the gut microbiota, as well as many of its physiological traits, can be modified by changes in diet (38, 55). This has generated interest in the development of functional foods that may influence gut microbiota composition to improve or maintain host health. One example is the prebiotic approach, recently defined as “The selective stimulation of growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host” (46). To date, the majority of studies on prebiotics have focused on inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), and lactulose. These groups of carbohydrates are at the forefront of commercial prebiotics due to their efficacy (22, 44), testing in humans (4, 57), and history of safe commercial use (36). However, there are nondigestible carbohydrates under investigation, such as glucooligosaccharides. According to Gibson (21), even though glucooligosaccharides exhibit promising characteristics, the evidence is not sufficient to classify them as prebiotics. Therefore, further investigation focusing on the structure-function relationship of these oligosaccharides will add to the current knowledge of their metabolism and prebiotic functionality.
Dextran is an extracellular bacterial polymer of d-glucopyranose that is composed of various chain lengths (from 10 to 1,000 kDa) with predominantly α-1,6 linkage in the main chain and a variable amount of α-1,2-, α-1,3-, and α-1,4-branched linkages (31). It is produced by lactic acid bacteria, primarily Leuconostoc strains, but other workers have reported dextran formation from different bacteria, such as Streptococcus, Lactococcus, and Lactobacillus, where the specificity of the synthesized linkages in the dextran is strain dependent (60). Commercially, dextran is produced by the fermentation of sucrose-rich media, mainly with Leuconostoc species, with the precipitation of dextran by chilled ethanol (52). Several research workers have optimized fermentation conditions for maximum dextran production (2, 62). It has been reported that molecular size and yield of dextran production depend on process variables, such as temperature, sucrose concentration, and acceptor concentration (52).
The strain Leuconostoc citreum NRRL B-1299 produces a dextran polymer containing 27 to 35% α-1,2 glycosidic branching linkages, besides a limited amount of α-1,3 glycosidic branching linkages (33). However, other strains producing α-1,2-branched dextrans have been described recently (5). Brison et al. (8) showed that the content of α-1,2 linkage and the molecular mass of branched dextrans produced by transglucosidase from Leuconostoc mesenteroides NRRL B-1299 can be controlled by varying the initial molarity of the sucrose/dextran ratio, producing dextrans with α-1,2 linkage contents ranging from 13 to 40%. This procedure resulted in the production of dextrans with the highest content of α-1,2 linkages ever reported (8). Such α-1,2 glycosidic linkages present at or near the nonreducing end result in high resistance to hydrolysis by digestive enzymes in both humans and animals (58). These properties make this type of dextran an interesting candidate prebiotic (15). Indeed, according to some studies, these glucooligosaccharides are metabolized by certain species of beneficial intestinal flora, e.g., bifidobacteria, lactobacilli, and particularly Bacteroides, and they are poorly metabolized by potentially detrimental strains (14, 15). Chung and Day have shown that branched-chain glucooligosaccharides produced using Leuconostoc mesenteroides B-742 are readily utilized by bifidobacteria and lactobacilli but not by Salmonella spp. or Escherichia coli in a pure-culture study (11). A study performed by Valette et al. (58) shows that the α-1,2-branched-chain glucooligosaccharides are not significantly metabolized by gnotobiotic rats. However, all of these studies were carried out with culture-dependent methods, which do not reflect the true potential of test substrates in mixed populations. Therefore, further investigation using culture-independent (DNA-based) methods following in vitro and in vivo experiments is needed to verify these initial findings.
The aim of this study was to carry out preliminary investigations on the fermentation selectivity of oligodextrans by human fecal microbiota to obtain structure-function information.
MATERIALS AND METHODS
Materials.
Unless stated otherwise, all reagents and chemicals used were purchased from Sigma Laboratories (Gillingham, Dorset, United Kingdom). The following nondigestible carbohydrates, synthesized as described in Brison et al. (8), were evaluated: dextran at 1 kDa, dextran at 1 kDa with 16% α-1,2 linkages (designated 1 kDa dextran + 16% α-1,2; molecular mass, 1,200 Da), dextran at 1 kDa with 32% α-1,2 linkages (1 kDa + 32% α-1,2; molecular mass, 1,500 Da), dextran at 6 kDa, dextran at 6 kDa with 33% α-1,2 linkages (6 kDa dextran + 33% α-1,2; molecular mass, 9,000 Da), dextran at 70 kDa, dextran at 70 kDa with 15% α-1,2 linkages (70 kDa dextran + 15% α-1,2; molecular mass, 82,000 Da), and dextran at 70 kDa with 37% α-1,2 linkages (70 kDa dextran + 37% α-1,2; molecular mass, 110,000 Da). Their main features, including degree of purity, total dietary fiber percentage, theoretical molecular sizes, and degrees of branching, are reported in Table 1. Inulin Frutafit TEX (Sensus, Roosendaal, The Netherlands) was used as a positive control. All test substrates were supplied by Tate & Lyle Innovation Centre, Lille, France.
Table 1.
Molecular mass properties of the oligodextrans
| Dextran compound | Avg size of backbone (Da) | Degree of α-1,2 branching (%) | Avg size of oligodextrana (Da) | Total dietary fiberb (%) | DP < 3 (%) | DP > 3 (%) |
|---|---|---|---|---|---|---|
| 1 kDa | 1,000 | 0 | 1,000 | 74 | 1.6 | 98.4 |
| 1 kDa + 16% α-1,2 | 1,000 | 16 | 1,190 | 101 | 0.1 | 99.9 |
| 1 kDa + 32% α-1,2 | 1,000 | 32 | 1,493 | 101 | 0.0 | 100 |
| 6 kDa | 6,000 | 0 | 6,000 | 62 | 0.4 | 99.6 |
| 6 kDa + 33% α-1,2 | 6,000 | 33 | 8,955 | 82 | 0.2 | 99.8 |
| 70 kDa | 70,000 | 0 | 70,000 | 88 | 1.4 | 98.6 |
| 70 kDa + 15% α-1,2 | 70,000 | 15 | 82,353 | NAc | 0.6 | 99.4 |
| 70 kDa + 37% α-1,2 | 70,000 | 37 | 111,111 | 102 | 2.2 | 97.8 |
Average size of oligodextrans is a theoretical molecular mass calculated from the total size of the backbone plus the total degree of branching.
Total dietary fibers of the compounds were determined by the total dietary fiber analysis method AOAC 2009.01.
NA, data not available.
Molecular mass determination.
Test substrates with low degrees of polymerization (DP) (dextran at 1 kDa) were analyzed by high-performance liquid chromatography (HPLC) performed with a Shimadzu RID10A connected to a Bio-Rad Aminex column HPX-87K. The column was heated at 80°C and the flow rate adjusted to 0.6 ml/min. High purity standards of fructose, sucrose, glucose, maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose, and maltoheptaose were used for external calibration.
Test substrates of higher DP (dextran at 6 and 70 kDa) were analyzed by gel permeation chromatography, which was performed with a TGA 300 series Viscotek chromatograph unit equipped with a refractive index detector connected to a Tosoh TSKGel G3000PWXL column. The column was heated at 30°C and the flow rate adjusted to 0.75 ml/min. High purity standards of dextran at 50, 12, 25, and 50 kDa from Leuconostoc mesenteroides were used for external calibration. Standards and samples were prepared by dissolution in the mobile phase (water) at 0.3%. The determination of the molecular mass and average molecular masses were made using conventional calibrations of the different standards expressed as a log function of the molecular mass versus the elution volume.
Determination of degree of branching determination.
The determination of the degree of branching of the test substrates was described in Brison et al. (8). The degree of α-1,2 branching is the number of glucose units in the backbone bearing, or coupled to, a glucose unit in an α-1,2 position versus the total number of glucose units present in the molecule, expressed as a percentage. Thus, the branching degree refers to the percentage of glucose units in an α-1,2 position of the entire molecule, not just of the backbone. The degree of branching was determined by nuclear magnetic resonance (NMR) spectra. 1H-NMR is preferred over 13C-NMR, as it provides a higher signal/noise ratio for signals from anomeric protons of the pure α-1,2-branched α-1,6 oligodextrans.
Fecal inocula.
Fecal samples were obtained from four healthy human volunteers (30 to 36 years old) who were free of known metabolic and gastrointestinal diseases (e.g., diabetes, ulcerative colitis, Crohn's disease, irritable bowel syndrome, peptic ulcers, and cancer). The samples were collected on site, kept in an anaerobic cabinet (10% H2, 10% CO2, and 80% N2), and used within a maximum of 15 min after collection. Samples were diluted 1/10 (wt/wt) in anaerobic phosphate-buffered saline (PBS; 0.1 mol/liter, pH 7.4) and homogenized in a stomacher (Stomacher 400; Seward, West Sussex, United Kingdom) for 2 min at normal speed.
In vitro fermentations.
Sterile stirred batch culture fermentation systems (50-ml working volume) were set up and aseptically filled with a 45-ml volume of sterile, prereduced, 2 g/liter basal medium peptone water (Oxoid), 2 g/liter yeast extract (Oxoid, Basingstoke, United Kingdom), 0.1 g/liter NaCl, 0.04 g/liter K2HPO4, 0.04 g/liter KH2PO4, 0.01 g/liter MgSO4·7H2O, 0.01 g/liter CaCl2·6H2O, 2 g/liter NaHCO3, 2 ml Tween 80 (BDH, Poole, United Kingdom), 0.05 g/liter hemin, 10 μl vitamin K1, 0.5 g/liter cysteine·HCl, 0.5 g/liter bile salts, pH 7.0, and gassed overnight with oxygen-free nitrogen (15 ml/min).
The carbohydrates (8 dextrans and inulin, 1/100 [wt/vol]) were added to the respective fermentation vessels just prior to the addition of the fecal slurry. The temperature was kept at 37°C, and the pH was kept between 6.7 and 6.9 using an automated pH controller (Fermac 260; Electrolab, Tewkesbury, United Kingdom). Each vessel was inoculated with 5 ml of fresh fecal slurry (1/10, wt/wt). The batch cultures were run for a period of 36 h, and 5-ml samples were obtained from each vessel at 0, 10, 24, and 36 h for fluorescent in situ hybridization (FISH) and short-chain fatty acid (SCFA) analysis.
Bacterial enumeration.
Synthetic oligonucleotide probes targeting specific regions of the 16S rRNA molecule and labeled with the fluorescent dye Cy3 were utilized for the enumeration of bacterial groups (Table 2). Labeled cells were visualized using fluorescent microscopy.
Table 2.
16S rRNA oligonucleotide probes used in this study
| Probe name | Specificity | Reference |
|---|---|---|
| Chis150 | Most of the Clostridium histolyticum group (Clostridium cluster I and II) | 20 |
| Lab158 | Lactobacillus-Enterococcus | 25 |
| Erec482 | Most of the Clostridium coccoides-Eubacterium rectale group (Clostridium cluster XIVa and XIVb) | 20 |
| Prop853 | Clostridium cluster IX | 64 |
| Fpra655 | Faecalibacterium prausnitzii and relatives | 28 |
| Rbro730 | Clostridium sporosphaeroides, Ruminococcus bromii, Clostridium leptum | 24 |
| Rfla729 | Ruminococcus albus, Ruminococcus flavefaciens | 24 |
| Bac303 | Most Bacteroidaceae and Prevotellaceae, some Porphyromonadaceae | 39 |
| Bif164 | Bifidobacterium spp. | 34 |
| Ato291 | Atopobium cluster | 23 |
Samples (375 μl) obtained from each vessel at each sampling time were fixed for 4 h (4°C) in 1,125 μl 4% (wt/vol) paraformaldehyde. Fixed cells were centrifuged at 13,000 × g for 5 min and washed twice in 1 ml filter-sterilized PBS. The washed cells were resuspended in 150 μl filtered PBS and stored in 150 μl ethanol (99%) at −20°C for at least 1 h before further processing. Samples (10 μl) were diluted in a suitable volume of PBS to obtain 20 to 100 fluorescent cells in each field of view, and 20 μl of the solution was added to each well of a six-well polytetrafluoroethylene/poly-l-lysine-coated slide (Tekdon Inc., Myakka City, FL). The samples were dried for 15 min in a drying chamber (46°C). They then were dehydrated using an alcohol series (50, 80, and 96% [vol/vol] ethanol) for 3 min in each solution. Slides were returned to the drying oven for 2 min to evaporate excess ethanol before adding the hybridization mixture. Hybridization mixture (50 μl consisting of 5 μl probe and 45 μl hybridization buffer) was added to each well and left to hybridize for 4 h in a microarray hybridization incubator (Grant-Boekel, Cambridge, United Kingdom). After hybridization, slides were washed in 50 ml washing buffer for 15 min. They then were dipped in cold water for a few seconds and dried with compressed air. Five microliters of polyvinyl alcohol mounting medium with 1,4-diazabicyclo(2.2.2)octane (DABCO) was added to each well, and a coverslip was placed on each slide (20 mm; thickness no. 1; VWR, Lutterworth, United Kingdom). Slides were examined under an epifluorescence microscope (Eclipse 400; Nikon, Surrey, United Kingdom) using a Fluor 100 lens. For each well, 15 different fields of view were enumerated.
Short-chain fatty acid analyses.
Analysis of SCFA was performed using an ion-exclusion HPLC system (LaChrom Merck Hitachi, Poole, Dorset, United Kingdom) equipped with a pump (L-7100), refractive index detector (L-7490), and autosampler (L-7200). Data were collected using software by Jones Chromatography Ltd. for Windows, version 2.0. The column used was an ion-exclusion Rezex ROA-Organic Acid H+ (8%) (300 by 7.80 mm; Phenomenex, Cheshire, United Kingdom). Guard columns were SecurityGuard Carbo-H+, 4- by 3.0-mm cartridges (Phenomenex, Cheshire, United Kingdom). The eluent used was 0.0025 mM sulfuric acid in HPLC-grade water.
Samples (1 ml) from each fermentation time point were centrifuged at 13,000 × g for 10 min. Supernatants were filtered through a 0.22-μm filter unit (Millipore, Cork, Ireland), and 20 μl was injected into the HPLC, operating at a flow rate of 0.5 ml/min with a heated column at 84.2°C. The sample run time was 35 min. Sample quantification was carried out using calibration curve standards for lactate, formate, acetate, propionate, isobutyrate, butyrate, isovalerate, and valerate at concentrations of 12.5, 25, 50, 75, and 100 mM.
Rate of gas production measurement.
Sterile glass Balch tubes (18 by 150 mm; Bellco, Vineland, NJ) containing 13.5 ml prereduced basal medium (peptone water at 2 g/liter [Oxoid], yeast extract at 2 g/liter [Oxoid, Basingstoke, United Kingdom], NaCl at 0.1 g/liter, K2HPO4 at 0.04 g/liter, KH2PO4 at 0.04 g/liter, MgSO4·7H2O at 0.01 g/liter, CaCl2·6H2O at 0.01 g/liter, NaHCO3 at 2 g/liter, 2 ml Tween 80 [BDH, Poole, United Kingdom], hemin at 0.05 g/liter, 10 μl vitamin K1, cysteine·HCl at 0.5 g/liter, and bile salts at 0.5 g/liter, pH 7.0) were placed in the anaerobic cabinet and kept overnight. Substrates (1/100, wt/vol) were added to the fermentation tubes just prior to the addition of the fecal inocula (1/10, wt/vol). The tubes then were sealed with a gas-impermeable butyl rubber septum (Bellco, Vineland, NJ) and an aluminum crimp (Sigma Aldrich, Gillingham, Dorset, United Kingdom). Tubes were incubated at 37°C with constant agitation.
The volume of gas generated by fecal bacteria from each substrate was measured every 3 h up to 36 h fermentation by inserting a sterile needle (23 gauge, 1 inch long) attached to a transducer (Gems Sensors, Basingtoke, United Kingdom) into the butyl rubber septum of each tube. The pressure buildup in the headspace was measured in pounds per square inch. After each measurement, the headspace of each tube was allowed to equilibrate with the atmosphere. The gas production experiments were performed in four replicates for each substrate. The quantification of gas volume (ml) was carried out using calibration curves of air pressure (pounds per square inch) by injecting known volumes of air into the culture tubes (0.5 to 7 ml).
Statistical analyses.
Statistical analysis was performed using SPSS for Windows, version 15.0. Univariate analysis of variance (ANOVA) and post hoc Tukey's test were used to determine the significant differences of substrates used on bacterial group populations, SCFA production, and gas production. Differences were deemed significant at P < 0.05.
RESULTS
Bacterial enumeration.
Bacterial group counts are shown in Table 3. There was a significant increase in the Bifidobacterium populations (enumerated by probe Bif164) in response to all 1-kDa dextran (linear and branched) tested at all time points. No significant changes were recorded in Bifidobacterium populations following the fermentation of all other test substrates. There were significant increases in the Bacteroides-Prevotella group (Bac303) at 10 h with linear 6-kDa dextran, linear 70-kDa dextran, and 70-kDa dextran + 15% α-1,2. A significant increase of the Bacteroides-Prevotella group also was detected on inulin (24 h). No significant changes were noted in Bacteroides-Prevotella group populations following the fermentation of all 1-kDa dextrans (linear and branched) and 70-kDa dextran + 37% α-1,2 at all time points. A significant decrease of Faecalibacterium prausnitzii (Fpra655) was observed in linear and highly branched dextran (70 kDa + 32% α-1,2), linear 70-kDa dextran, and inulin. No significant changes were detected in other bacterial populations with all substrates tested. Also, there were no significant changes in the total cell count (4′,6′-diamidino-2-phenylindole [DAPI] stain) in all cultures, except an increase after 10 h of fermentation of linear 1-kDa dextran.
Table 3.
Mean bacterial populations in pH-controlled batch cultures at 0, 10, 24, and 36 ha
| Probe/stain | Time point (h) | Bacterial population (log10 cells ml−1 batch culture fluid) with substrate: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dextran (1 kDa) | Dextran (1 kDa) + 16% α-1,2 | Dextran (1 kDa) + 32% α-1,2 | Dextran (6 kDa) | Dextran (6 kDa) + 33% α-1,2 | Dextran (70 kDa) | Dextran (70 kDa) + 15% α-1,2 | Dextran (70 kDa) + 37% α-1,2 | Inulin | ||
| Chis150 | 0 | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) | 7.55 (0.51) |
| 10 | 7.47 (0.33) | 7.53 (0.22) | 7.49 (0.21) | 7.57 (0.20) | 7.52 (0.30) | 7.68 (0.33) | 7.57 (0.26) | 7.72 (0.43) | 7.64 (0.38) | |
| 24 | 7.44 (0.34) | 7.32 (0.14) | 7.49 (0.30) | 7.53 (0.30) | 7.36 (0.31) | 7.34 (0.20) | 7.48 (0.32) | 7.43 (0.42) | 7.90 (0.44) | |
| 36 | 7.50 (0.26) | 7.61 (0.24) | 7.52 (0.78) | 7.80 (0.78) | 7.68 (0.37) | 7.58 (0.28) | 7.52 (0.53) | 7.54 (0.51) | 7.76 (0.51) | |
| Lab158 | 0 | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) | 7.77 (0.31) |
| 10 | 7.81 (0.29) | 7.80 (0.13) | 7.87 (0.12) | 8.08 (0.30) | 7.81 (0.19) | 7.82 (0.19) | 7.67 (0.13) | 7.64 (0.16) | 8.06 (0.46) | |
| 24 | 7.59 (0.29) | 7.51 (0.18) | 7.80 (0.63) | 7.94 (0.30) | 7.56 (0.15) | 7.56 (0.15) | 7.71 (0.08) | 7.60 (0.11) | 8.10 (0.63) | |
| 36 | 7.97 (0.53) | 7.74 (0.23) | 7.62 (0.28) | 8.03 (0.37) | 7.62 (0.22) | 7.92 (0.22) | 7.82 (0.29) | 7.54 (0.24) | 8.44 (0.29) | |
| Erec482 | 0 | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) | 8.53 (0.17) |
| 10 | 8.17 (0.33) | 8.30 (0.38) | 8.33 (0.33) | 8.20 (0.28) | 8.36 (0.18) | 8.52 (0.21) | 8.36 (0.16) | 8.25 (0.09) | 8.34 (0.33) | |
| 24 | 8.42 (0.18) | 8.38 (0.48) | 8.52 (0.29) | 8.49 (0.13) | 8.53 (0.21) | 8.42 (0.11) | 8.23 (0.17) | 8.22 (0.19) | 8.36 (0.30) | |
| 36 | 8.24 (0.13) | 8.36 (0.20) | 8.48 (0.31) | 8.74 (0.17) | 8.24 (0.32) | 8.28 (0.36) | 8.26 (0.23) | 8.33 (0.20) | 8.49 (0.28) | |
| Prop853 | 0 | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) | 8.41 (0.29) |
| 10 | 8.45 (0.30) | 8.25 (0.30) | 8.57 (0.18) | 8.46 (0.20) | 8.57 (0.20) | 8.34 (0.27) | 8.54 (0.33) | 8.44 (0.22) | 8.45 (0.17) | |
| 24 | 8.42 (0.19) | 8.59 (0.06) | 8.42 (0.27) | 8.62 (0.32) | 8.47 (0.37) | 8.44 (0.34) | 8.35 (0.49) | 8.51 (0.41) | 8.52 (0.16) | |
| 36 | 8.34 (0.16) | 8.12 (0.27) | 8.25 (0.15) | 8.24 (0.28) | 8.16 (0.38) | 8.10 (0.19) | 8.10 (0.32) | 8.31 (0.44) | 8.42 (0.22) | |
| Fpra655 | 0 | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) | 8.58 (0.34) |
| 10 | 8.13 (0.26) | 8.25 (0.19) | 8.30 (0.18) | 8.46 (0.20) | 8.28 (25) | 8.40 (0.30) | 8.25 (0.23) | 8.23 (0.26) | 7.83 (0.16)** | |
| 24 | 7.88 (0.04)** | 8.27 (0.30) | 8.08 (0.24)* | 8.35 (0.27) | 8.22 (0.26) | 8.34 (0.22) | 8.08 (0.41) | 8.39 (0.36) | 7.93 (0.19)** | |
| 36 | 7.77 (0.05)** | 8.12 (0.33) | 7.96 (0.13)** | 8.07 (0.24) | 8.34 (0.21) | 7.99 (0.15)* | 7.97 (0.29) | 7.99 (0.34) | 7.80 (0.09)** | |
| Rbro730/Rfla729 | 0 | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) | 8.36 (0.44) |
| 10 | 8.05 (0.32) | 8.25 (0.53) | 8.23 (0.53) | 8.57 (0.21) | 8.45 (0.25) | 8.16 (0.24) | 8.53 (0.30) | 8.29 (0.39) | 8.17 (0.45) | |
| 24 | 8.04 (0.44) | 8.01 (0.23) | 8.09 (0.23) | 8.19 (0.22) | 8.26 (0.25) | 8.05 (0.30) | 8.09 (0.33) | 8.07 (0.20) | 8.33 (0.56) | |
| 36 | 8.09 (0.44) | 8.16 (0.30) | 8.03 (0.30) | 8.23 (0.14) | 8.18 (0.23) | 8.13 (0.32) | 8.22 (0.29) | 7.95 (0.29) | 8.22 (0.45) | |
| Bac303 | 0 | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) | 8.48 (0.38) |
| 10 | 9.24 (0.30) | 9.12 (0.46) | 9.12 (0.57) | 9.37 (0.16)** | 8.67 (0.42) | 9.36 (0.15)* | 9.22 (0.36)* | 8.83 (0.45) | 9.07 (0.21) | |
| 24 | 9.09 (0.36) | 9.19 (0.53) | 9.18 (0.46) | 9.46 (0.12)** | 9.07 (0.32) | 9.18 (0.33) | 9.19 (0.12) | 9.02 (0.56) | 9.27 (0.28)* | |
| 36 | 8.74 (0.41) | 8.95 (0.58) | 8.98 (0.47) | 8.82 (0.39) | 9.10 (0.52) | 8.88 (0.29) | 8.85 (0.21) | 8.78 (0.64) | 8.98 (0.24) | |
| Bif164 | 0 | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) | 8.03 (0.14) |
| 10 | 9.26 (0.18)** | 8.78 (0.15)** | 8.67 (0.30)* | 8.14 (0.20) | 8.04 (0.25) | 8.53 (0.32) | 8.13 (0.22) | 8.05 (0.24) | 8.42 (0.43) | |
| 24 | 9.13 (0.22)** | 8.72 (0.25)** | 8.82 (0.37)** | 8.27 (0.31) | 7.91 (0.05) | 8.46 (0.14) | 8.41 (0.53) | 8.12 (0.29) | 8.59 (0.25) | |
| 36 | 9.26 (0.40)** | 8.94 (0.25)** | 8.79 (0.16)** | 7.90 (0.18) | 7.71 (0.24) | 8.65 (0.26) | 8.40 (0.51) | 7.83 (0.23) | 8.10 (0.47) | |
| Ato291 | 0 | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) | 8.15 (0.45) |
| 10 | 8.24 (0.30) | 8.36 (0.52) | 8.25 (0.23) | 8.46 (0.52) | 8.21 (0.30) | 8.45 (0.30) | 8.30 (0.29) | 8.16 (0.16) | 8.47 (0.66) | |
| 24 | 8.31 (0.50) | 8.30 (0.57) | 8.30 (0.66) | 8.53 (0.31) | 8.22 (0.23) | 8.36 (0.32) | 8.18 (0.27) | 8.33 (0.23) | 8.74 (0.34) | |
| 36 | 8.45 (0.17) | 8.44 (0.44) | 8.32 (0.47) | 8.63 (0.12) | 8.18 (0.13) | 8.54 (0.31) | 8.36 (0.36) | 8.15 (0.27) | 8.85 (0.20) | |
| DAPI | 0 | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) | 9.46 (0.12) |
| 10 | 9.81 (0.02)* | 9.76 (0.20) | 9.63 (0.33) | 9.63 (0.15) | 9.75 (0.31) | 9.76 (0.11) | 9.60 (0.28) | 9.41 (0.24) | 9.70 (0.11) | |
| 24 | 9.76 (0.19) | 9.72 (0.29) | 9.77 (0.27) | 9.87 (0120) | 9.72 (0.27) | 9.65 (0.28) | 9.50 (0.13) | 9.57 (0.22) | 9.74 (0.16) | |
| 36 | 9.69 (0.19) | 9.67 (0.33) | 9.57 (0.13) | 9.71 (0.35) | 9.80 (0.15) | 9.64 (0.32) | 9.80 (0.26) | 9.57 (0.27) | 9.69 (0.22) | |
The starting concentrations of the test substrates were 1% (wt/vol) of 50 ml batch culture fluid. **, significant difference from the 0-h value, P < 0.01; *, significant difference from the 0-h value, P < 0.05. Standard deviations are shown in parentheses (n = 4).
Short-chain fatty acid analyses.
Table 4 shows the SCFA concentrations in the cultures. The total SCFA concentrations determined from all batch culture fermentations are similar. However, depending on the substrates used, variations are observed in the ratios of each type of SCFA. Acetate production from the fermentation of the linear 1-kDa dextran is significantly higher than that observed from 70-kDa dextran + 32% α-1,2 fermentation (at all time points) and inulin at 24 h. Also, butyrate production from the fermentation of 70-kDa dextran + 32% α-1,2 are significantly lower than that obtained from the fermentation of 6-kDa dextran at 36 h. Meanwhile, lactate production from the fermentation of 1-kDa dextran at 10 h is significantly higher than that of all other dextrans, but not inulin.
Table 4.
Mean SCFA and lactic acid concentrations in pH-controlled batch cultures at 0, 10, 24 and 36 ha
| SCFA | Time point (h) | Mean SCFA and lactic acid concn (mM) in substrate: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dextran (1 kDa) | Dextran (1 kDa) + 16% α-1,2 | Dextran (1 kDa) + 32% α-1,2 | Dextran (6 kDa) | Dextran (6 kDa) + 33% α-1,2 | Dextran (70 kDa) | Dextran (70 kDa) + 15% α-1,2 | Dextran (70 kDa) + 37% α-1,2 | Inulin | ||
| Lactate | 0 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a |
| 10 | 8.98 (4.65)b** | 0.10 (0.46)a | 0.60 (1.19)a | 0.57 (1.14)a | 0.00 (0.00)a | 0.30 (0.50)a | 0.00 (0.00)a | 0.54 (1.08)a | 5.16 (5.12)ab | |
| 24 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.26 (0.53)a | 0.33 (0.66)a | 0.00 (0.00)a | 2.15 (2.69)a | 1.24 (2.47)a | |
| 36 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 1.14 (2.28)a | 0.40 (0.80)a | |
| Acetate | 0 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a |
| 10 | 45.13 (14.25)b** | 36.08 (15.68)ab | 20.66 (11.60)ab** | 15.30 (3.44)a | 12.90 (10.21)a | 23.43 (11.71)ab** | 15.58 (6.86)a | 8.44 (2.19)a | 25.27 (8.97)ab** | |
| 24 | 57.67 (6.13)b** | 36.77 (16.81)ab** | 42.45 (5.60)ab** | 35.04 (5.60)a** | 32.44 (7.33)a** | 37.86 (8.06)ab** | 40.17 (10.95)ab** | 30.70 (6.25)a** | 34.59 (3.96)a** | |
| 36 | 53.35 (6.86)b** | 52.67 (13.03)b** | 41.97 (5.47)ab** | 38.27 (11.61)ab** | 35.57 (8.92)ab** | 35.59 (3.94)ab** | 39.93 (12.50)ab** | 30.14 (7.55)a** | 35.43 (3.83)ab** | |
| Propionate | 0 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a |
| 10 | 7.70 (5.04)a | 10.90 (7.80)a | 9.09 (6.53)a* | 7.75 (3.60)a | 7.62 (5.75)a | 12.59 (5.79)a | 8.35 (2.86)a** | 4.55 (3.00)a | 10.97 (5.26)a | |
| 24 | 15.04 (5.12)a** | 20.56 (8.75)a** | 28.66 (3.40)a** | 27.74 (6.63)a** | 25.09 (2.74)a** | 25.07 (9.06)a** | 27.60 (1.69)a** | 25.37 (12.78)a** | 18.76 (7.72)a* | |
| 36 | 19.26 (5.87)a** | 22.60 (7.20)a** | 28.75 (4.52)a** | 31.21 (4.27)a** | 26.11 (4.40)a** | 27.17 (9.91)a** | 29.29 (1.85)a** | 29.12 (6.45)a** | 19.74 (7.04)a* | |
| Butyrate | 0 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a |
| 10 | 0.59 (1.18)a | 2.65 (2.13)a | 1.25 (1.58)a | 1.50 (0.56)a | 1.05 (2.10)a | 2.27 (1.87)a | 1.26 (1.60)a | 0.00 (0.00)a | 5.33 (6.96)a | |
| 24 | 3.71 (1.00)ab** | 2.66 (1.71)ab | 4.20 (1.37)ab* | 6.01 (0.84)ab** | 3.95 (2.31)ab* | 4.79 (0.74)ab** | 4.55 (2.05)ab* | 2.51 (1.75)a* | 8.30 (5.12)b | |
| 36 | 5.10 (1.96)ab** | 3.40 (1.14)ab | 4.92(1.39)ab** | 8.86 (1.73)b** | 5.33 (1.78)ab** | 6.42 (1.40)ab** | 5.18 (2.51)ab* | 3.02 (1.47)a** | 8.49 (4.29)b | |
| Total | 0 | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a |
| 10 | 53.42 (19.74)a** | 30.03 (25.51)a | 31.00 (19.56)a** | 24.55 (5.58)a** | 21.57 (18.01)a* | 38.29 (17.93)a** | 25.18 (10.56)a | 12.99 (5.16)a | 41.57 (16.10)a** | |
| 24 | 76.41 (10.01)a** | 59.98 (26.57)a** | 75.31 (4.09)a** | 68.79 (4.31)a** | 61.48 (7.76)a** | 67.72 (11.64)a** | 72.31 (11.44)a** | 58.57 (19.23)a** | 61.65 (3.92)a** | |
| 36 | 77.70 (9.09)a** | 59.00 (39.73)a** | 75.63 (1.06)a** | 78.34 (10.93)a** | 67.00 (9.45)a** | 69.18 (11.83)a** | 74.40 (15.56)a** | 62.28 (13.31)a** | 63.66 (2.03)a** | |
Starting concentration of the test substrates were 1% (wt/vol) of 50 ml batch culture fluid. **, significant difference from 0-h value, P < 0.01; *, significant difference from 0-h value, P < 0.05. Standard deviations are shown in parentheses (n = 4). Significant differences (P < 0.05) among treatments are indicated with different letters from the same row of data (a, lowest concentration; b, highest concentration).
The total SCFA concentration was significantly increased on all substrates tested after 24 h of fermentation (P < 0.01). Acetate was the most prevalent SCFA on all substrates tested, accounting for >48% of the SCFA produced, followed by propionate and butyrate. Significant increases in acetate were found on all substrates tested, and the highest concentration was observed in linear 1-kDa dextran fermentation at 10 h. A significant increase in propionate was found in all substrates tested, with the highest propionate levels being found with 6-kDa dextran. The results show a trend for higher proportions of propionate with higher-molecular-mass dextrans, namely 6 and 70 kDa, than with 1-kDa dextran. A significant increase in propionate concentration was observed beyond 10 h with all dextrans tested (except linear 1- and 70-kDa dextran) compared to the concentration with inulin. Low levels of butyrate were produced upon the fermentation of all test substrates. However, the highest significant butyrate levels were recorded for linear 6-kDa dextran, which persisted beyond 10 h. A significant increase in lactate concentration was observed only after the fermentation of linear 1-kDa dextran, which significantly decreased after 10 h.
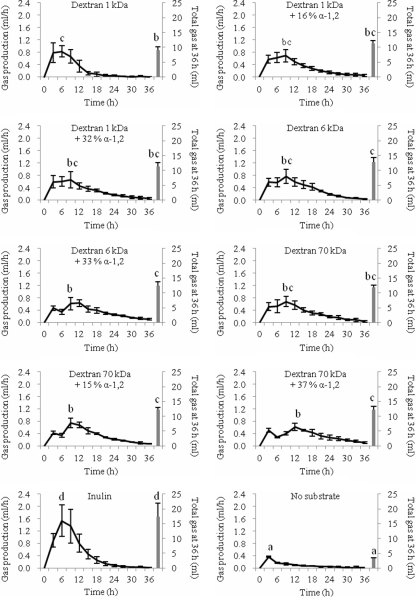
Total gas production.
The total gas production of each substrate incubated with healthy volunteer fecal slurries (n = 4) after 36 h is shown in Fig. 1. The largest amounts of gas were produced from the fermentation of inulin (P < 0.05). As for the unbranched dextrans, total gas produced significantly increased in the following sequence: 1-kDa dextran (unbranched) < 70-kDa dextran (unbranched) < 6-kDa dextran (unbranched), with linear 1-kDa dextran producing the smallest amount of total gas after 36 h (P < 0.05) compared to the amounts of all other substrates tested.
Fig. 1.
Gas production pattern expressed in milliliters per hour and total gas production in 36 h (shown as a single column) from non-pH-controlled batch culture. Significant differences (P < 0.05) among treatments are indicated with different letters (a, slowest gas rate/least total gas; d, fastest gas rate/most total gas) (n = 4).
Rate of gas production.
Figure 1 shows the gas production patterns of fecal fermentation on each substrate. Generally, at ≤36 h all of the dextrans tested produced gas at a lower rate than inulin (P < 0.05). The fermentation of inulin rapidly produced gas, which peaked at 6 h. A relationship between a higher rate of gas production and high total gas production was seen on the fermentation of inulin. However, this is not the case for dextran, where a high rate of gas production was observed on the fermentation of linear 1-kDa dextran, which at the same time produced the least total gas of all substrates. This shows that the linear 1-kDa dextran is rapidly fermented to produce smaller amounts of gas, and this fermentation ended earlier than that for any other substrate tested (approximately at 24 h). All other dextrans showed a more gradual increase and subsequent decrease in gas production. All dextrans tested reached peak gas production rates at 9 h, except linear 1-kDa dextran and branched 70-kDa dextran + 37% α-1,2, which peaked at 3 and 12 h, respectively.
DISCUSSION
In this study, the 1-kDa dextrans (linear and branched) gave rise to significant increases in Bifidobacterium populations and high selectivity for this genus. This may be because the low molecular mass means more nonreducing ends per unit of mass, which are susceptible to attack by various exo-acting α- and β-glucosidases produced by colonic bacteria such as Bifidobacterium spp. (59). α-Glucosidase enzyme activities were commonly observed among bifidobacteria (54). A recent study of Pokusaeva et al. (43) shows that α-glucosidase from Bifidobacterium breve UCC2003 belongs to a subgroup of the glycoside hydrolase family 13, the α-1,6-glucosidases (EC 3.2.1.10), which exhibit hydrolytic activity toward α-1,6-linked carbohydrates such as panose, isomaltose, and isomaltotriose.
A greater increase in glycosidase activities following the fermentation of α-glucooligosaccharides compared to that of β-fructooligosaccharides and β-galactooligosaccharides has been shown by Djouzi and Andrieux (14). α-Glucooligosaccharides led to a nonspecific induction of β-galactosidase (EC 3.2.1.23), β-glucosidase (EC 3.2.1.21), and α-glucosidase (EC 3.2.1.20) activities, whereas the induction of β-glucuronidase (EC 3.2.1.31) was lowered, which could be considered beneficial for the host. β-Glucuronidase is involved in the generation of toxic and carcinogenic metabolites (49), whereas β-galactosidase and α-glucosidase activities can improve carbohydrate fermentation to SCFA (37).
Our current understanding of prebiotic substances is that low-molecular-mass oligosaccharides are more rapidly fermented than high-molecular-mass carbohydrates by bifidobacteria and lactobacilli (21). Most of the findings that relate to such structure-function relationships are based on in vitro experiments. A limitation to performing in vivo study is the need for large carbohydrate quantities, which usually involves complex production.
Olano-Martin et al. (41) demonstrated that lower-DP oligodextrans produced by the controlled enzymatic hydrolysis of high-molecular-mass industrial dextran resulted in higher fermentation selectivity for bifidobacteria compared to that of the parent molecule and other oligodextran fractions with higher average DP. Kaneko et al. (32) also reported an influence of DP with the structurally related isomaltooligosaccharides (IMO) on human fecal bifidobacteria with a DP of 3, which gives a higher prebiotic activity than a DP of 2. In addition, using in vitro batch cultures, maltose-based oligosaccharides with a DP of 3 resulted in the highest selectivity toward bifidobacteria, with oligosaccharides with a DP of >7 not displaying selectivity for bifidobacteria (51). Furthermore, Olano-Martin et al. (41) found that low-molecular-mass oligodextran was the best substrate for bifidobacteria and lactobacilli in all three vessels of an in vitro gut model.
Our study demonstrated that the 1-kDa linear and branched dextrans are the best selective inducers of bifidobacteria, and the linear and highly branched 1-kDa dextrans also specifically suppressed the Faecalibacterium prausnitzii group of bacteria. However, no significant changes in Bifidobacterium populations were detected with inulin supplementation. This is because most bifidobacteria were unable to grow on longer chain inulin (48). Only limited species of bifidobacteria are capable of inulin degradation, such as Bifidobacterium thermophilum and B. adolescentis, which possess extracellular fructofuranosidases (48). This also may be due to the type of inulin used (Frutafit TEX; Sensus, Roosendaal, The Netherlands), which has a higher DP than other commercial inulin-type fructans, such as Raftilin ST, Raftilose P95 (Orafti, Tienen, Belgium), and Frutafit IQ (Sensus, Roosedaal, The Netherlands) (3). The degree of polymerization is a determining factor in the Bifidobacterium metabolism of FOS. Generally, as chain length increased, consumption by bifidobacteria decreased (56).
Here, we found that an increase in α-1,2 branching did not lead to the higher selectivity of bifidobacteria. However, α-1,2 branching may decrease the digestibility of the 1-kDa dextrans, as assessed by the higher total dietary fiber content of branched 1-kDa dextrans than linear 1-kDa dextrans (Table 1). Kinetic studies of glucooligosaccharide hydrolysis in pH-regulated fermenters found that α-1,2 glucosidic bonds were more resistant than α-1,6 linkages (15). Sanz et al. (51) found that α-1,2-linked disaccharides were more selective for Bifidobacterium spp. using in vitro fermentation experiments, and α-1,6-linked disaccharides also were selective. These observations are not consistent with the results of our current study. The Sanz et al. study (51), however, used a range of disaccharides in an attempt to obtain structure-function information. In addition, that study used non-pH-controlled fermentation to assess selectivity due to the limited availability of substrates.
The fermentation of all substrates tested did not induce the production of lactate, with the exception of unbranched 1-kDa dextran at 10 h, which significantly differs from all dextrans tested. This correlates with the significant increase of Bifidobacterium populations on 1-kDa dextran. Bifidobacterium spp. and lactic acid bacteria such as Lactobacillus and Enterococcus spp. produce lactate as a major product (12, 47). Lactate levels, however, subsequently declined beyond 10 h on all substrates tested. This is expected, as lactate is utilized by other bacteria (27) which then produce acetate, butyrate, and propionate (18). In addition, the direct conversion of lactate to butyrate and/or propionate can occur in the mixed-gut community, as shown by stable isotopic tracer studies (6). Lactate generally does not accumulate in healthy subjects. However, it does accumulate in certain gut disorders, e.g., ulcerative colitis, where concentrations of up to 100 mM lactate have been observed (63).
There was a considerable increase in propionate concentration following the fermentation of all tested dextrans and inulin. The highest significant increase was seen with 6-kDa dextran. This is in accord with a significant increase in the Bacteroides-Prevotella group, as these are known to be propionate producers (41). Bacteroides fragilis-type bacteria are a prevalent group in the human gut microbial community, and they produce substantial amounts of propionate from succinate and fumarate (61). Previous in vitro and in vivo studies show that Bacteroides thetaiotamicron is highly efficient in utilizing α-1,2 and α-1,6 glycosidic linkages in α-glucooligosaccharides (15). A number of bacterial groups could be implicated in propionate production, including clostridia. Some species within the Clostridium histolyticum group can produce propionate, such as C. homopropionicum (50, 65). Further, clostridial cluster IX contains known propionate producers (65), and species within it (e.g., Succiniclasticum ruminis and Succinispira mobilis) have been reported to convert succinate into propionate (30, 61). We did not, however, see a significant increase of C. histolyticum and Clostridium cluster IX. Djouzi et al. (15) also saw increased propionate production on the fermentation of glucooligosaccharides, possibly correlated to Bacteroides populations.
The amount of butyrate produced with 6-kDa dextran was higher than that with other tested glucooligosaccharides and inulin. The populations of Faecalibacterium prausnitzii and the Clostridium coccoides-Eubacterium rectale group, major butyrate producers (1), were not stimulated by the supplementation of this substrate. Therefore, it is suspected that the significant amount of butyrate following the fermentation of 6-kDa dextran was due to the significant increase of levels of the Bacteroides-Prevotella group.
Gas production in the large intestine is part of a normal digestive function caused by the fermentation of carbohydrate by the gut microbiota. The major gases produced in the gastrointestinal tract are CO2 and H2 by bacterial groups such as clostridia and enterobacteria (9). These gases are inevitable fermentation products but also are the major clinical disincentive to the consumption of prebiotics. Unwanted symptoms relating to gas production in the gut are widely reported in human prebiotic feeding studies (26, 29, 57).
It is known that bifidobacteria do not produce gas; however, many clostridial cluster IV and XIVa butyrate producers have been shown to produce gases, mainly CO2 and H2 (16, 18, 19, 53). Consequently, these organisms might produce gas as a result of either cross-feeding or the direct degradation of inulin-type fructans (17). Indeed, inulin-type fructan consumption has been reported to cause some gastrointestinal discomfort, i.e., flatulence and bloating (45). At a dose of 14 g/day, highly significant increases in flatulence, rumbling, stomach and gut cramps, and bloating were seen in a group of 64 women taking inulin in a double-blind crossover study for 4-week periods. Twelve percent of the volunteers considered the flatulence severe and unacceptable (42). Compared to results of our in vitro study, the fermentation of inulin produced the largest amount of gas at a higher rate than that of the dextrans. The dextrans produced less total gas and did so at a lower rate.
Gas production probably is influenced by the chemical structure of carbohydrates, such as differing chain length and monosaccharide composition (10, 13), which suggest that molecules with longer chain lengths are fermented more slowly (7, 35). However, to date, this has not been demonstrated in vivo. Nevertheless, our findings showed that the lower-molecular-mass dextran, i.e., 1-kDa dextran (linear), is more rapidly fermented than 70-kDa dextran (branched). Unlike β-fructooligosaccharides and β-galactooligosaccharides, α-glucooligosaccharides reduced H2 production (40). We report here, for the first time, the study of gas rate production following α-glucooligosaccharide fermentation. In this study, all substrates generated some gas after 3 h of fermentation, and the patterns of gas production were similar among all dextrans tested but were significantly lower than levels for inulin. The linear 1-kDa dextran produced the lowest total gas and shortest time to attain maximal gas production (3 to 6 h) of all the dextrans. Complex dextrans (higher-molecular-mass and/or highly branched), showing slower fermentation and gas production rates, may persist to the distal part of the colon and encourage beneficial saccharolytic fermentation.
The in vitro studies described in this paper suggest that low-molecular-mass (1-kDa) α-1,6-glucans have potential bifidogenic activity and are worthy of further in vivo study. This, in combination with the fact that the α-1,2 glycosidic linkages result in a higher total dietary fiber and a very high resistance to hydrolysis by digestive enzymes in both humans and animals (58), makes these types of branched dextrans interesting prebiotic candidates.
ACKNOWLEDGMENTS
The Sarawak Foundation of the Malaysian government funded a scholarship for Shahrul R. Sarbini.
This research is partly funded by Tate & Lyle and is considered proprietary data in accordance with EC Regulation no. 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods.
Footnotes
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Barcenilla A., et al. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Behravan J., Bazzaz B. S. F., Salimi Z. 2003. Optimization of dextran production by Leuconostoc mesenteroides NRRL B-512 using cheap and local sources of carbohydrates and nitrogen. Biotechnol. Appl. Biochem. 38:267–269 [DOI] [PubMed] [Google Scholar]
- 3. Borromei C., et al. 2009. Evaluation of fructooligosaccharides and inulins as potentially health benefiting food ingredients by HPAEC-PED and MALDI-TOF MS. Int. J. Anal. Chem. 2009:530639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouhnik Y., et al. 2004. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 80:1658–1664 [DOI] [PubMed] [Google Scholar]
- 5. Bounaix M., et al. 2010. Characterization of glucan-producing Leuconostoc strains isolated from sourdough. Int. J. Food Microbiol. 144:1–9 [DOI] [PubMed] [Google Scholar]
- 6. Bourriaud C., et al. 2005. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 99:201–212 [DOI] [PubMed] [Google Scholar]
- 7. Brighenti F., et al. 1995. Comparison of lactulose and inulin as reference standard for the study of resistant starch fermentation using hydrogen breath test. Ital. J. Gastroenterol. 27:122–128 [PubMed] [Google Scholar]
- 8. Brison Y., et al. 2010. Synthesis of dextrans with controlled amounts of α-1,2 linkages using the transglucosidase GBD-CD2. Appl. Microbiol. Biotechnol. 86:545–554 [DOI] [PubMed] [Google Scholar]
- 9. Chaia A. P., Oliver G. 2008. Intestinal microflora and metabolic activity. In Fuller R., Perdigón G. (ed.), Gut flora, nutrition, immunity and health. Blackwell Publishing Ltd., Oxford, United Kingdom [Google Scholar]
- 10. Christl S. U., Murgatroyd P. R., Gibson G. R., Cummings J. H. 1992. Production, metabolism and excretion of hydrogen in the large intestine. Gastroenterology 102:1424–1426 [PubMed] [Google Scholar]
- 11. Chung C. H., Day D. F. 2002. Glucooligosaccharides from Leuconostoc mesenteroides B-742 (ATCC 13146): a potential prebiotic. J. Ind. Microbiol. Biotechnol. 29:196–199 [DOI] [PubMed] [Google Scholar]
- 12. Cummings J. H. 1995. Short chain fatty acids, p. 101–130 In Gibson G. R., Macfarlane G. T. (ed.), Human colonic bacteria: role in nutrition, physiology and pathology. CRC Press, Boca Raton, FL [Google Scholar]
- 13. Cummings J. H., Macfarlane G. T., Englyst H. N. 2001. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 73:415S–420S [DOI] [PubMed] [Google Scholar]
- 14. Djouzi Z., Andrieux C. 1997. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with human faecal flora. Br. J. Nutr. 78:313–324 [DOI] [PubMed] [Google Scholar]
- 15. Djouzi Z., et al. 1995. Degradation and fermentation of α-glucooligosaccharides by bacterial strains from human colon: in vitro and in vivo studies in gnotobiotic rats. J. Appl. Bacteriol. 79:117–127 [DOI] [PubMed] [Google Scholar]
- 16. Duncan S. H., Hold G. L., Barcenilla A., Stewart C. S., Flint H. J. 2002. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 52:1615–1620 [DOI] [PubMed] [Google Scholar]
- 17. Duncan S. H., et al. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermenter system. Appl. Environ. Microbiol. 69:1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duncan S. H., Louis P., Flint H. J. 2004. Lactate-utilizing bacteria, isolated from human feces that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 70:5810–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falony G., Vlachou A., Verbrugghe K., De Vuyst L. 2006. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate producing colon bacteria during growth on oligofructose. Appl. Environ. Microbiol. 72:7835–7841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franks A. H., et al. 1998. Variations of bacterial populations in human faeces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibson G. R. 2004. Fibre and effects on probiotics (the prebiotic concept). Clin. Nutr. Suppl. 1:25–31 [Google Scholar]
- 22. Gibson G. R., Rastall R. A., Roberfroid M. B. 1999. Prebiotics, p. 101–124 In Gibson G. R., Roberfroid M. B. (ed.), Colonic microbiota, nutrition and health. Kluwer Academic Press, Doordrecht, The Netherlands [Google Scholar]
- 23. Harmsen H. J. M., et al. 2000. Development of 16S rRNA-based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 66:4523–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harmsen H. J. M., Raangs G. C., He T., Degener J. E., Welling G. W. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harmsen H. J. M., Elfferich P., Schut F., Welling G. W. 1999. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 11:3–12 [Google Scholar]
- 26. Hartemink R., Rombouts F. M. 1997. Gas formation from oligosaccharides by the intestinal microflora, p. 57–66 In Hartemink R. (ed.), Non-digestible oligosaccharides: healthy food for the colon? Proc. Int. Symp., Wageningen Grad. School. VLAG, Wageningen, Germany [Google Scholar]
- 27. Hashizume K., Tsukahara T., Yamada K., Koyama H., Ushida K. 2003. Megasphaera elsdenii JCM1772T normalizes hyperlactate production in the large intestine of fructooligosaccharide-fed rats by stimulating butyrate production. J. Nutr. 133:3187–3190 [DOI] [PubMed] [Google Scholar]
- 28. Hold G. L., Schwiertz A., Aminov R. I., Blaut M., Flint H. J. 2003. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 69:4320–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito M., et al. 1990. Effects of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microb. Ecol. Health Dis. 3:285–292 [Google Scholar]
- 30. Janssen P. H., O'Farrell K. A. 1999. Succinispira mobilis gen. nov., sp. nov., a succinate-decarboxylating anaerobic bacterium. Int. J. Syst. Bacteriol. 49:1009–1013 [DOI] [PubMed] [Google Scholar]
- 31. Jeanes A., et al. 1954. Characterization and classification of dextrans from ninety-six strains of bacteria. J. Am. Chem. Soc. 76:5041–5052 [Google Scholar]
- 32. Kaneko T., et al. 1994. Effects of isomaltooligosaccharides with different degrees of polymerization on human fecal bifidobacteria. Biosci. Biotechnol. Biochem. 58:2288–2290 [Google Scholar]
- 33. Kobayashi M., Matsuda K. K. 1977. Structural characteristics of dextran synthesized by dextransucrases from Leuconostoc mesenteroides NRRL B-1299. Agric. Biol. Chem. 41:1937 [Google Scholar]
- 34. Langendijk P. S., et al. 1995. Quantitative fluorescence in situ hybridisation of Bifidobacterium with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livesey G., et al. 1993. Determination of sugar alcohol and polydextrose absorption in humans by the breath hydrogen (H2) technique: the stoichiometry of hydrogen production and the interaction between carbohydrates assessed in vivo and in vitro. Eur. J. Clin. Nutr. 47:419–430 [PubMed] [Google Scholar]
- 36. Macfarlane G. T., Steed H., Macfarlane S. 2008. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 104:305–344 [DOI] [PubMed] [Google Scholar]
- 37. Macfarlane G. T., Cummings J. H. 1991. The colonic flora, fermentation and large bowel digestive function, p. 51–92 In Phillips S. F., Pemberton J. H., Shorter R. G. (ed.), The large intestine: physiology, pathophysiology and disease. Raven Press, New York, NY [Google Scholar]
- 38. Macfarlane S., Macfarlane G. T. 2003. Food and the large intestine, p. 24–51 In Fuller R., Perdigon G. (ed.), Gut flora, nutrition, immunity and health. Blackwell Publishing, Oxford, United Kingdom [Google Scholar]
- 39. Manz W., Amman R., Ludwig W., Vancanneyt M., Schleifer K. H. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142:1097–1106 [DOI] [PubMed] [Google Scholar]
- 40. Monsan P. F., Paul F. 1995. Oligosaccharide feed additives, p. 233–235 In Wallace R. J., Chesson A. (ed.), Biotechnology in animal feeds and feeding. VCH, Weinheim, Germany [Google Scholar]
- 41. Olano-Martin E., Mountzouris K. C., Gibson G. R., Rastall R. A. 2000. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br. J. Nutr. 83:247–255 [DOI] [PubMed] [Google Scholar]
- 42. Pedersen A., Sandstrom B., Van Amelsvoort J. M. M. 1997. The effect of ingestion of inulin on blood lipids and gastrointestinal symptoms in healthy females. Br. J. Nutr. 78:215–222 [DOI] [PubMed] [Google Scholar]
- 43. Pokusaeva K., Zomer M. O.-M. A., Fitzgerald G. F., van Sinderen D. 2009. Characterization of two novel α-glucosidases from Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 75:1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roberfroid M. B. 1998. Prebiotics and synbiotics: concepts and nutritional properties. Br. J. Nutr. 80:S197–S202 [PubMed] [Google Scholar]
- 45. Roberfroid M. B. 2005. The digestive functions: inulin and oligofructose as dietary fiber, p. 103–131 In Roberfroid M. B., Wolinsky I. (ed.), Inulin type fructans: functional food ingredients. CRC Press, Boca Raton, FL [Google Scholar]
- 46. Roberfroid M. B., et al. 2010. Prebiotic concept: definition, metabolic and health benefits. Br. J. Nutr. 104:S1–S63 [DOI] [PubMed] [Google Scholar]
- 47. Rolfe R. D. 2000. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 130:396–402 [DOI] [PubMed] [Google Scholar]
- 48. Rossi M., et al. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 71:6150–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rowland I. R. 1988. Factors affecting metabolic activity of the intestinal microflora. Drug Metab. Rev. 19:243–261 [DOI] [PubMed] [Google Scholar]
- 50. Salminen S., et al. 1998. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 80:S147–S171 [DOI] [PubMed] [Google Scholar]
- 51. Sanz M. L., Gibson G. R., Rastall R. A. 2005. Influence of disaccharide structure on prebiotic selectivity in vitro. J. Agric. Food Chem. 53:5192–5199 [DOI] [PubMed] [Google Scholar]
- 52. Sarwat F., Qader S. A. U., Aman A., Ahmed N. 2008. Production and characterization of a unique dextran from an indigenous Leuconostoc mesenteroides CMG713. Int. J. Biol. Sci. 4:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwiertz A., et al. 2002. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilizing, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 25:46–51 [DOI] [PubMed] [Google Scholar]
- 54. Sidebotham R. L. 1974. Dextrans. Adv. Carbohydr. Chem. Biochem. 30:371–444 [DOI] [PubMed] [Google Scholar]
- 55. Steer T., Carpenter H., Tuohy K., Gibson G. R. 2000. Perspectives on the role of the human gut microbiota and its modulation by pro- and prebiotics. Nutr. Res. Rev. 13:229–254 [DOI] [PubMed] [Google Scholar]
- 56. Stewart M. L., Timm D. A., Slavin J. L. 2008. Fructooligosaccharides exhibit more rapid fermentation than long-chain inulin in an in vitro fermentation system. Nutr. Res. 28:329–334 [DOI] [PubMed] [Google Scholar]
- 57. Tuohy K. M., Kolida S., Lustenberger A. M., Gibson G. R. 2001. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides—a human volunteer study. Br. J. Nutr. 86:341–348 [DOI] [PubMed] [Google Scholar]
- 58. Valette P., et al. 1993. Bioavailability of new synthesized glucooligosaccharides in the intestinal tract of gnotobiotic rats. J. Sci. Food Agric. 62:121–127 [Google Scholar]
- 59. Van den Broek L. A., Hinz S. W., Beldman G., Vincken J. P., Voragen A. G. 2008. Bifidobacterium carbohydrases—their role in breakdown and synthesis of (potential) prebiotics. Mol. Nutr. Food Res. 52:146–163 [DOI] [PubMed] [Google Scholar]
- 60. Van Geel-Schutten G. H., et al. 1999. Biochemical and structural characterization of the glucan and fructan exopolysaccharides synthesized by the Lactobacillus reuteri wild-type strain and by mutant strains. Appl. Environ. Microbiol. 65:3008–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Van Gylswyk N. O. 1995. Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 45:29–300 [DOI] [PubMed] [Google Scholar]
- 62. Vedyashkina T. A., Revin V., Gogotov I. N. 2005. Optimizing the conditions of dextran synthesis by the bacterium Leuconostoc mesenteroides grown in a molasses-containing medium. Prickl. Biokhim. Mikrobiol. 41:361–364 [PubMed] [Google Scholar]
- 63. Vernia P., et al. 1988. Fecal lactate and ulcerative colitis. Gastroenterology 95:1564–1568 [DOI] [PubMed] [Google Scholar]
- 64. Walker A. W., Duncan S. H., Leitch E. C. M., Child M. W., Flint H. J. 2005. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71:3692–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zigova J., Sturdk E., Vandak D., Schlosser S. 1999. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 34:835–843 [Google Scholar]